Limited Antioxidant Effect of Rosemary in Lipid Oxidation of Pan-Fried Salmon
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Fish Samples, Cooking Oils, and Condiments
2.3. Sample Preparation and Analysis
2.3.1. Condiments and Cooking Oils
2.3.2. Condiment-Infused Oil
2.3.3. Oil from Salmon Meat
2.3.4. Salmon Meat
2.4. Statistical Analysis
3. Results and Discussion
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish Consumption, Fish Oil, Omega-3 Fatty Acids, and Cardiovascular Disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Bouwens, M.; Van De Rest, O.; Dellschaft, N.; Bromhaar, M.G.; De Groot, L.C.; Geleijnse, J.M.; Muller, M.; A Afman, L. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am. J. Clin. Nutr. 2009, 90, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. Omega-3 polyunsaturated fatty acids in the brain: Metabolism and neuroprotection. Front. Biosci. 2011, 16, 2653–2670. [Google Scholar] [CrossRef]
- Thomas, J.; Thomas, C.J.; Radcliffe, J.; Itsiopoulos, C. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed]
- Rajaei, E.; Mowla, K.; Ghorbani, A.; Bahadoram, S.; Bahadoram, M.; Dargahi-Malamir, M. The Effect of Omega-3 Fatty Acids in Patients with Active Rheumatoid Arthritis Receiving DMARDs Therapy: Double-Blind Randomized Controlled Trial. Glob. J. Health Sci. 2015, 8, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Calder, P.C. Conversion of α-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef]
- Al-Saghir, S.; Thurner, K.; Wagner, K.-H.; Frisch, G.; Luf, W.; Razzazi-Fazeli, E.; Elmadfa, I. Effects of Different Cooking Procedures on Lipid Quality and Cholesterol Oxidation of Farmed Salmon Fish (Salmo salar). J. Agric. Food Chem. 2004, 52, 5290–5296. [Google Scholar] [CrossRef]
- Leung, K.S.; Galano, J.M.; Durand, T.; Lee, J.C. Profiling of Omega-Polyunsaturated Fatty Acids and Their Oxidized Products in Salmon after Different Cooking Methods. Antioxidants 2018, 7, 96. [Google Scholar] [CrossRef]
- Pokorny, J. Changes of nutrients at frying temperature. In Frying of Food; Technomic Publishing CO.: Lancaster, PA, USA, 1999; pp. 66–103. [Google Scholar]
- Ohshima, T.; Shozen, K.-I.; Ushio, H.; Koizumi, C. Effects of Grilling on Formation of Cholesterol Oxides in Seafood Products Rich in Polyunsaturated Fatty Acids. LWT Food Sci. Technol. 1996, 29, 94–99. [Google Scholar] [CrossRef]
