Inactivation of Aldehyde Dehydrogenase by Disulfiram in the Presence and Absence of Lipoic Acid or Dihydrolipoic Acid: An in Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Homogenate Preparation
2.3. Determination of Yeast ALDH Activity
2.4. Determination of ALDH Activity in the Rat Liver Homogenate
2.5. Series of Mixtures
2.5.1. Assay Using Yeast ALDH
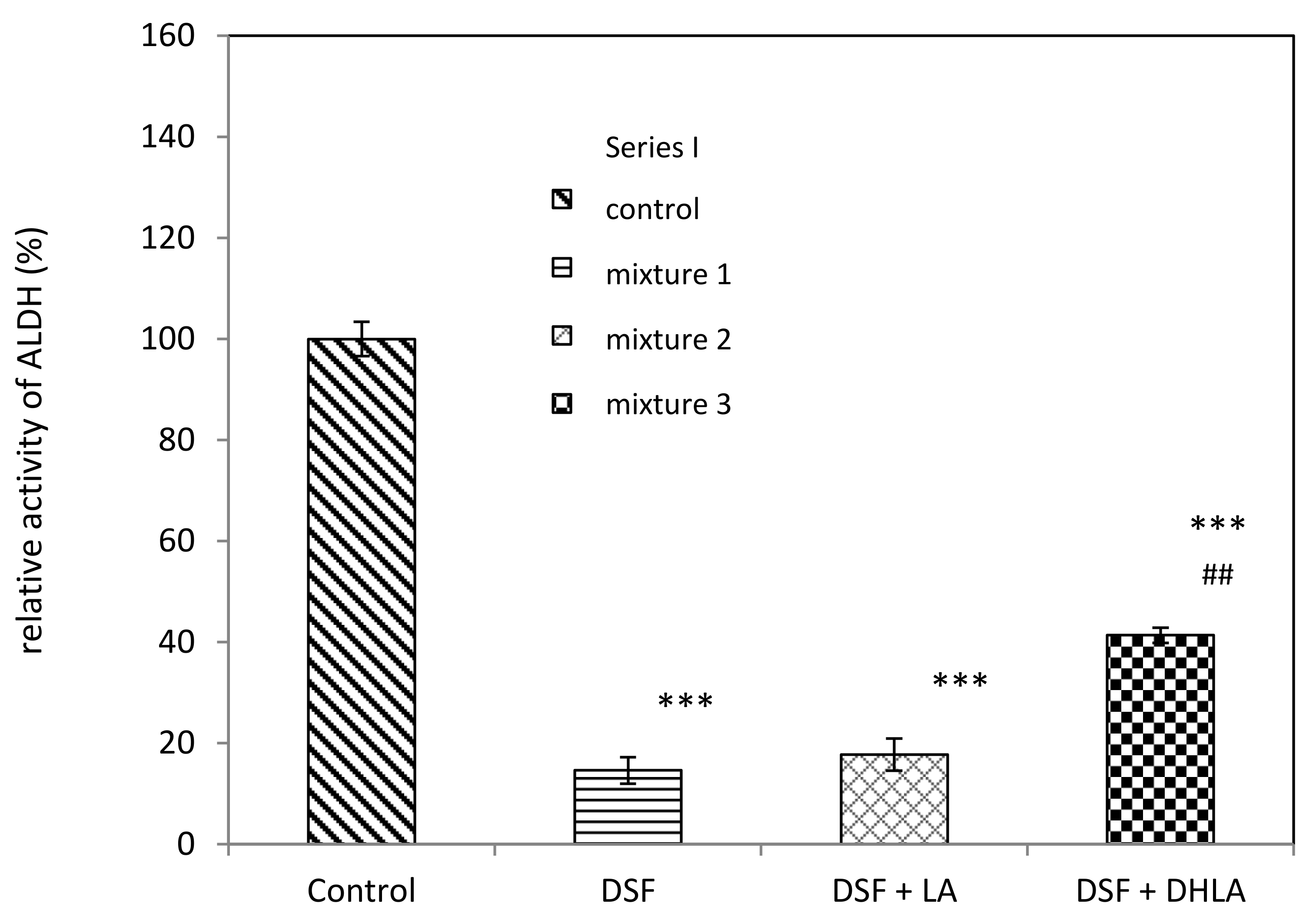
- Mixture 1. ALDH was incubated with 0.1 mM DSF in 50 mM sodium phosphate buffer for 20 min.
