Vaccine Adjuvants Derived from Marine Organisms
Abstract
:1. Introduction
2. Adjuvant Families
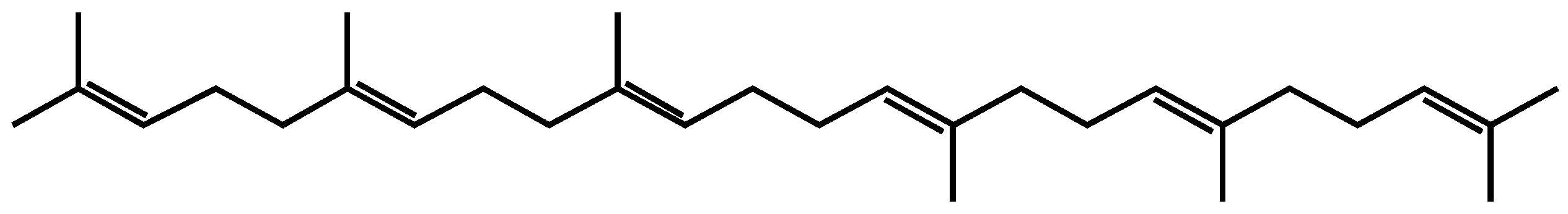
2.1. Squalene
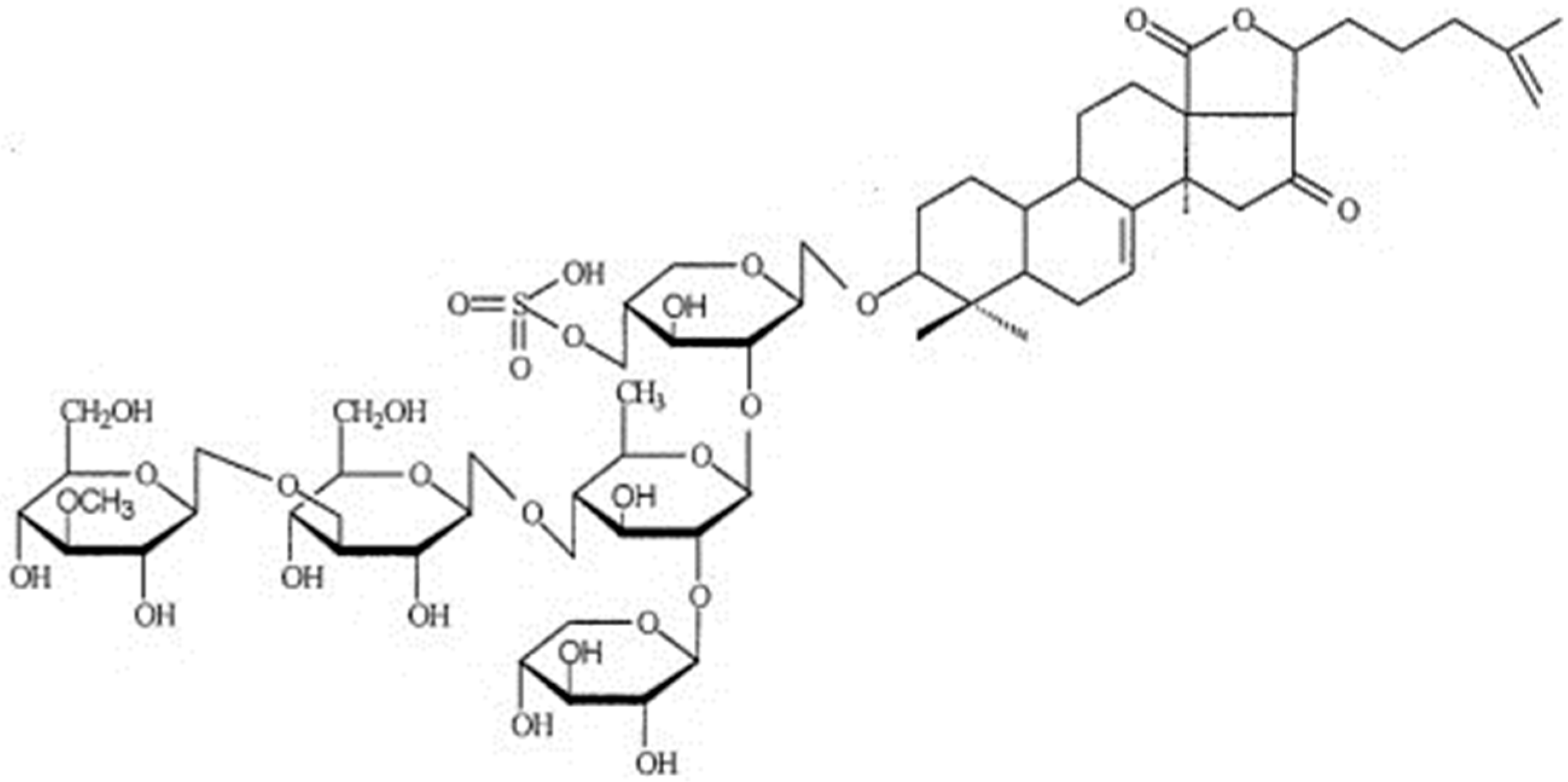
2.2. Saponins
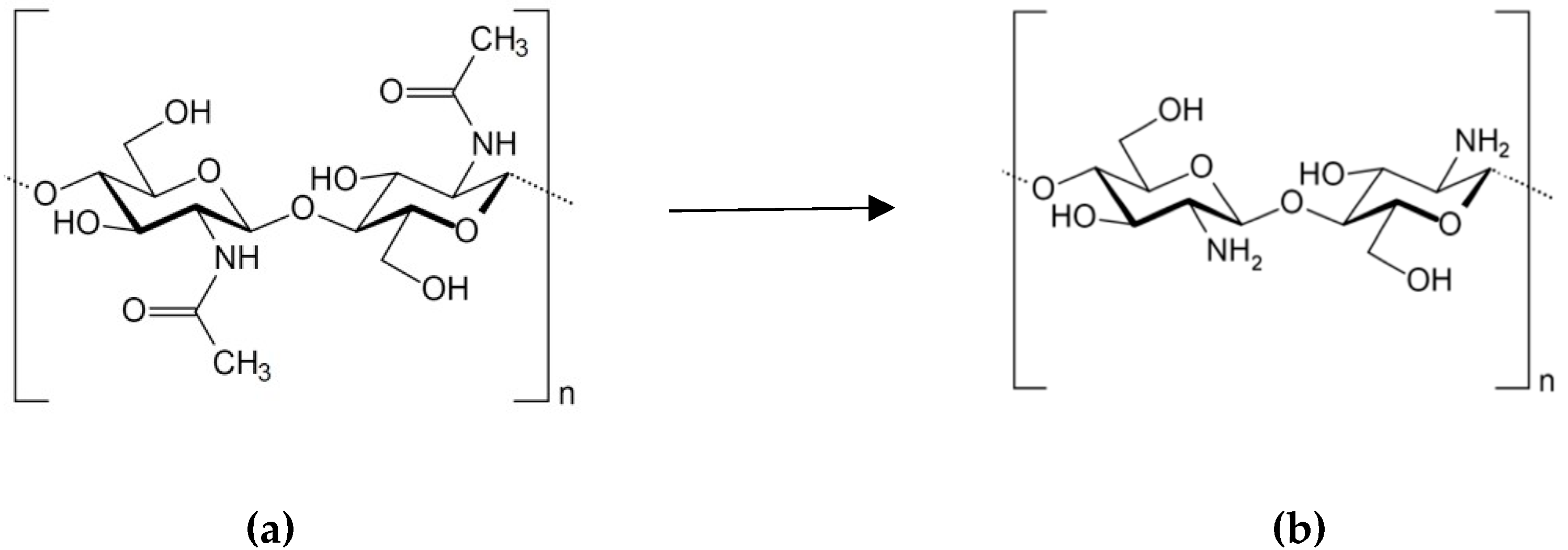
2.3. Chitosan
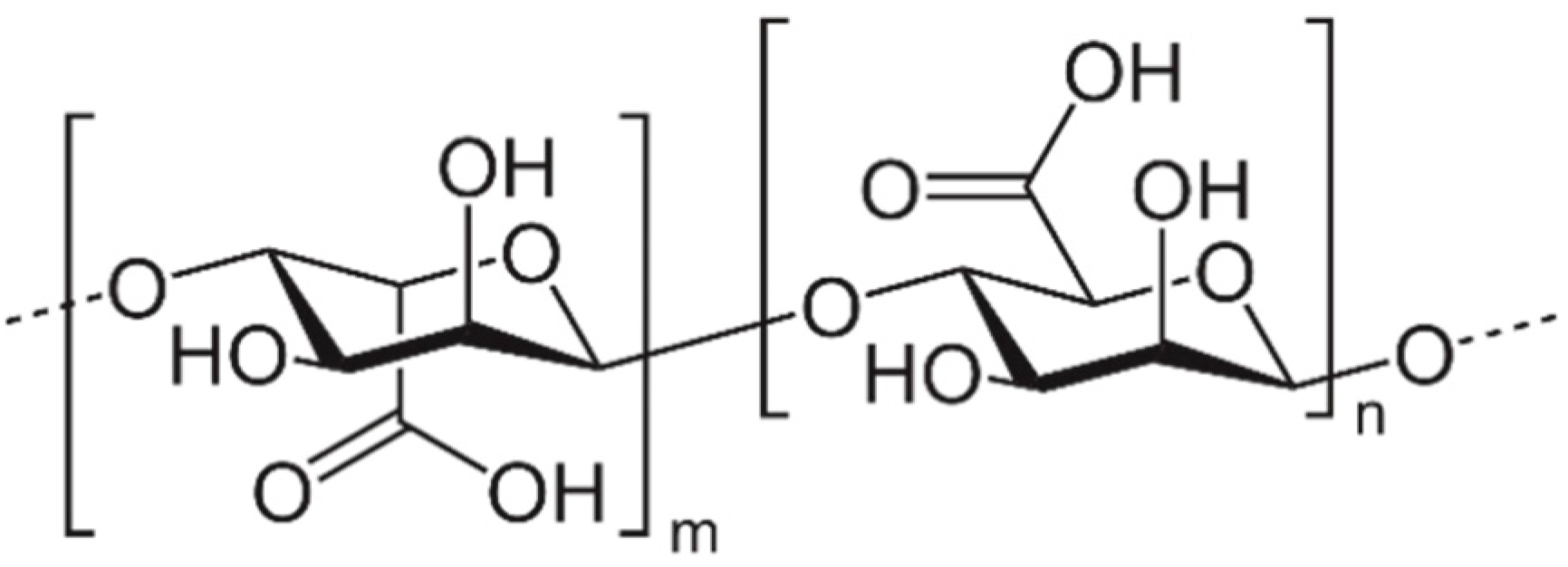
2.4. Seaweed Polysaccharides
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Foged, C. Subunit vaccines of the future: The need for safe, customized and optimized particulate delivery systems. Ther. Deliv. 2011, 2, 1057–1077. [Google Scholar] [CrossRef]
- Afrough, B.; Dowall, S.; Hewson, R. Emerging viruses and current strategies for vaccine intervention. Clin. Exp. Immunol. 2019, 196, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Walkeral, E.J.; MacDonald, N.E.; Islam, N.; Le Sauxe, N.; Top, K.A.; Fell, D.B. Completeness and timeliness of diphtheria-tetanus-pertussis, measles-mumps-rubella, and polio vaccines in young children with chronic health conditions: A systematic review. Vaccine 2019, 37, 1725–1735. [Google Scholar] [CrossRef]
- Rueckert, C.; Guzmán, C.A. Vaccines: From empirical development to rational design. PLoS Pathog. 2012, 8, e1003001. [Google Scholar] [CrossRef]
- Francis, M.J. Recent advances in vaccine technologies. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 231–241. [Google Scholar] [CrossRef]
- Moyle, P.M. Biotechnology approaches to produce potent, self-adjuvanting antigen-adjuvant fusion protein subunit vaccines. Biotechnol. Adv. 2017, 35, 375–389. [Google Scholar] [CrossRef]
- Chua, B.Y.; Sekiya, T.; Jackson, D.C. Opinion: Making Inactivated and Subunit-Based Vaccines Work. Viral Immunol. 2018, 31, 150–158. [Google Scholar] [CrossRef]
- Karch, C.P.; Burkhard, P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016, 120, 1–14. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Ott, G.S.; Nest, G.V.; Rappuoli, R.; Giudice, G.D. The history of MF59® adjuvant: A phoenix that arose from the ashes. Expert Rev. Vaccines 2013, 12, 13–30. [Google Scholar] [CrossRef]
- Kim, S.K.; Karadeniz, F. Biological importance and applications of squalene and squalane. Adv. Food Nutr. Res. 2012, 65, 224–233. [Google Scholar] [CrossRef]
- Popa, I.; Băbeanu, N.; Niță, S.; Popa, O. Squalene-natural resources and applications. Farmacia 2014, 62, 840–862. [Google Scholar]
- Chang, M.H.; Kim, H.J.; Jahng, K.Y.; Hong, S.C. The isolation and characterization of Pseudozyma sp. JCC 207, a novel producer of squalene. Appl. Microbiol. Biotechnol. 2008, 78, 963–972. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Tan, Y.; Feng, Y.; Li, W.; Cui, Q. High production of squalene using a newly isolated yeast-like strain Pseudozyma sp. SD301. J. Agric. Food Chem. 2015, 63, 8445–8451. [Google Scholar] [CrossRef]
- Ko, E.J.; Kang, S.M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccin. Immunother. 2018, 14, 3041–3045. [Google Scholar] [CrossRef]
- Wilson, K.L.; Xiang, S.D.; Plebanski, M. Inflammatory/noninflammatory adjuvants and nanotechnology—The secret to vaccine design. In Micro-and Nanotechnology in Vaccine Development; Skwarczynski, M., Toth, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 99–125. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Ott, G.S.; De Gregorio, E.; Seubert, A. The mechanism of action of MF59—An innately attractive adjuvant formulation. Vaccine 2012, 30, 4341–4348. [Google Scholar] [CrossRef]
