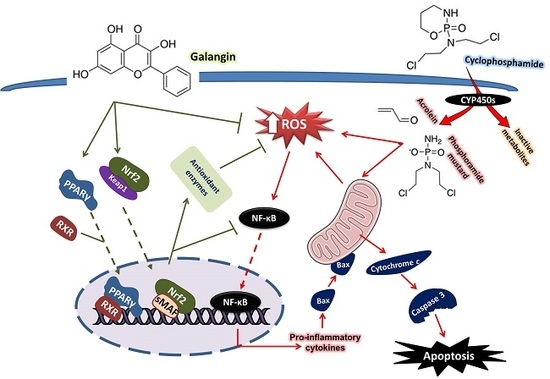
Galangin Activates Nrf2 Signaling and Attenuates Oxidative Damage, Inflammation, and Apoptosis in a Rat Model of Cyclophosphamide-Induced Hepatotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Determination of Liver Function Markers and Cytokines
2.3. Determination of Reactive Oxidative Species, Lipid Peroxidation, Nitric Oxide, and Antioxidants
2.4. Determination of 8-Oxo-2′-Deoxyguanosine (8-Oxo-dG) and Caspase-3
2.5. Histological Preparation of Liver Sections
2.6. Gene Expression
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. Galangin Protects against CP-Induced Hepatic Injury
3.2. Galangin Prevents CP-Induced Alterations in the Expression of CYPs
3.3. Galangin Attenuates Oxidative Stress and DNA Damage, and Enhances Antioxidants in Liver of CP-Induced Rats
3.4. Galangin Suppresses NF-κB and Inflammation in CP-Intoxicated Rats
3.5. Galangin Attenuates CP-Induced Hepatic Apoptosis
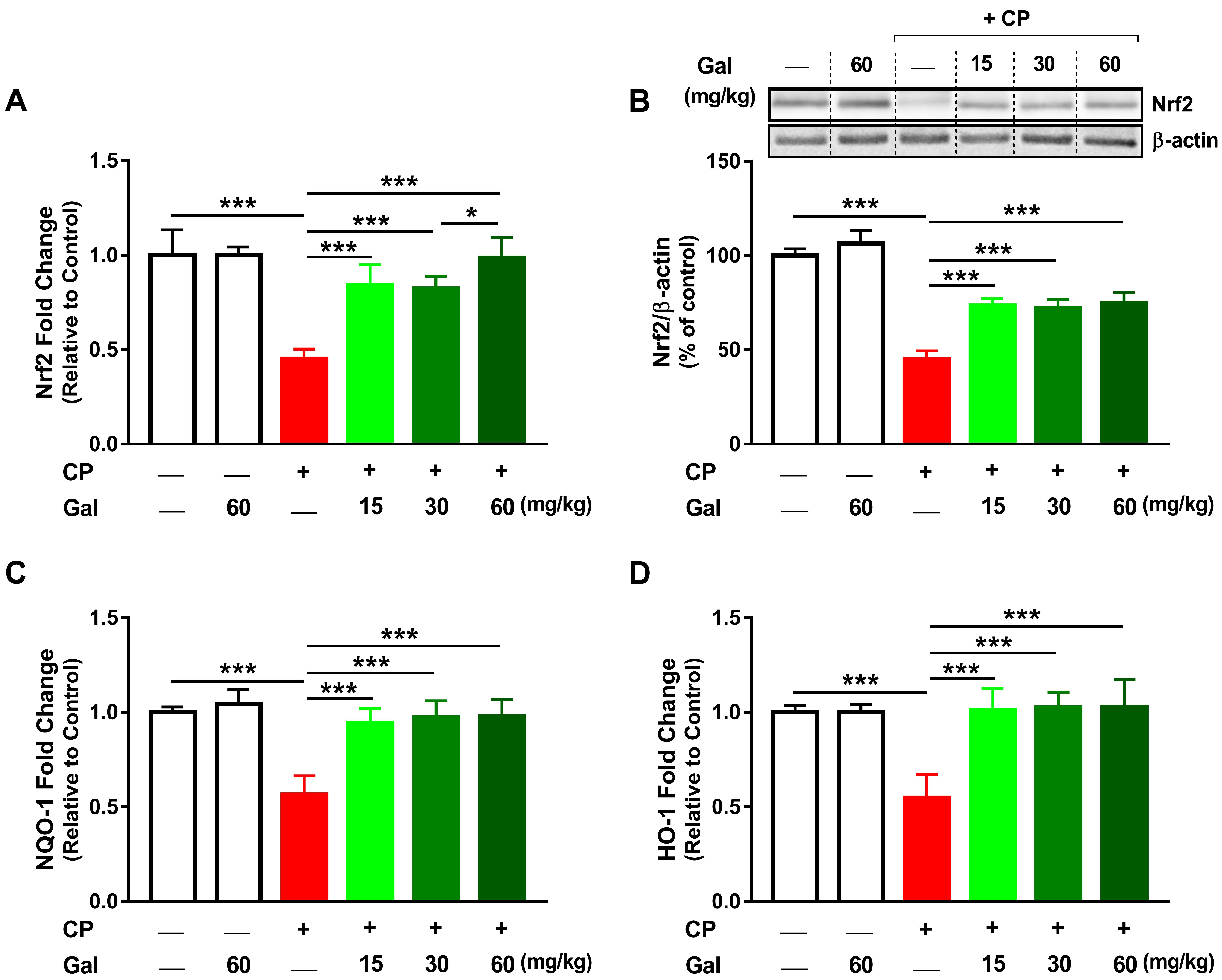
3.6. Galangin Activates Nrf2/HO-1 Signaling and Increases PPARγ Expression Liver of CP-Intoxicated Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mahmoud, A.M.; Germoush, M.O.; Alotaibi, M.F.; Hussein, O.E. Possible involvement of nrf2 and pparγ up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed. Pharmacother. 2017, 86, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Moignet, A.; Hasanali, Z.; Zambello, R.; Pavan, L.; Bareau, B.; Tournilhac, O.; Roussel, M.; Fest, T.; Awwad, A.; Baab, K. Cyclophosphamide as a first-line therapy in lgl leukemia. Leukemia 2014, 28, 1134–1136. [Google Scholar] [CrossRef] [PubMed]
- Omole, J.G.; Ayoka, O.A.; Alabi, Q.K.; Adefisayo, M.A.; Asafa, M.A.; Olubunmi, B.O.; Fadeyi, B.A. Protective effect of kolaviron on cyclophosphamide-induced cardiac toxicity in rats. J. Evid. Based Integr. Med. 2018, 23, 2156587218757649. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.F.; Saleh, D.O.; Mostafa, R.E. Genistein ameliorates cyclophosphamide-induced hepatotoxicity by modulation of oxidative stress and inflammatory mediators. Open Access Maced. J. Med. Sci. 2017, 5, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh, M.; Hosseinimehr, S.J.; Khalatbary, A.R.; Mohammadi, H.R.; Dashti, A.; Amiri, F.T. Atorvastatin mitigates cyclophosphamide-induced hepatotoxicity via suppression of oxidative stress and apoptosis in rat model. Res. Pharm. Sci. 2018, 13, 440–449. [Google Scholar] [PubMed]
- Fahmy, S.R.; Amien, A.I.; Abd-Elgleel, F.M.; Elaskalany, S.M. Antihepatotoxic efficacy of mangifera indica l. Polysaccharides against cyclophosphamide in rats. Chem. Biol. Interact. 2016, 244, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular mechanisms of acrolein toxicity: Relevance to human disease. Toxicol. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Long, M.H.; Wu, J.; Wang, M.M.; Li, X.Y.; Shen, H.; Xu, J.D.; Zhou, L.; Fang, Z.J.; Luo, Y. Ginseng alleviates cyclophosphamide-induced hepatotoxicity via reversing disordered homeostasis of glutathione and bile acid. Sci. Rep. 2015, 5, 17536. [Google Scholar] [CrossRef] [PubMed]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The role of nrf2 in cardiovascular function and disease. Oxid. Med. Cell Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Germoush, M.O.; Al-Anazi, K.M.; Mahmoud, A.H.; Farah, M.A.; Allam, A.A. Commiphora molmol protects against methotrexate-induced nephrotoxicity by up-regulating nrf2/are/ho-1 signaling. Biomed. Pharmacother. 