- Mixture 2. ALDH was preincubated with 0.1 mM DSF in 50 mM sodium phosphate buffer for 5 min, then LA was added (final concentration 1 mM) and the mixture was incubated for a further 15 min.
- Mixture 3. ALDH was preincubated with 0.1 mM DSF in 50 mM sodium phosphate buffer for 5 min, then DHLA was added (final concentration 1 mM) and the mixture was incubated further for 15 min.
- Mixture 1. ALDH was preincubated alone for 15 min, then 0.1 mM DSF and the mixture was incubated for a further 5 min.
- Mixture 2. ALDH was preincubated with 1mM LA in 50 mM sodium phosphate buffer for 15 min, then 0.1 mM DSF was added and the mixture was incubated for a further 5 min.
- Mixture 3. ALDH was preincubated with 1 mM DHLA in 50 mM sodium phosphate buffer for 15 min, then 0.1 mM DSF was added and the mixture was incubated for a further 5 min.
- Mixture 1. DSF (0.1 mM) in 50 mM sodium phosphate buffer was preincubated for 15 min, then ALDH was added and the mixture was incubated for a further 5 min.
- Mixture 2. DSF (0.1 mM) in 50 mM sodium phosphate buffer was preincubated with 1 mM LA for 15 min, then ALDH was added and the mixture was incubated for a further 5 min.
- Mixture 3. DSF (0.1 mM) in 50 mM sodium phosphate buffer was preincubated with 1 mM DHLA for 15 min, then ALDH was added and the mixture was incubated for a further 5 min.
2.5.2. Assay Using Rat Liver Homogenate-Derived ALDH
2.6. Statistical Analysis
3. Results
3.1. Yeast ALDH
3.1.1. Series I
3.1.2. Series II
3.1.3. Series III
3.2. Rat Liver-Derived ALDH
3.2.1. Series I
3.2.2. Series II
3.2.3. Series III
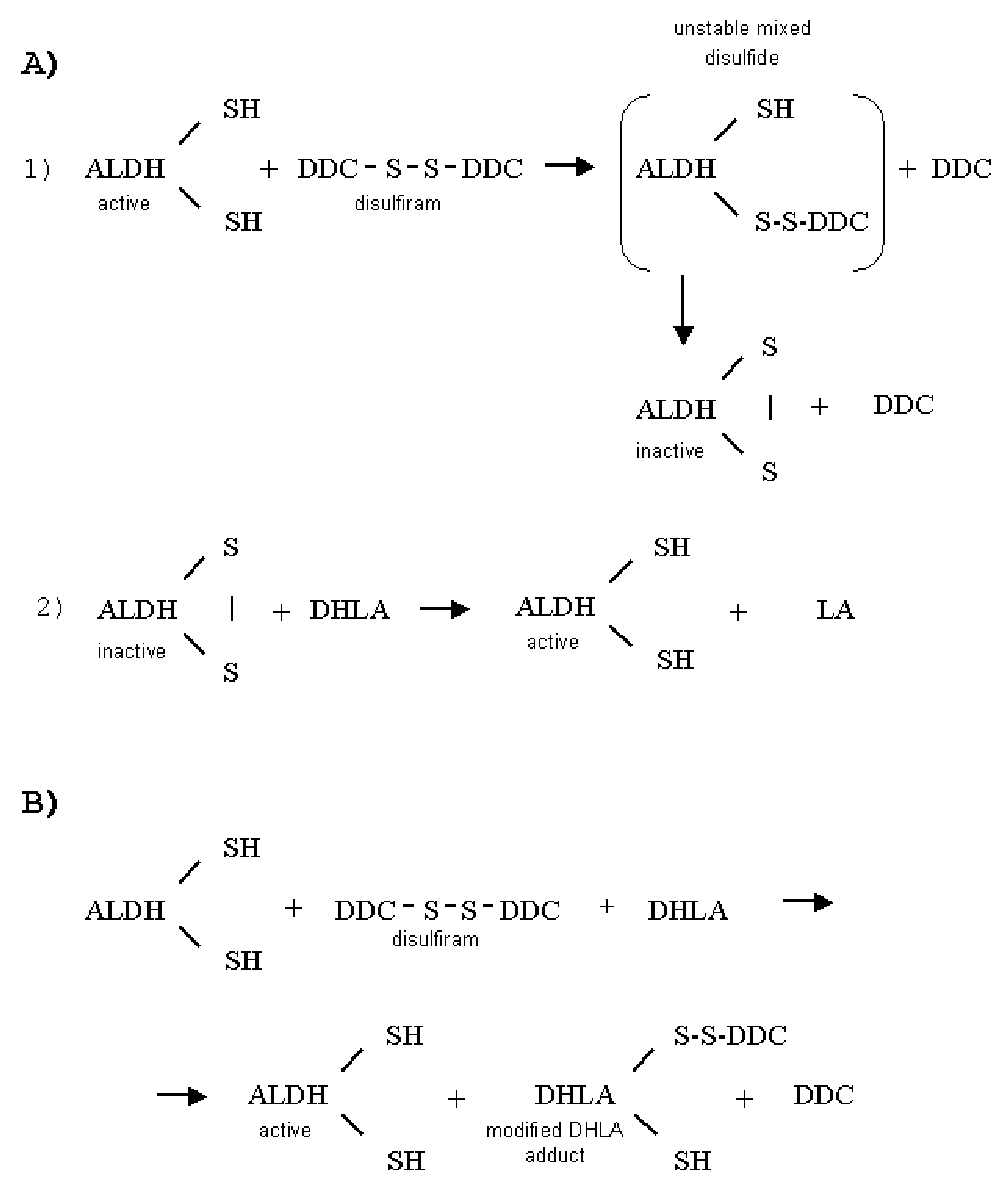
4. Discussion




5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koppaka, V.; Thompson, D.C.; Chen, Y.; Ellermann, M.; Nicolaou, K.C.; Juvonen, R.O.; Petersen, D.; Deitrich, R.A.; Hurley, T.D.; Vasiliou, V. Aldehyde dehydrogenase inhibitors: A comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol. Rev. 2012, 64, 520–539. [Google Scholar] [CrossRef] [PubMed]
- Kitson, T.M. The effect of disulfiram on the aldehyde dehydrogenases of sheep liver. Biochem. J. 1975, 151, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, S.M.; Bacher, R.S.; Jost, S.A. Disulfiram-like reaction involving ceftriaxone in a pediatric patient. J. Pediatr. Pharmacol. Ther. 2018, 23, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Guler, S.; Aytar, H.; Soyuduru, M.; Ramadan, H. Disulfiram-like reaction with ornidazole. Am. J. Emerg. Med. 2015, 33, e7–e8. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Cao, Y.; Zhang, X.; Jiao, S.; Qian, S.; Liu, P. Cephalosporin induced disulfiram-like reaction: A retrospective review of 78 cases. Int. Surg. 2014, 99, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, Y.; Zhu, W.; Shi, J.; Liu, Y.; Ling, W.; Kosten, T.R. Traditional medicine in the treatment of drug addiction. Am. J. Drug Alcohol Abus. 2009, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- De Haro, L. Disulfiram-like syndrome after hydrogen cyanamide professional skin exposure: Two case reports in France. J. Agromed. 2009, 14, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Górny, M.; Bilska-Wilkosz, A.; Kowalczyk-Pachel, D. Is aldehyde dehydrogenase inhibited by sulfur compounds? In vitro and in vivo studies. Acta Biochim. Pol. 2018, 65, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, M.L.; Lipsky, J.J.; Naylor, S. Role of disulfiram in the in vitro inhibition of rat liver mitochondrial aldehyde dehydrogenase. Biochem. Pharmacol. 2000, 60, 947–953. [Google Scholar] [CrossRef]
- Lipsky, J.J.; Shen, M.L.; Naylor, S. Overview-in vitro inhibition of aldehyde dehydrogenase by disulfiram and metabolites. Chem. Biol. Interact. 2001, 130–132, 81–91. [Google Scholar] [CrossRef]
- Dworacka, M.; Iskakova, S.; Krzyżagórska, E.; Wesołowska, A.; Kurmambayev, Y.; Dworacki, G. Alpha-lipoic acid modifies circulating angiogenic factors in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2015, 107, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Biewenga, G.P.; Haenen, G.R.; Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997, 29, 315–331. [Google Scholar] [CrossRef]
- Papanas, N.; Ziegler, D. Efficacy of α-lipoic acid in diabetic neuropathy. Expert Opin. Pharmacother. 2014, 15, 2721–2731. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Marcykiewicz, B.; Bilska-Wilkosz, A.; Sokołowska-Jeżewicz, M.; Kłapcińska, J. The effect of lipoate on anaerobic cysteine metabolism in erythrocytes of patients treated with peritoneal dialysis. Pharmacol. Rep. 2014, 66, 325–328. [Google Scholar] [CrossRef]
- Ghibu, S.; Richard, C.; Vergely, C.; Zeller, M.; Cottin, Y.; Rochette, L. Antioxidant properties of an endogenous thiol: Alpha-lipoic acid, useful in the prevention of cardiovascular diseases. J. Cardiovasc. Pharmacol. 2009, 54, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Bilska, A.; Dubiel, M.; Sokołowska-Jeżewicz, M.; Lorenc-Koci, E.; Włodek, L. Alpha-lipoic acid differently affects the reserpine-induced oxidative stress in the striatum and prefrontal cortex of rat brain. Neuroscience 2007, 146, 1758–1771. [Google Scholar] [CrossRef] [PubMed]
- Gorąca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic acid–biological activity and therapeutic potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef]