- Christensen, D. Vaccine adjuvants: Why and how. Hum. Vaccin. Immunother. 2016, 12, 2709–2711. [Google Scholar] [CrossRef] [Green Version]
- Cohet, C.; Van Der Most, R.; Bauchau, V.; Bekkat-Berkani, R.; Doherty, T.M.; Schuind, A.; Da Silva, F.T.; Rappuoli, R.; Garçon, N.; Innis, B.L. Safety of AS03-adjuvanted influenza vaccines: A review of the evidence. Vaccine 2019, 37, 3006–3021. [Google Scholar] [CrossRef]
- Vogel, G. Narcolepsy link to pandemic flu vaccine becomes clearer. Science 2015, 349, 17. [Google Scholar] [CrossRef]
- Lippi, G.; Targher, G.; Franchini, M. Vaccination, squalene and anti-squalene antibodies: Facts or fiction? Eur. J. Intern. Med. 2010, 21, 70–73. [Google Scholar] [CrossRef]
- Baldwin, S.L.; Bertholet, S.; Reese, V.A.; Ching, L.K.; Reed, S.G.; Coler, R.N. The importance of adjuvant formulation in the development of a tuberculosis vaccine. J. Immunol. 2012, 188, 2189–2197. [Google Scholar] [CrossRef]
- Orr, M.T.; Beebe, E.A.; Hudson, T.E.; Moon, J.J.; Fox, C.B.; Reed, S.G.; Coler, R.N. A dual TLR agonist adjuvant enhances the immunogenicity and protective efficacy of the tuberculosis vaccine antigen ID93. PLoS ONE 2014, 9, e83884. [Google Scholar] [CrossRef]
- Fox, C.B.; Baldwin, S.L.; Vedvick, T.S.; Angov, E.; Reed, S.G. Effects on immunogenicity by formulations of emulsion-based adjuvants for malaria vaccines. Clin. Vaccine Immunol. 2012, 19, 1633–1640. [Google Scholar] [CrossRef]
- Pedersen, G.K.; Andersen, P.; Christensen, D. Immunocorrelates of CAF family adjuvants. Semin. Immunol. 2018, 39, 4–13. [Google Scholar] [CrossRef]
- Rao, A.V.; Gurfinkel, D.M. The bioactivity of saponins: Triterpenoid and steroidal glycosides. Drug Metabol. Drug Interact. 2000, 17, 211–235. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, H.P.S.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Pedebos, C.; Pol-Fachin, L.; Pons, R.; Teixeira, C.V.; Verli, H. Atomic model and micelle dynamics of QS-21 saponin. Molecules 2014, 19, 3744–3760. [Google Scholar] [CrossRef]
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Anticancer activity of sea cucumber triterpene glycosides. Mar. Drugs 2015, 13, 1202–1223. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Dyshlovoy, S.A.; Kicha, A.A.; Ivanchina, N.V.; Malyarenko, T.V.; Carsten, B.; Gunhild, V.A.; Stonik, V.A.; Ermakova, S.P. The inhibitory activity of luzonicosides from the starfish Echinaster luzonicus against human melanoma cells. Mar. Drugs 2017, 15, 227. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Ivanchina, N.V.; Krasokhin, V.B.; Makarieva, T.N.; Stonik, V.A. Glycosides from marine sponges (porifera, demospongiae): Structures, taxonomical distribution, biological activities and biological roles. Mar. Drugs 2012, 10, 1671–1710. [Google Scholar] [CrossRef]
- Li, Y.X.; Li, Y.; Lee, S.H.; Qian, Z.J.; Kim, S.K. Inhibitors of oxidation and matrix metalloproteinases, floridoside, and D-isofloridoside from marine red alga Laurencia undulata. J. Agric. Food Chem. 2010, 58, 578–586. [Google Scholar] [CrossRef]