2018, 106, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Wilkinson, F.L.; McCarthy, E.M.; Moreno-Martinez, D.; Langford-Smith, A.; Romero, M.; Duarte, J.; Alexander, M.Y. Endothelial microparticles prevent lipid-induced endothelial damage via akt/enos signaling and reduced oxidative stress. FASEB J. 2017, 31, 4636–4648. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Xiao, Q.; Zhou, J.; Feng, H.; Liu, G.; Ci, X. Licochalcone a upregulates nrf2 antioxidant pathway and thereby alleviates acetaminophen-induced hepatotoxicity. Front. Pharmacol. 2018, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Al Dera, H.S. 18β-glycyrrhetinic acid exerts protective effects against cyclophosphamide-induced hepatotoxicity: Potential role of pparγ and nrf2 upregulation. Genes Nutr. 2015, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hozayen, W.G.; Ramadan, S.M. Berberine ameliorates methotrexate-induced liver injury by activating nrf2/ho-1 pathway and ppargamma, and suppressing oxidative stress and apoptosis in rats. Biomed. Pharmacother. 2017, 94, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Mohammed, H.M.; Khadrawy, S.M.; Galaly, S.R. Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of nrf2/are/ho-1, ppargamma and tgf-beta1/smad3 signaling, and amelioration of oxidative stress and inflammation. Chem. Biol. Interact. 2017, 277, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hozayen, W.G.; Hasan, I.H.; Shaban, E.; Bin-Jumah, M. Umbelliferone ameliorates ccl4-induced liver fibrosis in rats by upregulating ppargamma and attenuating oxidative stress, inflammation, and tgf-beta1/smad3 signaling. Inflammation 2019, 42, 1103–1116. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, J.; Park, K.W. The multifaceted factor peroxisome proliferator-activated receptor gamma (ppargamma) in metabolism, immunity, and cancer. Arch. Pharm. Res. 2015, 38, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Okuno, Y.; Matsuda, M.; Miyata, Y.; Fukuhara, A.; Komuro, R.; Shimabukuro, M.; Shimomura, I. Human catalase gene is regulated by peroxisome proliferator activated receptor-gamma through a response element distinct from that of mouse. Endocr. J. 2010, 57, 303–309. [Google Scholar] [CrossRef]
- Mak, K.K.; Tan, J.J.; Marappan, P.; Balijepalli, M.K.; Choudhury, H.; Ramamurthy, S.; Pichika, M.R. Galangin’s potential as a functional food ingredient. J. Funct. Foods 2018, 46, 490–503. [Google Scholar] [CrossRef]
- Aloud, A.A.; Veeramani, C.; Govindasamy, C.; Alsaif, M.A.; El Newehy, A.S.; Al-Numair, K.S. Galangin, a dietary flavonoid, improves antioxidant status and reduces hyperglycemia-mediated oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2017, 22, 290–300. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tsai, M.S.; Hsieh, P.C.; Shih, J.H.; Wang, T.S.; Wang, Y.C.; Lin, T.H.; Wang, S.H. Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of erk and nf-kappab signaling. Toxicol. Appl. Pharmacol. 