- Tarley, C.R.; Visentainer, J.V.; Matsushita, M.; E De Souza, N. Proximate composition, cholesterol and fatty acids profile of canned sardines (Sardinella brasiliensis) in soybean oil and tomato sauce. Food Chem. 2004, 88, 1–6. [Google Scholar] [CrossRef]
- Gladyshev, M.; Sushchik, N.; Gubanenko, G.; Demirchieva, S.; Kalachova, G. Effect of boiling and frying on the content of essential polyunsaturated fatty acids in muscle tissue of four fish species. Food Chem. 2007, 101, 1694–1700. [Google Scholar] [CrossRef]
- Stephen, N.M.; Shakila, R.J.; Jeyasekaran, G.; Sukumar, D. Effect of different types of heat processing on chemical changes in tuna. J. Food Sci. Technol. 2010, 47, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Flaskerud, K.; Bukowski, M.; Golovko, M.; Johnson, L.; Brose, S.; Ali, A.; Cleveland, B.; Picklo, M.; Raatz, S. Effects of cooking techniques on fatty acid and oxylipin content of farmed rainbow trout (Oncorhynchus mykiss). Food Sci. Nutr. 2017, 5, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Dai, Q.; Roberts, L.J., 2nd. The isoprostanes—25 years later. Biochim. Biophys. Acta 2015, 1851, 433–445. [Google Scholar] [CrossRef]
- Gao, L.; Yin, H.; Milne, G.L.; Porter, N.A.; Morrow, J.D. Formation of F-ring Isoprostane-like Compounds (F3-Isoprostanes) in Vivofrom Eicosapentaenoic Acid. J. Boil. Chem. 2006, 281, 14092–14099. [Google Scholar] [CrossRef]
- Roy, J.; Oger, C.; Thireau, J.; Roussel, J.; Mercier-Touzet, O.; Faure, D.; Pinot, E.; Farah, C.; Taber, D.F.; Cristol, J.-P.; et al. Nonenzymatic lipid mediators, neuroprostanes, exert the antiarrhythmic properties of docosahexaenoic acid. Free. Radic. Boil. Med. 2015, 86, 269–278. [Google Scholar] [CrossRef]
- Alkazemi, D.; Jackson, R.L., 2nd; Chan, H.M.; Kubow, S. Increased F3-Isoprostanes in the Canadian Inuit Population Could Be Cardioprotective by Limiting F2-Isoprostane Production. J. Clin. Endocrinol. Metab. 2016, 101, 3264–3271. [Google Scholar] [CrossRef]
- Roy, J.; Fauconnier, J.; Oger, C.; Farah, C.; Angebault-Prouteau, C.; Thireau, J.; Bideaux, P.; Scheuermann, V.; Bultel-Poncé, V.; Demion, M.; et al. Non-enzymatic oxidized metabolite of DHA, 4 (RS)-4-F 4t -neuroprostane protects the heart against reperfusion injury. Free Radic. Boil. Med. 2017, 102, 229–239. [Google Scholar] [CrossRef]