- Garcia, M.M.; Van Der Maarel, M.J.E.C. Floridoside production by the red microalga Galdieria sulphuraria under different conditions of growth and osmotic stress. AMB Express 2016, 6, 71. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hwang, T.L.; Lin, M.-R.; Chen, Y.H.; Chang, Y.C.; Fang, L.S.; Wang, W.H.; Wu, Y.C.; Sung, P.J.; Carijoside, A. A bioactive sterol glycoside from an octocoral Carijoa sp. (Clavulariidae). Mar. Drugs 2010, 8, 2014–2020. [Google Scholar] [CrossRef]
- Lasley, B.J.; Nigrelli, R.F. The effect of crude holothurin on leucocyte phagocytosis. Toxicon 1970, 8, 301–306. [Google Scholar] [CrossRef]
- Bahrami, Y.; Zhang, W.; Franco, C. Discovery of novel saponins from the viscera of the sea cucumber Holothuria lessoni. Mar. Drugs 2014, 12, 2633–2667. [Google Scholar] [CrossRef]
- Sedov, A.M.; Apollonin, A.V.; Sevast'ianova, E.K.; Alekseeva, I.A.; Batrakov, S.G.; Sakandelidze, O.G.; Likhoded, V.G.; Stonik, V.A.; Avilov, S.A.; Kupera, E.V. Stimulation of nonspecific antibacterial resistance of mice to opportunistic gram-negative microorganisms with triterpene glycosides from Holothuroidea. Antibiot. Khimioter. 1990, 35, 23–26. [Google Scholar]
- Sedov, A.M.; Shepeleva, I.B.; Zakharova, N.S.; Sakandelidze, O.G.; Sergeev, V.V. Effect of cucumariosides (a triterpene glycoside from the holothurian Cucumaria japonica) on the development of an immune response in mice to corpuscular pertussis vaccine. Zhurnal Mikrobiol. Epidemiol. I Immunobiol. 1984, 9, 100–104. [Google Scholar]
- Aminin, D.; Pislyagin, E.; Astashev, M.; Es’kov, A.; Kozhemyako, V.; Avilov, S.; Zelepuga, E.; Yurchenko, E.; Kaluzhskiy, L.; Kozlovskaya, E.; et al. Glycosides from edible sea cucumbers stimulate macrophages via purinergic receptors. Sci. Rep. 2016, 6, 39683. [Google Scholar] [CrossRef]
- Lee, L.A.; Popov, A.M.; Kostetsky, E.Y.; Sanina, N.M.; Mazeyka, A.N.; Boguslavskii, V.M. Membranotropic effect of some triterpene glycosides possessing immunostimulating properties. Biofizika 2008, 53, 462–469. [Google Scholar]
- Kalinin, V.I.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S.; Stonik, V.A. Triterpene glycosides from sea cucumbers (Holothurioidea, Echinodermata). Biological activities and functions. Stud. Nat. Prod. Chem. 2008, 35, 135–196. [Google Scholar] [CrossRef]
- Mazeika, A.N.; Popov, A.M.; Kalinin, V.I.; Avilov, S.A.; Sil’chenko, A.S.; Kostetsky, E.Y. The complexation between holothurian triterpene glycosides and Chl as the basis for lipid-saponin carriers of subunit protein antigens. Biophysics 2008, 53, 409–416. [Google Scholar] [CrossRef]
- Kostetsky, E.Y.; Sanina, N.M.; Mazeika, A.N.; Tsybulsky, A.V.; Vorobyeva, N.S.; Shnyrov, V.L. Tubular immunostimulating complex based on cucumarioside A2-2 and monogalactosyldiacylglycerol from marine macrophytes. J. Nanobiotechnol. 2011, 9, 35. [Google Scholar] [CrossRef]
- Sanina, N.; Chopenko, N.; Mazeika, A.; Kostetsky, E. Nanoparticulate tubular immunostimulating complexes: Novel formulation of effective adjuvants and antigen delivery systems. BioMed Res. Int. 2017, 2017, 4389525. [Google Scholar] [CrossRef]
- Lee, I.A.; Popov, A.M.; Sanina, N.M.; Kostetsky, E.Y.; Novikova, O.D.; Reunov, A.V.; Nagorskaya, V.P.; Shnyrov, V.L. Morphological and immunological characterization of immunostimulatory complexes based on glycoglycerolipids from Laminaria japonica. Acta Biochim. Pol. 2004, 51, 263–272. [Google Scholar]