2017, 329, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, A.S.; Anuradha, C.V. Effect of galangin supplementation on oxidative damage and inflammatory changes in fructose-fed rat liver. Chem. Biol. Interact. 2011, 193, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, G.; Yang, W.; Li, Y.; Jiang, M.; Li, L. Antifibrotic activity of galangin, a novel function evaluated in animal liver fibrosis model. Environ Toxicol. Pharmacol. 2013, 36, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tong, L.; Zhang, J.; Zhang, Y.; Zhang, F. Galangin alleviates liver ischemia-reperfusion injury in a rat model by mediating the pi3k/akt pathway. Cell Physiol. Biochem. 2018, 51, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Hewage, S.R.K.M.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Fernando, P.M.D.J.; Oh, M.C.; Park, J.E.; Shilnikova, K.; Moon, Y.J.; Shin, D.O. Galangin activates the erk/akt-driven nrf2 signaling pathway to increase the level of reduced glutathione in human keratinocytes. Biomol. Ther. 2017, 25, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Aloud, A.A.; Veeramani, C.; Govindasamy, C.; Alsaif, M.A.; Al-Numair, K.S. Galangin, a natural flavonoid reduces mitochondrial oxidative damage in streptozotocin-induced diabetic rats. Redox Rep. 2018, 23, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Jarrell, S.T.; Scheckenbach, R.; Lieberman, S.; Anderson, R.A. Comparative effects of chromium, vanadium and gymnema sylvestre on sugar-induced blood pressure elevations in shr. J. Am. Coll Nutr. 1998, 17, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R., Jr. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996, 268, 237–246. [Google Scholar]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Dembiec, D.; Marcus, J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970, 34, 30–38. [Google Scholar] [CrossRef]
- Matkovics, B.; Szabo, L.; Varga, I.S. Determination of enzyme activities in lipid peroxidation and glutathione pathways (in hungarian). Lab. Diagn. 1998, 15, 248–249. [Google Scholar]
- Mahmoud, A.M. Hesperidin protects against cyclophosphamide-induced hepatotoxicity by upregulation of pparγ and abrogation of oxidative stress and inflammation. Can. J. Physiol. Pharmacol. 2014, 92, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar] [PubMed]
- Alqahtani, S.; Mahmoud, A.M. Gamma-glutamylcysteine ethyl ester protects against cyclophosphamide-induced liver injury and hematologic alterations via upregulation of pparγ and attenuation of oxidative stress, inflammation, and apoptosis. Oxid. Med. Cell Longev. 2016, 2016, 4016209. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhu, L.; Ding, J.; Zhuang, X.; Xu, L.; Chen, F. Protective effect of galangin in concanavalin a-induced hepatitis in mice. Drug Des. Devel. Ther. 2015, 9, 2983–2992. [Google Scholar]
- Nebert, D.W.; Russell, D.W. Clinical importance of the cytochromes p450. Lancet 2002, 360, 1155–1162. [Google Scholar] [CrossRef]
- Sheweita, S.A.; El-Hosseiny, L.S.; Nashashibi, M.A. Protective effects of essential oils as natural antioxidants against hepatotoxicity induced by cyclophosphamide in mice. PLoS ONE 2016, 11, e0165667. [Google Scholar] [CrossRef]
