Kinematic Optimization for the Design of a Collaborative Robot End-Effector for Tele-Echography †
Abstract
:1. Introduction
2. Background
2.1. Ultrasonography
2.2. Tele-USG Systems
3. End-Effector Design Optimization
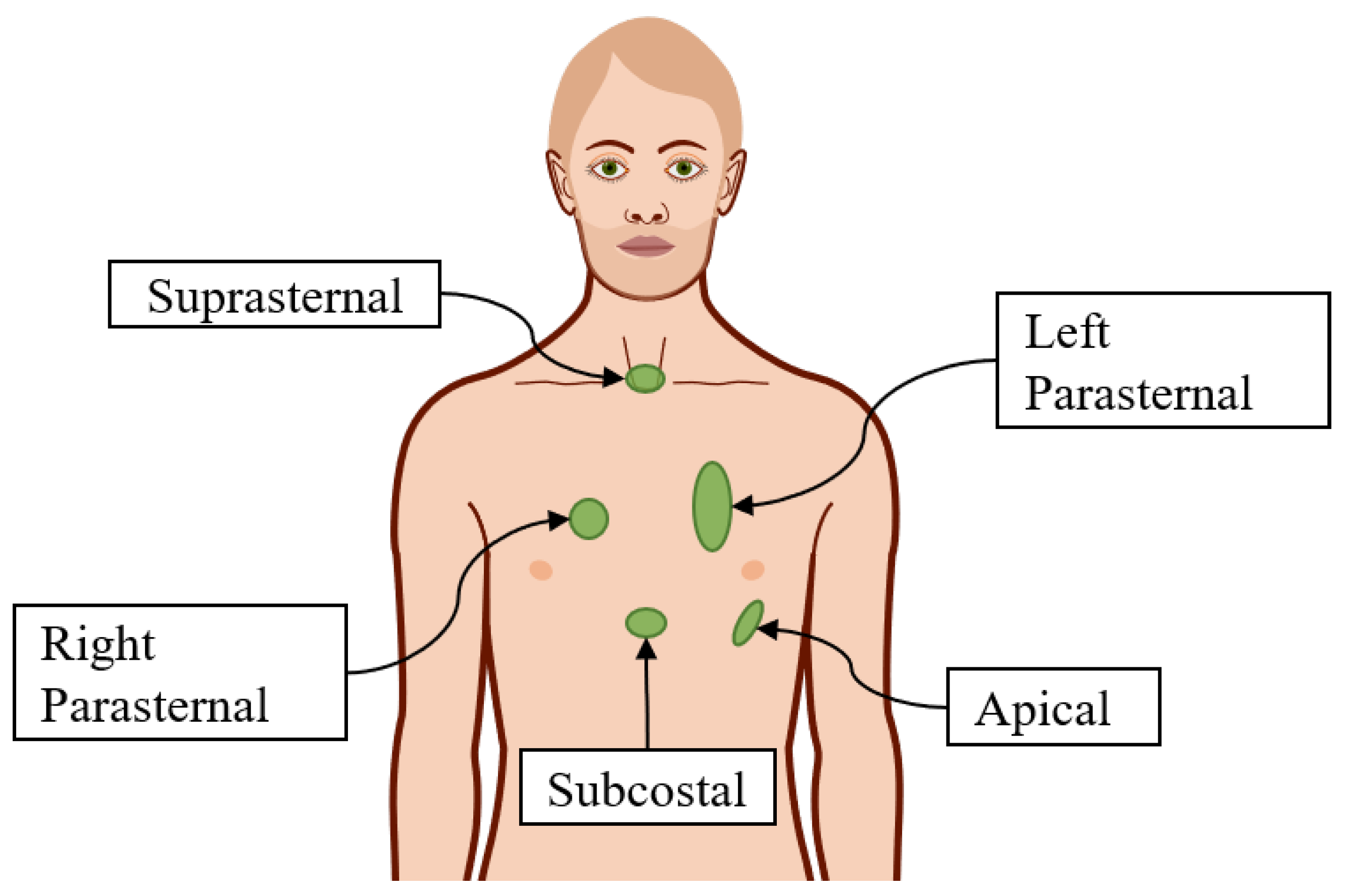
3.1. Requirements
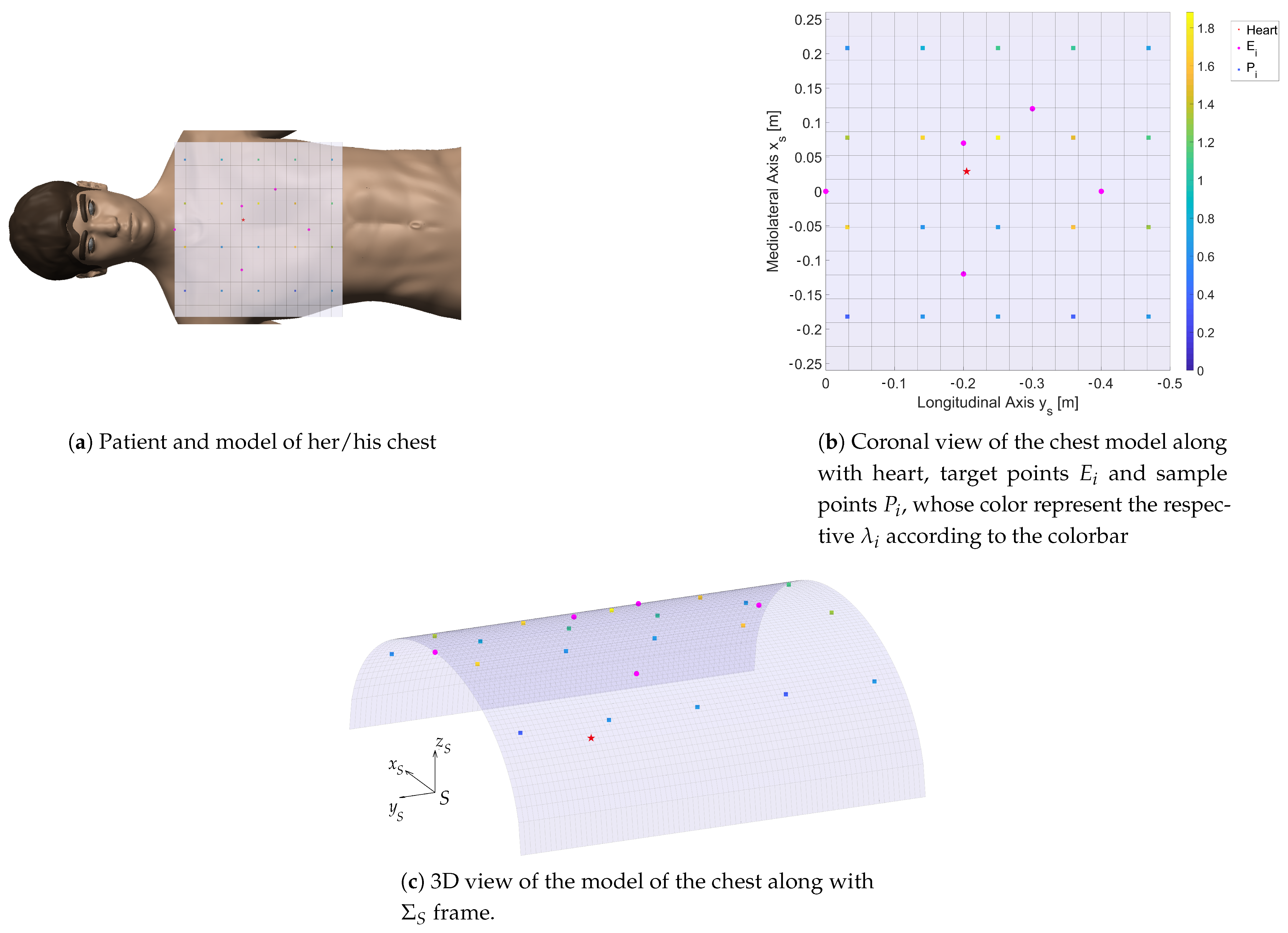
Model of the Patient and Target of the ECG
3.2. UR5 and Panda Kinematics
3.3. End-Effector Kinematics
3.4. Optimization Variables
3.5. Objective Function
3.6. Constraints
4. Implementation
| Algorithm 1: Optimization algorithm |
| Data: sample points , weights |
| Result: optimal |
| create the manipulator MN; |
| set , , ; |
| compute , ; |
| compute inverse kinematics starting points ; |
| set bounds in MultiStart according to Equations (13)–(15); |
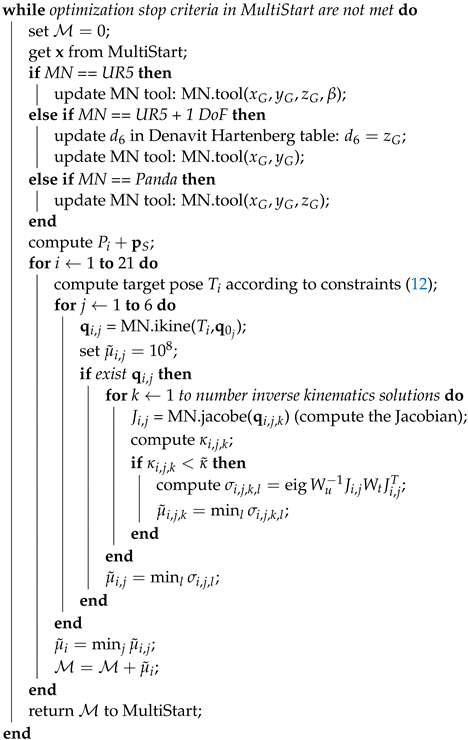 |
5. Results
Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Telemedicine: Opportunities and Developments in Member States: Report on the Second Global Survey on eHealth; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Valdastri, P.; Simi, M.; Webster, R.J., III. Advanced technologies for gastrointestinal scopy. Annu. Rev. Biomed. Eng. 2012, 14, 397–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbeille, P.; Capri, A.; Ayoub, J.; Kieffer, V.; Georgescu, M.; Poisson, G. Use of a robotic arm to perform remote abdominal telesonography. Am. J. Roentgenol. 2007, 188, W317–W322. [Google Scholar] [CrossRef] [PubMed]