- Sanina, N.M.; Kostetsky, E.Y.; Shnyrov, V.L.; Tsybulsky, A.V.; Novikova, O.D.; Portniagina, O.Y.; Vorobieva, N.S.; Mazeika, A.N.; Bogdanov, M.V. The influence of monogalactosyldiacylglycerols from different marine macrophytes on immunogenicity and conformation of protein antigen of tubular immunostimulating complex. Biochimie 2012, 94, 1048–1056. [Google Scholar] [CrossRef]
- Vorobyeva, N.S.; Mazeika, A.N.; Davydova, L.A.; Velansky, P.V.; Tsybulsky, A.V.; Kostetsky, E.Y.; Sanina, N.M. The effects of triterpene glycosides and phospholipids from marine invertebrates in the composition of tubular immunostimulating complexes on the immunogenicity of human serum albumin. Russ. J. Mar. Biol. 2015, 41, 69–77. [Google Scholar] [CrossRef]
- Sanina, N.M.; Vorobieva, N.S.; Novikova, O.D.; Portniagina, O.Y.; Davydova, L.A.; Shnyrov, V.L.; Kostetsky, E.Y. Lipid induced changes in protein conformation as a means to regulate the immunogenicity of antigens incorporated in tubular immunostimulating complexes. Biophysics 2016, 61, 380–386. [Google Scholar] [CrossRef]
- Bahrami, Y.; Franco, C.M.M. Acetylated triterpene glycosides and their biological activity from Holothuroidea reported in the past six decades. Mar. Drugs 2016, 14, 147. [Google Scholar] [CrossRef]
- Aminin, D.L.; Agafonova, I.G.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Collin, P.D.; Woodward, C. Immunomodulatory properties of frondoside A, a major triterpene glycoside from the North Atlantic commercially harvested sea cucumber Cucumaria frondosa. J. Med. Food 2008, 11, 443–453. [Google Scholar] [CrossRef]
- Aminin, D.L.; Silchenko, A.S.; Avilov, S.A.; Stepanov, V.G.; Kalinin, V.I. Immunomodulatory action of monosulfated triterpene glycosides from the sea cucumber Cucumaria okhotensis: Stimulation of activity of mouse peritoneal macrophages. Nat. Prod. Commun. 2010, 5, 1877–1880. [Google Scholar] [CrossRef]
- Aminin, D.L.; Agafonova, I.G.; Berdyshev, E.V.; Isachenko, E.G.; Avilov, S.A.; Stonik, V.A. Immunomodulatory properties of cucumariosides from the edible far-eastern holothurian Cucumaria japonica. J. Med. Food 2001, 4, 127–135. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Menchinskaya, E.S.; Aminin, D.L.; Kalinin, V.I. Structure of cucumarioside I2 from the sea cucumber Eupentacta fraudatrix (Djakonov et Baranova) and cytotoxic and immunostimulatory activities of this saponin and relative compounds. Nat. Prod. Res. 2013, 27, 1776–1783. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Arca, H.C.; Günbeyaz, M.; Senel, S. Chitosan-based systems for the delivery of vaccine antigens. Expert Rev. Vaccines 2009, 8, 937–953. [Google Scholar] [CrossRef]
- Muñoz-Wolf, N.; McCluskey, S.; Lavelle, E.C. The role of inflammasomes in adjuvant-driven humoral and cellular immune responses. In Immunopotentiators in Modern Vaccines, 2nd ed.; Schijns, V.E.J.C., O’Hagan, D.T., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 23–42. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Saikia, C.; Gogoi, P.; Maji, T.K. Chitosan: A promising biopolymer in drug delivery applications. J. Mol. Genet. Med. 2015, 4. [Google Scholar] [CrossRef]