- Xie, H.; Afsharian, P.; Terelius, Y.; Mirghani, R.A.; Yasar, U.; Hagbjork, A.L.; Lundgren, S.; Hu, Y.; Rane, A.; Hassan, M. Cyclophosphamide induces mrna, protein and enzyme activity of cytochrome p450 in rat. Xenobiotica Fate Foreign Compd. Biol. Syst. 2005, 35, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Bardbori, A.; Bengtsson, J.; Rannug, U.; Rannug, A.; Wincent, E. Quercetin, resveratrol, and curcumin are indirect activators of the aryl hydrocarbon receptor (ahr). Chem. Res. Toxicol. 2012, 25, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Murakami, A.; Urushizaki, S.; Matsuda, M.; Kawazoe, K.; Ishizawa, K. Extracts of immature orange (aurantii fructus immaturus) and citrus unshiu peel (citri unshiu pericarpium) induce p-glycoprotein and cytochrome p450 3a4 expression via upregulation of pregnane x receptor. Front. Pharmacol. 2017, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Carazo Fernandez, A.; Smutny, T.; Hyrsova, L.; Berka, K.; Pavek, P. Chrysin, baicalein and galangin are indirect activators of the human constitutive androstane receptor (car). Toxicol. Lett. 2015, 233, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.L.; Zhao, F.; Yin, J.T.; Liang, C.J.; Niu, X.L.; Qiu, Z.H.; Zhang, L.T. Two approaches for evaluating the effects of galangin on the activities and mrna expression of seven cyp450. Molecules 2019, 24, 1171. [Google Scholar] [CrossRef] [PubMed]
- Smathers, R.L.; Galligan, J.J.; Stewart, B.J.; Petersen, D.R. Overview of lipid peroxidation products and hepatic protein modification in alcoholic liver disease. Chem. Biol. Interact. 2011, 192, 107–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Germoush, M.O.; Mahmoud, A.M. Berberine mitigates cyclophosphamide-induced hepatotoxicity by modulating antioxidant status and inflammatory cytokines. J. Cancer Res. Clin. Oncol. 2014, 140, 1103–1109. [Google Scholar] [CrossRef]
- Kamel, E.M.; Mahmoud, A.M.; Ahmed, S.A.; Lamsabhi, A.M. A phytochemical and computational study on flavonoids isolated from trifolium resupinatum l. And their novel hepatoprotective activity. Food Funct. 2016, 7, 2094–2106. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Amić, D.; Davidović-Amić, D.; Bešlo, D.; Trinajstić, N. Structure-radical scavenging activity relationships of flavonoids. Croat. Chem. Acta 2003, 76, 55–61. [Google Scholar]
- Caglayan, C.; Temel, Y.; Kandemir, F.M.; Yildirim, S.; Kucukler, S. Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ. Sci. Pollut. Res. Int. 2018, 25, 20968–20984. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Valatas, V.; Kouroumalis, E. Role of kupffer cells in the pathogenesis of liver disease. World J. Gastroenterol. 2006, 12, 7413–7420. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Prakash, C.; Tiwari, K.N.; Mishra, S.K.; Kumar, V. Premna integrifolia ameliorates cyclophosphamide-induced hepatotoxicity by modulation of oxidative stress and apoptosis. Biomed. Pharmacother. 2018, 107, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Gao, Y.; Zhao, H.; Wang, Z.; Wang, J. Galangin protects human rheumatoid arthritis fibroblast-like synoviocytes via suppression of the nf-κb/nlrp3 pathway. Mol. Med. Rep. 2018, 18, 3619–3624. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Zaki, A.R.; Hassan, M.E.; Mostafa-Hedeab, G. Commiphora molmol resin attenuates diethylnitrosamine/phenobarbital-induced hepatocarcinogenesis by modulating oxidative stress, inflammation, angiogenesis and nrf2/are/ho-1 signaling. Chem. Biol. Interact. 2017, 270, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Twab, S.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Mahmoud, A.M. Chicoric acid prevents methotrexate-induced kidney injury by suppressing nf-kappab/nlrp3 inflammasome activation and up-regulating nrf2/are/ho-1 signaling. Inflamm. Res. 2019, 68, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Twab, S.M.; Hozayen, W.G.; Hussein, O.E.; Mahmoud, A.M. 18beta-glycyrrhetinic acid protects against methotrexate-induced kidney injury by up-regulating the nrf2/are/ho-1 pathway and endogenous antioxidants. Ren. Fail. 2016, 38, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between nrf2 and nf-κb response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wang, H.; Wang, X.; Zhu, L.; Mao, L. The absence of nrf2 enhances nf-kappab-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat. Inflamm. 2012, 2012, 217580. [Google Scholar] [CrossRef]
- Choi, M.J.; Lee, E.J.; Park, J.S.; Kim, S.N.; Park, E.M.; Kim, H.S. Anti-inflammatory mechanism of galangin in lipopolysaccharide-stimulated microglia: Critical role of ppar-γ signaling pathway. Biochem. Pharmacol. 2017, 144, 120–131. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; El-Twab, S.M.A. Methotrexate hepatotoxicity is associated with oxidative stress, and down-regulation of pparγ and nrf2: Protective effect of 18β-glycyrrhetinic acid. Chem. Biol. Interact. 2017, 270, 59–72. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.A.; Rifaai, R.A. Peroxisome proliferator activator receptor (ppar)-γ ligand, but not ppar-α, ameliorates cyclophosphamide-induced oxidative stress and inflammation in rat liver. PPAR Res. 2014, 2014, 626319. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S.; Desvergne, B.; Wahli, W. Roles of ppars in health and disease. Nature 2000, 405, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Remels, A.H.; Langen, R.C.; Gosker, H.R.; Russell, A.P.; Spaapen, F.; Voncken, J.W.; Schrauwen, P.; Schols, A.M. Ppargamma inhibits nf-kappab-dependent transcriptional activation in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E174–E183. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kleinhenz, D.J.; Lassegue, B.; Griendling, K.K.; Dikalov, S.; Hart, C.M. Peroxisome proliferator-activated receptor-gamma ligands regulate endothelial membrane superoxide production. Am. J. Physiol. Cell Physiol. 2005, 288, C899–C905. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Park, J.S.; Park, J.E.; Kim, H.S.; Kim, H.S. Galangin suppresses pro-inflammatory gene expression in polyinosinic-polycytidylic acid-stimulated microglial cells. Biomol. Ther. 2017, 25, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.C.; Standiford, T.J. Nrf2 and pparγ: Ppartnering against oxidant-induced lung injury. Am. J. Respir Crit. Care Med. 2010, 182, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Polvani, S.; Tarocchi, M.; Galli, A. Pparγ and oxidative stress: Con (β) catenating nrf2 and foxo. PPAR Res. 2012, 2012, 641087. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hussein, O.; Abd El-Twab, S.; Hozayen, W. Ferulic acid protects against methotrexate nephrotoxicity via activation of nrf2/are/ho-1 signaling and pparγ, and suppression of nf-κb/nlrp3 inflammasome axis. Food Funct. 2019. [Google Scholar] [CrossRef] [PubMed]









| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| BAX | AGGACGCATCCACCAAGAAG | CAGTTGAAGTTGCCGTCTGC |
| BCL2 | ACTCTTCAGGGATGGGGTGA | TGACATCTCCCTGTTGACGC |
| Casp3 | GAGCTTGGAACGCGAAGAAA | TAACCGGGTGCGGTAGAGTA |
| TNFa | AAATGGGCTCCCTCTCATCAGTTC | TCTGCTTGGTGGTTTGCTACGAC |
| IL1b | GACTTCACCATGGAACCCGT | GGAGACTGCCCATTCTCGAC |
| NOS2 | ATTCCCAGCCCAACAACACA | GCAGCTTGTCCAGGGATTCT |
| COX2 | TGATCTACCCTCCCCACGTC | ACACACTCTGTTGTGCTCCC |
| NRF2 | TTGTAGATGACCATGAGTCGC | TGTCCTGCTGTATGCTGCTT |
| NQO1 | GGCCATCATTTGGGCAAGTC | TCCTTGTGGAACAAAGGCGA |
| HO-1 | GTAAATGCAGTGTTGGCCCC | ATGTGCCAGGCATCTCCTTC |
| PPARg | GGACGCTGAAGAAGAGACCTG | CCGGGTCCTGTCTGAGTATG |
| Cyp2b1 | AGGACCATGGAGCCCAGTAT | GAGGTCCTGGTGGGAAGTTG |
| Cyp2b2 | GTCCTGCATGGATGAGAGAGG | ATCATCAAGGGATGGTGGCCT |
| Cyp2e1 | GCGGAGGTTTTCCCTAAGCA | GCGCAGCCAATCAGAAATGT |
| Cyp2c11 | GGTCCAACACCTCTCCCAAT | CAAAGGGCTTCATGCCCAAA |
| Cyp3a2 | GGAGTTGGCAAGGTCTGTGA | GATGTGGATGGAGATGGTCCC |
| Actb | AGGAGTACGATGAGTCCGGC | CGCAGCTCAGTAACAGTCCG |
| Control | 60 mg/kg Gal | CP | ||||
|---|---|---|---|---|---|---|
| 0 mg/kg Gal | 15 mg/kg Gal | 30 mg/kg Gal | 60 mg/kg Gal | |||
| Cytoplasmic vacuolations | - | - | +++ | + | + | + |
| Congestion | - | - | +++ | + | + | - |
| Hemorrhage | - | - | ++ | + | + | - |
| Fatty changes | - | - | + | - | - | - |
| Leukocyte infiltration | - | - | ++ | + | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aladaileh, S.H.; Abukhalil, M.H.; Saghir, S.A.M.; Hanieh, H.; Alfwuaires, M.A.; Almaiman, A.A.; Bin-Jumah, M.; Mahmoud, A.M. Galangin Activates Nrf2 Signaling and Attenuates Oxidative Damage, Inflammation, and Apoptosis in a Rat Model of Cyclophosphamide-Induced Hepatotoxicity. Biomolecules 2019, 9, 346. https://doi.org/10.3390/biom9080346
Aladaileh SH, Abukhalil MH, Saghir SAM, Hanieh H, Alfwuaires MA, Almaiman AA, Bin-Jumah M, Mahmoud AM. Galangin Activates Nrf2 Signaling and Attenuates Oxidative Damage, Inflammation, and Apoptosis in a Rat Model of Cyclophosphamide-Induced Hepatotoxicity. Biomolecules. 2019; 9(8):346. https://doi.org/10.3390/biom9080346
Chicago/Turabian StyleAladaileh, Saleem H., Mohammad H. Abukhalil, Sultan A. M. Saghir, Hamza Hanieh, Manal A. Alfwuaires, Amer A. Almaiman, May Bin-Jumah, and Ayman M. Mahmoud. 2019. "Galangin Activates Nrf2 Signaling and Attenuates Oxidative Damage, Inflammation, and Apoptosis in a Rat Model of Cyclophosphamide-Induced Hepatotoxicity" Biomolecules 9, no. 8: 346. https://doi.org/10.3390/biom9080346
APA StyleAladaileh, S. H., Abukhalil, M. H., Saghir, S. A. M., Hanieh, H., Alfwuaires, M. A., Almaiman, A. A., Bin-Jumah, M., & Mahmoud, A. M. (2019). Galangin Activates Nrf2 Signaling and Attenuates Oxidative Damage, Inflammation, and Apoptosis in a Rat Model of Cyclophosphamide-Induced Hepatotoxicity. Biomolecules, 9(8), 346. https://doi.org/10.3390/biom9080346