- Zygmunt, M.; Dudek, M.; Bilska-Wilkosz, A.; Bednarski, M.; Mogilski, S.; Knutelska, J.; Sapa, J. Anti-inflammatory activity of lipoic acid in mice peritonitis model. Acta Pol. Pharm. 2013, 70, 899–904. [Google Scholar]
- Hernández-Rabaza, V.; López-Pedrajas, R.; Almansa, I. Progesterone, Lipoic Acid, and Sulforaphane as Promising Antioxidants for Retinal Diseases: A Review. Antioxidants 2019, 8, 53. [Google Scholar] [CrossRef]
- Sokołowska, M.; Niedzielska, E.; Iciek, M.; Bilska, A.; Lorenc-Koci, E.; Włodek, L. The effect of the uremic toxin cyanate (CNO−) on anaerobic cysteine metabolism and oxidative processes in the rat liver: A protective effect of lipoate. Toxicol. Mech. Methods 2001, 21, 473–478. [Google Scholar] [CrossRef]
- Shindyapina, A.; Komarova, T.; Sheshukova, E.; Ershova, N.; Tashlitsky, V.; Kurkin, A.; Yusupov, I.; Mkrtchyan, G.; Shagidulin, M.; Dorokhov, Y. The Antioxidant Cofactor Alpha-Lipoic Acid May Control Endogenous Formaldehyde Metabolism in Mammals. Front. Neurosci. 2017, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Ji, W.Q.; Pang, J.J.; Wang, J.L.; Chen, Y.G.; Zhang, Y. Alpha-lipoic acid ameliorates oxidative stress by increasing aldehyde dehydrogenase-2 activity in patients with acute coronary syndrome. Tohoku J. Exp. Med. 2013, 229, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Ju, G.X.; Jiang, J.L.; Li, N.S.; Peng, J.; Luo, X.J. Lipoic acid protects gastric mucosa from ethanol-induced injury in rat through a mechanism involving aldehyde dehydrogenase 2 activation. Alcohol 2016, 56, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.; Lodge, J.K.; Marcocci, L.; Tritschler, H.J.; Packer, L.; Rihn, B.H. α-Lipoic acid in liver metabolism and disease. Free Radic. Biol. Med. 1998, 24, 1023–1039. [Google Scholar] [CrossRef]
- Bilska-Wilkosz, A.; Górny, M.; Dudek, M.; Nowiński, L.; Bednarski, M.; Iciek, M.; Kowalczyk-Pachel, D.; Sokołowska-Jeżewicz, M.; Filipek, B.; Włodek, L. Inactivation of aldehyde dehydrogenase by nitroglycerin in the presence and absence of lipoic acid and dihydrolipoic acid. Implications for the problem of differential effects of lipoic acid in vitro and in vivo. Acta Pol. Pharm. 2016, 73, 1531–1538. [Google Scholar] [PubMed]
- Tottmar, S.O.; Pettersson, H.; Kiessling, K.H. The subcellular distribution and properties of aldehyde dehydrogenases in rat liver. Biochem. J. 1975, 135, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.I. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Gilbert, H.F. Molecular and cellular aspects of thiol-disulfide exchange. Adv. Enzymol. Relat. Areas Mol. Biol. 1990, 63, 69–172. [Google Scholar]