- Smith, A.; Perelman, M.; Hinchcliffe, M. Chitosan: A promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum. Vaccin. Immunother. 2014, 10, 797–807. [Google Scholar] [CrossRef]
- Islam, M.A.; Firdous, J.; Choi, Y.J.; Yun, C.H.; Cho, C.S. Design and application of chitosan microspheres as oral and nasal vaccine carriers: An updated review. Int. J. Nanomed. 2012, 7, 6077–6093. [Google Scholar] [CrossRef]
- Bueter, C.L.; Lee, C.K.; Rathinam, V.A.; Healy, G.J.; Taron, C.H.; Specht, C.A.; Levitz, S.M. Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J. Biol. Chem. 2011, 286, 35447–35455. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, W.; Peng, Y.; Han, B.; Yang, Y. Toll like receptor 4 (TLR4) mediates the stimulating activities of chitosan oligosaccharide on macrophages. Int. Immunopharmacol. 2014, 23, 254–261. [Google Scholar] [CrossRef]
- Bueter, C.L.; Specht, C.A.; Levitz, S.M. Innate sensing of chitin and chitosan. PLoS Pathog. 2013, 9, e1003080. [Google Scholar] [CrossRef]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef]
- Riteau, N.; Sher, A. Chitosan: An adjuvant with an unanticipated STING. Immunity 2016, 44, 522–524. [Google Scholar] [CrossRef]
- Xing, L.; Fan, Y.T.; Zhou, T.J.; Gong, J.H.; Cui, L.H.; Cho, K.H.; Choi, Y.J.; Jiang, H.L.; Cho, C.S. Chemical modification of chitosan for efficient vaccine delivery. Molecules 2018, 23, 229. [Google Scholar] [CrossRef]
- Saravana, K.A.; Ramaswamy, N.M. Chitosan microspheres as potential vaccine delivery systems. Int. J. Drug Deliv. 2011, 3, 43–50. [Google Scholar] [CrossRef]
- Singh, B.; Maharjan, S.; Cho, K.H.; Cui, L.; Park, I.K.; Choi, Y.J.; Cho, C.S. Chitosan-based particulate systems for the delivery of mucosal vaccines against infectious diseases. Int. J. Biol. Macromol. 2018, 110, 54–64. [Google Scholar] [CrossRef]
- Chua, B.Y.; Al Kobaisi, M.; Zeng, W.; Mainwaring, D.; Jackson, D.C. Chitosan microparticles and nanoparticles as biocompatible delivery vehicles for peptide and protein-based immunocontraceptive vaccines. Mol. Pharm. 2019, 9, 81–90. [Google Scholar] [CrossRef]
- Barbosa, J.S.; Costa, M.S.S.P.; Melo, L.F.M.; Medeiros, M.J.C.; Pontes, D.L.; Scortecci, K.C.; Rocha, H.A.O. In vitro immunostimulating activity of sulfated polysaccharides from Caulerpa cupressoides var. flabellata. Mar. Drugs 2019, 17, 105. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Usov, A.I.; Bilan, M.I. Fucoidans—Sulfated polysaccharides of brown algae. Russ. Chem. Rev. 2009, 78, 785–799. [Google Scholar] [CrossRef]
- Kim, S.Y.; Joo, H.G. Evaluation of adjuvant effects of fucoidan for improving vaccine efficacy. J. Vet. Sci. 2015, 16, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsova, T.A.; Ivanushko, L.A.; Persiyanova, E.V.; Shutikova, A.L.; Ermakova, S.P.; Khotimchenko, M.Y.; Besednova, N.N. Evaluation of adjuvant effects of fucoidane from brown seaweed Fucus evanescens and its structural analogues for the strengthening vaccines effectiveness. Biomed. Khim. 2017, 63, 553–558. [Google Scholar] [CrossRef]
- Hwang, P.A.; Lin, H.V.; Lin, H.Y.; Lo, S.K. Dietary supplementation with low-molecular-weight fucoidan enhances innate and adaptive immune responses and protects against Mycoplasma pneumoniae antigen stimulation. Mar. Drugs 2019, 17, 175. [Google Scholar] [CrossRef]