- Filippeschi, A.; Brizzi, F.; Ruffaldi, E.; Jacinto Villegas, J.M.; Landolfi, L.; Avizzano, C.A. Evaluation of diagnostician user interface aspects in a virtual reality-based tele-ultrasonography simulation. Adv. Robot. 2019, 33, 840–852. [Google Scholar] [CrossRef]
- Endo, T.; Kawasaki, H.; Mouri, T.; Doi, Y.; Yoshida, T.; Ishigure, Y.; Koketsu, K. Five-fingered haptic interface robot: HIRO III. In Proceedings of the World Haptics 2009—Third Joint EuroHaptics Conference and Symposium on Haptic Interfaces for Virtual Environment and Teleoperator Systems, Salt Lake City, UT, USA, 18–20 March 2009; pp. 458–463. [Google Scholar]
- Coles, T.R.; John, N.W.; Gould, D.; Caldwell, D.G. Integrating haptics with augmented reality in a femoral palpation and needle insertion training simulation. IEEE Trans. Haptics 2011, 4, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Filippeschi, A.; Brizzi, F.; Ruffaldi, E.; Jacinto, J.M.; Avizzano, C.A. Encountered-type haptic interface for virtual interaction with real objects based on implicit surface haptic rendering for remote palpation. In Proceedings of the 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Hamburg, Germany, 28 September–2 October 2015; pp. 5904–5909. [Google Scholar]
- Arent, K.; Cholewinski, M.; Domski, W.; Drwiega, M.; Jakubiak, J.; Janiak, M.; Szczesniak-Stanczyk, D. Selected Topics in Design and Application of a Robot for Remote Medical Examination with Use of Ultrasonography and Auscultation from the Perspective of the ReMeDi Project. J. Autom. Mob. Robot. Intell. Syst. 2017, 11, 82–94. [Google Scholar]