- Long, E.K.; Murphy, T.C.; Leiphon, L.J.; Watt, J.; Morrow, J.D.; Milne, G.L.; Howard, J.R.; Picklo, M.J., Sr. Trans-4-hydroxy-2-hexenal is a neurotoxic product of docosahexaenoic (22:6; n-3) acid oxidation. J. Neurochem. 2008, 105, 714–724. [Google Scholar] [CrossRef]
- Ansorena, D.; Guembe, A.; Mendizábal, T.; Astiasarán, I. Effect of Fish and Oil Nature on Frying Process and Nutritional Product Quality. J. Food Sci. 2010, 75, H62–H67. [Google Scholar] [CrossRef] [PubMed]
- Agbor, G.A.; Vinson, J.A.; Oben, J.E.; Ngogang, J.Y. In Vitro antioxidant activity of three Piper species. J. Herb. Pharmacother. 2007, 7, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Kaleem, M.S.; Sarmad, H.; Bano, B. Protective effects of Piper nigrum and Vinca rosea in alloxan induced diabetic rats. Indian J. Physiol. Pharmacol. 2005, 49, 65–71. [Google Scholar] [PubMed]
- Vijayakumar, R.S.; Nalini, N. Efficacy of piperine, an alkaloidal constituent fromPiper nigrum on erythrocyte antioxidant status in high fat diet and antithyroid drug induced hyperlipidemic rats. Cell Biochem. Funct. 2006, 24, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Aeschbach, R.; Löligers, J. Antioxidant and pro-oxidant properties of active rosemary constituents: Carnosol and carnosic acid. Xenobiotica 1992, 22, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, S.S.K.; Cuppett, S.L. Potential of Rosemary (Rosemarinus officinalisL.) Diterpenes in Preventing Lipid Hydroperoxide-Mediated Oxidative Stress in Caco-2 Cells. J. Agric. Food Chem. 2007, 55, 1193–1199. [Google Scholar] [CrossRef]
- Posadas, S.; Caz, V.; Largo, C.; De La Gándara, B.; Matallanas, B.; Reglero, G.; De Miguel, E. Protective effect of supercritical fluid rosemary extract, Rosmarinus officinalis, on antioxidants of major organs of aged rats. Exp. Gerontol. 2009, 44, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Van Hecke, T.; Ho, P.; Goethals, S.; De Smet, S. The potential of herbs and spices to reduce lipid oxidation during heating and gastrointestinal digestion of a beef product. Food Res. Int. 2017, 102, 785–792. [Google Scholar] [CrossRef]