- Bi, D.; Yu, B.; Han, Q.; Lu, J.; White, W.L.; Lai, Q.; Cai, N.; Luo, W.; Gu, L.; Li, S.; et al. Immune activation of RAW264.7 macrophages by low molecular weight fucoidan extracted from New Zealand Undaria pinnatifida. J. Agric. Food Chem. 2018, 66, 10721–10728. [Google Scholar] [CrossRef]
- Park, S.; Hwang, E.; Shin, Y.; Lee, D.; Yang, J.; Park, J.; Yi, T. Immunostimulatory effect of enzyme-modified Hizikia fusiforme in a mouse model in vitro and ex vivo. Mar. Biotechnol. 2017, 19, 65–75. [Google Scholar] [CrossRef]
- Borazjani, N.J.; Tabarsa, M.; You, S.; Rezaei, M. Improved immunomodulatory and antioxidant properties of unrefined fucoidans from Sargassum angustifolium by hydrolysis. J. Food Sci. Technol. 2017, 54, 4016–4025. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A Review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Lee, J.B.; Ohta, Y.; Hayashi, K.; Hayashi, T. Immunostimulating effects of a sulfated galactan from Codium fragile. Carbohydr. Res. 2010, 345, 1452–1454. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217. [Google Scholar] [CrossRef]
- Thomson, A.W.; Fowler, E.F. Carrageenan: A review of its effects on the immune system. Agents Actions 1981, 11, 265–273. [Google Scholar] [CrossRef]
- Barth, C.R.; Funchal, G.A.; Luft, C.; De Oliveira, J.R.; Porto, B.N.; Donadio, M.V. Carrageenan-induced inflammation promotes ROS generation and neutrophil extracellular trap formation in a mouse model of peritonitis. Eur. J. Immunol. 2016, 46, 964–970. [Google Scholar] [CrossRef] [Green Version]
- Mizokami, S.S.; Hohmann, M.S.; Staurengo-Ferrari, L.; Carvalho, T.T.; Zarpelon, A.C.; Possebon, M.; de Souza, A.R.; Veneziani, R.C.; Arakawa, N.S.; Casagrande, R.; et al. Pimaradienoic acid inhibits carrageenan-induced inflammatory leukocyte recruitment and edema in mice: Inhibition of oxidative stress, nitric oxide and cytokine production. PLoS ONE 2016, 11, e0149656. [Google Scholar] [CrossRef]
- Chan, W.; Zhang, G.; Li, X.; Leung, C.H.; Ma, D.L.; Dong, L.; Wang, C. Carrageenan activates monocytes via type-specific binding with interleukin-8: An implication for design of immunoactive biomaterials. Biomater. Sci. 2017, 5, 403–407. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Tsai, Y.C.; Monie, A.; Hung, C.F.; Wu, T.C. Carrageenan as an adjuvant to enhance peptide-based vaccine potency. Vaccine 2010, 28, 5212–5219. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Shao, B.; Nie, W.; Wei, X.W.; Li, Y.L.; Wang, B.L.; He, Z.Y.; Liang, X.; Ye, T.H.; Wei, Y.Q. Antitumor and adjuvant activity of λ-carrageenan by stimulating immune response in cancer immunotherapy. Sci. Rep. 2015, 5, 11062. [Google Scholar] [CrossRef]
- Huang, G.J.; Pan, C.H.; Wu, C.H. Sclareol exhibits anti-inflammatory activity in both lipopolysaccharide-stimulated macrophages and the λ-carrageenan-induced paw edema model. J. Nat. Prod. 2012, 75, 54–59. [Google Scholar] [CrossRef]
- Kalitnik, A.A.; Karetin, Y.A.; Kravchenko, A.O.; Khasina, E.I.; Yermak, I.M. Influence of carrageenan on cytokine production and cellular activity of mouse peritoneal macrophages and its effect on experimental endotoxemia. J. Biomed. Mater. Res. A 2017, 105, 1549–1557. [Google Scholar] [CrossRef]