- Lenartowicz, E.; Wudarczyk, J.; Dębska, G. Regulation of thiol group redox state in animal cells. Postep. Biochem. 1996, 42, 154–161. [Google Scholar]
- Veverka, K.A.; Johnson, K.L.; Mays, D.C.; Lipsky, J.J.; Naylor, S. Inhibition of aldehyde dehydrogenase by disulfiram and its metabolite methyl diethylthiocarbamoyl-sulfoxide. Biochem. Pharmacol. 1997, 53, 511–518. [Google Scholar] [CrossRef]
- Deitrich, R.A.; Hellerman, L. Diphosphopridine nucleotide-linked aldehyde dehydrogenase. II. Inhibitors. J. Biol. Chem. 1963, 238, 1683–1689. [Google Scholar] [PubMed]
- Johnston, C.D. The in vitro reaction between tetraethylthiuram disulfide (antabuse) and glutathione. Arch. Biochem. Biophys. 1953, 44, 249–251. [Google Scholar] [CrossRef]
- Kitson, T.M. The inactivation of aldehyde dehydrogenase by disulfiram in the presence of glutathione. Biochem. J. 1981, 199, 255–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallari, R.C.; Pietruszko, R. Human aldehyde dehydrogenase: Mechanism of inhibition of disulfiram. Science 1982, 216, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Gitler, C.; Mogyoros, M.; Kalef, E. Labelling of protein vicinal dithiols: Role of protein-S2 to protein-(SH)2 conversion in metabolic regulation and oxidative stress. Methods Enzymol. 1994, 233, 403–415. [Google Scholar] [PubMed]
- Li, T.K.; Vallee, B.L. Alcohol dehydrogenase and ethanol metabolism. Surg. Clin. N. Am. 1969, 49, 577–582. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilska-Wilkosz, A.; Górny, M.; Iciek, M. Inactivation of Aldehyde Dehydrogenase by Disulfiram in the Presence and Absence of Lipoic Acid or Dihydrolipoic Acid: An in Vitro Study. Biomolecules 2019, 9, 375. https://doi.org/10.3390/biom9080375
Bilska-Wilkosz A, Górny M, Iciek M. Inactivation of Aldehyde Dehydrogenase by Disulfiram in the Presence and Absence of Lipoic Acid or Dihydrolipoic Acid: An in Vitro Study. Biomolecules. 2019; 9(8):375. https://doi.org/10.3390/biom9080375
Chicago/Turabian StyleBilska-Wilkosz, Anna, Magdalena Górny, and Małgorzata Iciek. 2019. "Inactivation of Aldehyde Dehydrogenase by Disulfiram in the Presence and Absence of Lipoic Acid or Dihydrolipoic Acid: An in Vitro Study" Biomolecules 9, no. 8: 375. https://doi.org/10.3390/biom9080375
APA StyleBilska-Wilkosz, A., Górny, M., & Iciek, M. (2019). Inactivation of Aldehyde Dehydrogenase by Disulfiram in the Presence and Absence of Lipoic Acid or Dihydrolipoic Acid: An in Vitro Study. Biomolecules, 9(8), 375. https://doi.org/10.3390/biom9080375