- Colombi, B.; Salvatore, S.; Bianchi, M.; Pellegrini, N.; Serafini, M.; Del Rio, D.; Brighenti, F. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Ninfali, P.; Aluigi, G.; Bacchiocca, M.; Magnani, M. Antioxidant capacity of extra-virgin olive oils. J. Am. Oil Chem. Soc. 2001, 78, 243–247. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Boil. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Mitsuno, Y.; Masumura, M. Determination of peroxide value by the colorimetric iodine method with protection of iodide as cadmium complex. Lipids 1978, 13, 147–151. [Google Scholar] [CrossRef]
- Ochrem, A.Z.P.; Żychlińska-Buczek, J.; Maj, D.; Czerniejewska-Surma, B.; Pokorska, J.; Kułaj, D. Antioxidant capacity and lipid peroxidation products of carp (Cyprinus carpio L.) meat stored in refrigeration conditions with addition of herbs or vegetables. Czech J. Food Sci. 2017, 35, 251–258. [Google Scholar]
- Quehenberger, O.; Armando, A.M.; Dennis, E.A. High Sensitivity Quantitative Lipidomics Analysis of Fatty Acids in Biological Samples by Gas Chromatography-Mass Spectrometry. Biochim. Biophys. Acta (BBA) Mol. Cell Boil. Lipids 2011, 1811, 648–656. [Google Scholar] [CrossRef]
- Dupuy, A.; Le Faouder, P.; Vigor, C.; Galano, J.-M.; Dray, C.; Lee, J.C.-Y.; Valet, P.; Gladine, C.; Durand, T.; Bertrand-Michel, J. Simultaneous quantitative profiling of 20 isoprostanoids from omega-3 and omega-6 polyunsaturated fatty acids by LC-MS/MS in various biological samples. Anal. Chim. Acta 2016, 921, 46–58. [Google Scholar] [CrossRef]
- Douny, C.; Bayram, P.; Brose, F.; Degand, G.; Scippo, M.-L.; Scippo, M. Development of an LC-MS/MS analytical method for the simultaneous measurement of aldehydes from polyunsaturated fatty acids degradation in animal feed. Drug Test. Anal. 2016, 8, 458–464. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L. extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Afonso, M.D.S.; Sant’Ana, L. Effects of pretreatment with rosemary (rosmarinus officinalisl.) In the prevention of lipid oxidation in salted tilapia fillets. J. Food Qual. 2008, 31, 586–595. [Google Scholar] [CrossRef]
- Gokoglu, N.; Yerikaya, P.; Topuz, O.K. Effects of tomato and garlic extracts on oxidative stability in marinated anchovy. J. Food Proc. Preserv. 2012, 36, 191–197. [Google Scholar] [CrossRef]