- Bondu, S.; Deslandes, E.; Fabre, M.S.; Berthou, C.; Guangli, Y. Carrageenan from Solieria chordalis (Gigartinales): Structural analysis and immunological activities of the low molecular weight fractions. Carbohydr. Polym. 2010, 81, 448–460. [Google Scholar] [CrossRef]
- Kidgella, J.T.; Magnusson, M.; de Nysa, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Pérez-recalde, M.; Matulewicz, M.C.; Pujol, C.A.; Carlucci, M.J. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from red seaweed Nemalion helminthoides. Int. J. Biol. Macromol. 2014, 63, 38–42. [Google Scholar] [CrossRef]
- Liu, Q.M.; Xu, S.S.; Li, L.; Pan, T.M.; Shi, C.L.; Liu, H.; Cao, M.J.; Su, W.J.; Liu, G.M. In vitro and in vivo immunomodulatory activity of sulfated polysaccharide from Porphyra haitanensis. Carbohydr. Polym. 2017, 165, 189–196. [Google Scholar] [CrossRef]
- Barsanti, L.; Passarelli, V.; Evangelista, V.; Frassanito, A.M.; Gualtieri, P. Chemistry, physico-chemistry and applications linked to biological activities of β-glucans. Nat. Prod. Rep. 2011, 28, 457–466. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.J.; Kim, H.J.; Kim, Y.S.; Park, W. Immunostimulatory effect of laminarin on RAW 264.7 mouse macrophages. Molecules 2012, 17, 5404–5411. [Google Scholar] [CrossRef]
- Vetvicka, V. Glucan-immunostimulant, adjuvant, potential drug. World J. Clin. Oncol. 2011, 2, 115–119. [Google Scholar] [CrossRef]
- Jin, Y.; Li, P.; Wang, F. β-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef]
- Dobakhti, F.; Naghibi, T.; Taghikhani, M.; Ajdary, S.; Rafinejad, A.; Bayati, K.; Rafiei, S.; Rafiee-Tehrani, M. Adjuvanticity effect of sodium alginate on subcutaneously injected BCG in BALB/c mice. Microbes Infect. 2009, 11, 296–301. [Google Scholar] [CrossRef]
- Powell, B.S.; Andrianov, A.K.; Fusco, P.C. Polyionic vaccine adjuvants: Another look at aluminum salts and polyelectrolytes. Clin. Exp. Vaccine Res. 2015, 4, 23–45. [Google Scholar] [CrossRef]
- Ge, F.; Zhu, L.; Yang, L.; Li, W.; Wei, S.; Tao, Y.; Du, G. The soluble and particulate form of alginates positively regulate immune response. Iran J. Immunol. 2018, 15, 228–238. [Google Scholar] [CrossRef]
- Sarei, F.; Dounighi, N.M.; Zolfagharian, H.; Khaki, P.; Bidhendi, S.M. Alginate nanoparticles as a promising adjuvant and vaccine delivery system. Indian J. Pharm. Sci. 2013, 75, 442–449. [Google Scholar] [CrossRef]
- Mata, E.; Igartua, M.; Patarroyo, M.E.; Pedraz, J.L.; Hernández, R.M. Enhancing immunogenicity to PLGA microparticulate systems by incorporation of alginate and RGD-modified alginate. Eur. J. Pharm. Sci. 2011, 44, 32–40. [Google Scholar] [CrossRef]
- Ibe, M.I.; Odimegwu, D.C.; Onuigbo, E.B. Alginate-coated chitosan microparticles encapsulating an oral plasmid-cured live Salmonella enterica serovar Gallinarum vaccine cause a higher expression of interferon-gamma in chickens compared to the parenteral live vaccine. Avian Pathol. 2019, 14, 1–6. [Google Scholar] [CrossRef]
- Mosafer, J.; Sabbaghi, A.-H.; Badiee, A.; Dehghan, S.; Tafaghodi, M. Preparation, characterization and in vivo evaluation of alginate-coated chitosan and trimethylchitosan nanoparticles loaded with PR8 influenza virus for nasal immunization. Asian J. Pharm. Sci. 2019, 14, 216–221. [Google Scholar] [CrossRef]