- Tural, S.; Turhan, S. Effect of anchovy by-product protein coating incorporated with thyme essential oil on the shelf life of anchovy (Engraulis encrasicolus L.) fillets. Food Sci. Biotechnol. 2017, 26, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Effect of Capsicum annuum (Red Sweet and Cayenne) and Piper nigrum (Black and White) Pepper Powders on the Shelf Life of Fresh Pork Sausages Packaged in Modified Atmosphere. J. Food Sci. 2006, 71, S48–S53. [Google Scholar] [CrossRef]
- Gantner, M.; Brodowska, M.; Górska-Horczyczak, E.; Wojtasik-Kalinowska, I.; Najda, A.; Pogorzelska, E.; Godziszewska, J. Antioxidant effect of sage (Salvia officinalis L.) extract on turkey meatballs packed in cold modified atmosphere. CyTA J. Food 2018, 16, 628–636. [Google Scholar] [CrossRef]
- Sioen, I.; Haak, L.; Raes, K.; Hermans, C.; De Henauw, S.; De Smet, S.; Van Camp, J. Effects of pan-frying in margarine and olive oil on the fatty acid composition of cod and salmon. Food Chem. 2006, 98, 609–617. [Google Scholar] [CrossRef]
- Kaleem, A.; Aziz, S.; Iqtedar, M. Investigating changes and effect of peroxide values in cooking oils subject to light and heat. FUUAST J. Biol. 2015, 5, 191–196. [Google Scholar]
- Ishikado, A.; Morino, K.; Nishio, Y.; Nakagawa, F.; Mukose, A.; Sono, Y.; Yoshioka, N.; Kondo, K.; Sekine, O.; Yoshizaki, T.; et al. 4-Hydroxy Hexenal Derived from Docosahexaenoic Acid Protects Endothelial Cells via Nrf2 Activation. PLoS ONE 2013, 8, e69415. [Google Scholar] [CrossRef]
- Zhang, Y.; Sano, M.; Shinmura, K.; Tamaki, K.; Katsumata, Y.; Matsuhashi, T.; Morizane, S.; Ito, H.; Hishiki, T.; Endo, J.; et al. 4-Hydroxy-2-nonenal protects against cardiac ischemia–reperfusion injury via the Nrf2-dependent pathway. J. Mol. Cell. Cardiol. 2010, 49, 576–586. [Google Scholar] [CrossRef]
- Long, E.K.; Picklo, M.J. Trans-4-hydroxy-2-hexenal, a product of n-3 fatty acid peroxidation: Make some room HN. Free Radic. Boil. Med. 2010, 49, 1–8. [Google Scholar] [CrossRef]
- Xiao, M.; Zhong, H.; Xia, L.; Tao, Y.; Yin, H. Pathophysiology of mitochondrial lipid oxidation: Role of 4-hydroxynonenal (4-HNE) and other bioactive lipids in mitochondria. Free Radic. Biol. Med. 2017, 111, 316–327. [Google Scholar] [CrossRef]
- Masuda, T.; Inaba, Y.; Takeda, Y. Antioxidant mechanism of carnosic acid: Structural identification of two oxidation products. J. Agric. Food Chem. 2001, 49, 5560–5565. [Google Scholar] [CrossRef]
- Gordon, J.A.; Broekemeier, K.M.; Spector, A.A.; Pfeiffer, D.R. Mitochondrial metabolism of 12- and 15-hydroxyeicosatetraenoic acids. J. Lipid Res. 1994, 35, 698–708. [Google Scholar]
- Tironi, V.; Tomás, M.; Añón, M. Lipid and protein changes in chilled sea salmon (Pseudopercis semifasciata): Effect of previous rosemary extract (Rossmarinus officinalis L.) application. Int. J. Food Sci. Technol. 2009, 44, 1254–1262. [Google Scholar] [CrossRef]
- Zunin, P.; Leardi, R.; Bisio, A.; Boggia, R.; Romussi, G. Oxidative stability of virgin olive oil enriched with carnosic acid. Food Res. Int. 2010, 43, 1511–1516. [Google Scholar] [CrossRef]
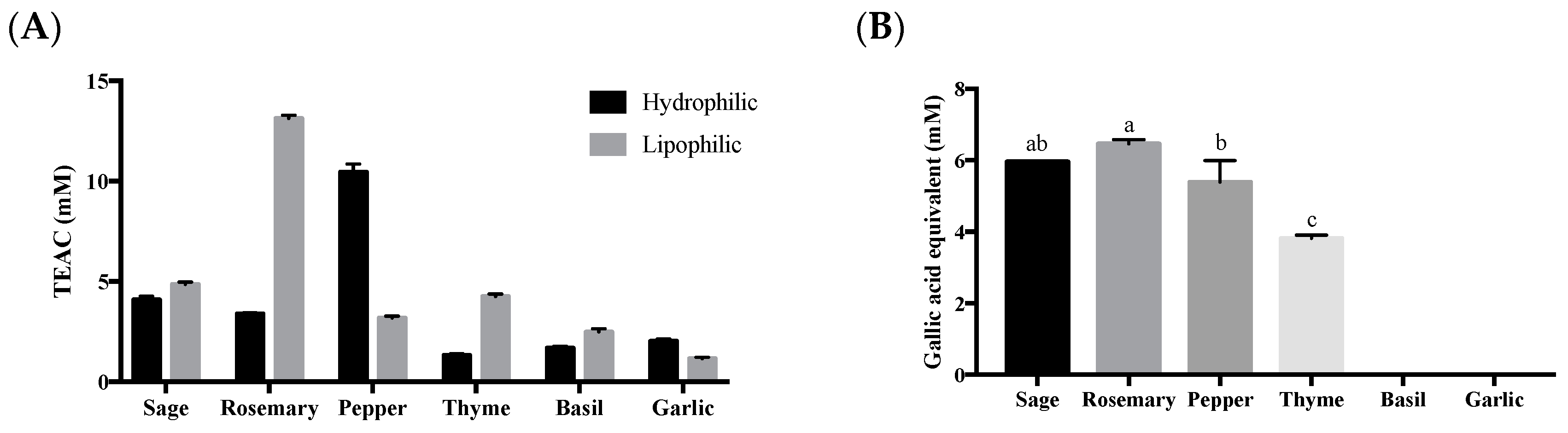

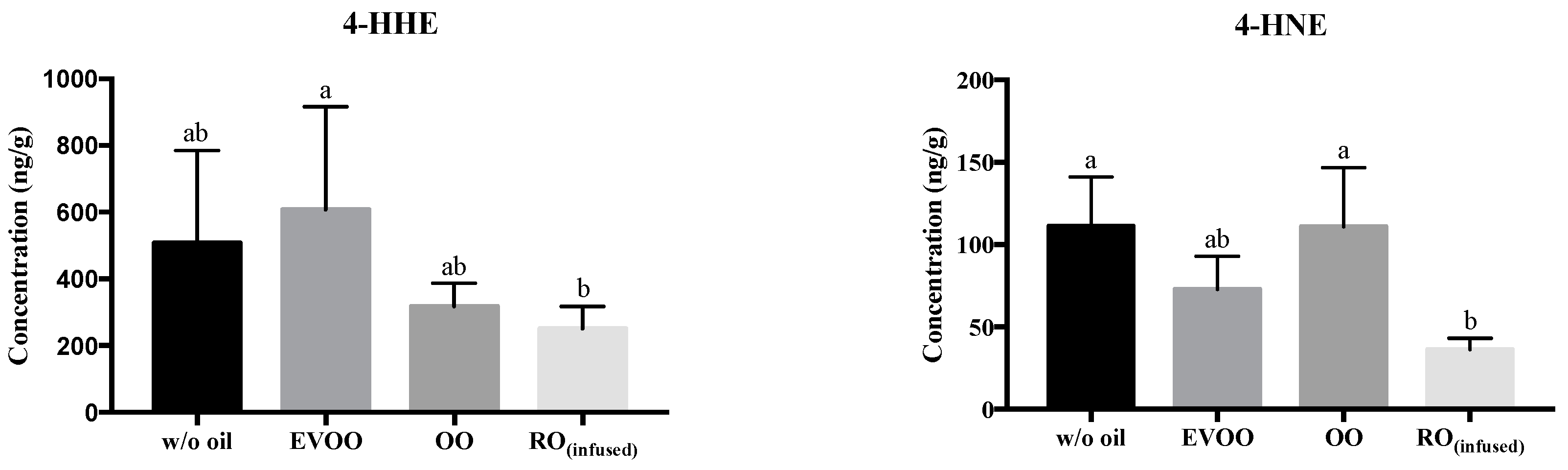

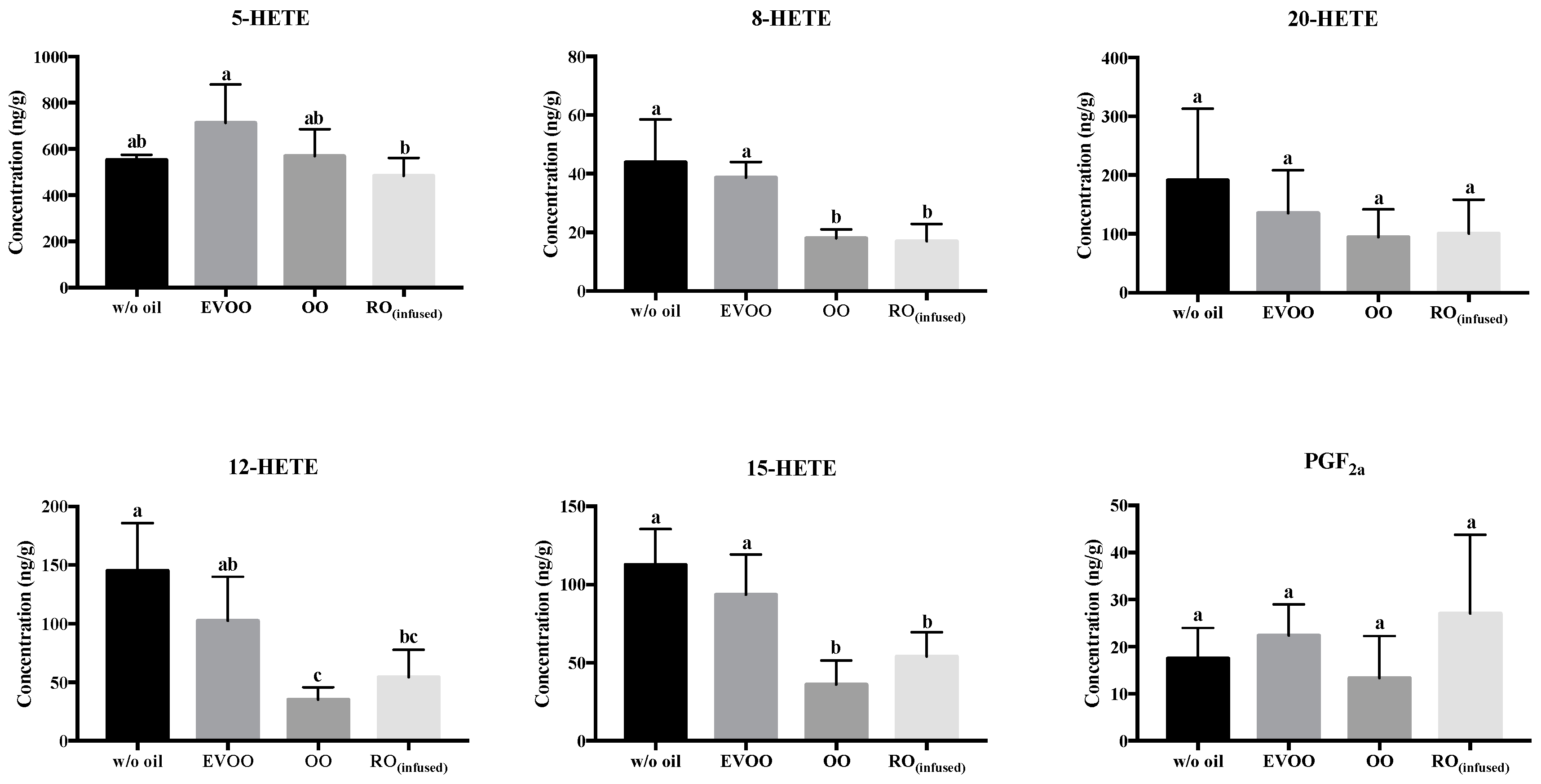
| Salmon (n = 6) | ||||
|---|---|---|---|---|
| w/o oil | EVOO | OO | RO(infused) | |
| Total | 14034.2 ± 1856.9 a | 14651.7 ± 3588.7 a | 12140.9 ± 1059.4 a | 9869.2 ± 1714.8 b |
| ∑ SFA (%) | 8.69 ± 0.14 a | 8.76 ± 0.18 a | 8.45 ± 0.30 ab | 7.94 ± 0.73 b |
| ∑ MUFA (%) | 25.50 ± 1.14 a | 24.91 ± 1.09 a | 24.75 ± 2.01 a | 23.38 ± 1.10 a |
| ∑ n-6 PUFA (%) | 20.74 ± 0.65 a | 19.11 ± 0.95 b | 20.00 ± 0.52 ab | 19.95 ± 1.33 ab |
| ∑ n-3 PUFA (%) | 45.06 ± 1.67 a | 47.22 ± 1.82 a | 46.81 ± 2.51 a | 48.72 ± 3.13 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, K.S.; Leung, H.H.; Wu, C.Y.; Galano, J.-M.; Durand, T.; Lee, J.C.-Y. Limited Antioxidant Effect of Rosemary in Lipid Oxidation of Pan-Fried Salmon. Biomolecules 2019, 9, 313. https://doi.org/10.3390/biom9080313
Leung KS, Leung HH, Wu CY, Galano J-M, Durand T, Lee JC-Y. Limited Antioxidant Effect of Rosemary in Lipid Oxidation of Pan-Fried Salmon. Biomolecules. 2019; 9(8):313. https://doi.org/10.3390/biom9080313
Chicago/Turabian StyleLeung, Kin Sum, Ho Hang Leung, Ching Yu Wu, Jean-Marie Galano, Thierry Durand, and Jetty Chung-Yung Lee. 2019. "Limited Antioxidant Effect of Rosemary in Lipid Oxidation of Pan-Fried Salmon" Biomolecules 9, no. 8: 313. https://doi.org/10.3390/biom9080313
APA StyleLeung, K. S., Leung, H. H., Wu, C. Y., Galano, J.-M., Durand, T., & Lee, J. C.-Y. (2019). Limited Antioxidant Effect of Rosemary in Lipid Oxidation of Pan-Fried Salmon. Biomolecules, 9(8), 313. https://doi.org/10.3390/biom9080313