| Adjuvant Name | Advantages | Disadvantages | Licensed Vaccines |
|---|---|---|---|
| MF59® | Compared to aluminum salts, MF59® causes stronger immune response, stimulating both antibody production and T-cell immune response. | Reactogenicity. Pain at injection site. Induces inflammatory arthritis. | MF59® is included in the Fluad® influenza vaccine which is now licensed in 30 countries worldwide. |
| AS03 | The strong stimulation of both antibody production and Th1 and Th2 immune responses. | An association between the AS03-adjuvanted Pandemrix vaccine and narcolepsy cannot yet be excluded. | It is approved in the United States and Europe and used in various vaccine products. For example, in H1N1 pandemic flu vaccine Pandemrix and Arepanrix. |
| GLA-SE | Strong Th1-type immune responses. The enhancement of the magnitude and polyfunctional cytokine profile of CD4+ T cells. | Mild or moderate adverse events (mainly pain at injection site). | - |
| CAF19, CAF24 | Promising adjuvant for induction of cytotoxic T lymphocytes (CTLs) responses upon intracutaneous and intramuscular immunization. | Specific lysis of antigen-pulsed splenocytes. | - |
| Cucumarioside A2-2 | Immunostimulating activity expressed primarily in the activation of cellular immunity. | Individual form possesses membranotropic properties which is absent in complex with cholesterol. | - |
| Chitosan | Non-toxic, biocompatible, biodegradable, non-allergenic. Parenteral and mucosal administrations. Controlled antigen release. Mucosal administration elicits robust antibody and T-cell responses. | Poor reproducibility of the results due to the variability of the chemical structure. Poor solubility above pH 6. | - |
| Fucoidans | Almost complete absence of toxicity, safety, and excellent biocompatibility. Regulation of cellular and humoral immunity as well as hematopoietic mobilization. Potentiation of the function of immune cells. Anti-cancer effect. | Difficulties with obtaining structurally characterized and homogeneous samples or oligomeric fractions. | - |
| Carrageenans | No adverse side effects at intranasal use. An activation of macrophages. Induction of the generation of pro-inflammatory cytokines. Significant ability to enhance antigen specific immune responses as well as antitumor effects. | Limited solubility. Anticoagulant properties. Prolonged oral administration can lead to the development of inflammation of the gastrointestinal tract. | - |
| Alginate | Non-toxic, biocompatible, biodegradable. Mucoadhesive nature and a relatively low cost. Stimulation of Th1 response and production of specific antibodies. Anti-cancer and anti-allergic properties. An ability to form hydrogel microspheres and nanospheres which possess higher immunostimulating effect. | Variable chemical structures. | - |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanina, N. Vaccine Adjuvants Derived from Marine Organisms. Biomolecules 2019, 9, 340. https://doi.org/10.3390/biom9080340
Sanina N. Vaccine Adjuvants Derived from Marine Organisms. Biomolecules. 2019; 9(8):340. https://doi.org/10.3390/biom9080340
Chicago/Turabian StyleSanina, Nina. 2019. "Vaccine Adjuvants Derived from Marine Organisms" Biomolecules 9, no. 8: 340. https://doi.org/10.3390/biom9080340