- Griffa, P.; Filippeschi, A.; Avizzano, C.A. Kinematic Optimization for the Design of a UR5 Robot End-Effector for Cardiac Tele-Ultrasonography. In The International Conference of IFToMM ITALY; Springer: Berlin/Heidelberg, Germany, 2020; pp. 423–430. [Google Scholar]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.J.; Burbridge, B.; Obaid, H.; Stoneham, G.; Babyn, P.; Mendez, I. Telerobotic Sonography for Remote Diagnostic Imaging: Narrative Review of Current Developments and Clinical Applications. J. Ultrasound Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Salcudean, S.; Bell, G.; Bachmann, S.; Zhu, W.H.; Abolmaesumi, P.; Lawrence, P.D. Robot-assisted diagnostic ultrasound–design and feasibility experiments. In International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1062–1071. [Google Scholar]
- Abolmaesumi, P.; Salcudean, S.E.; Zhu, W.H.; Sirouspour, M.R.; DiMaio, S.P. Image-guided control of a robot for medical ultrasound. IEEE Trans. Robot. Autom. 2002, 18, 11–23. [Google Scholar] [CrossRef]
- Delgorge, C.; Courreges, F.; Bassit, L.A.; Novales, C.; Rosenberger, C.; Smith-Guerin, N.; Bru, C.; Gilabert, R.; Vannoni, M.; Poisson, G.; et al. A tele-operated mobile ultrasound scanner using a light-weight robot. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Vieyres, P.; Poisson, G.; Courrèges, F.; Mérigeaux, O.; Arbeille, P. The TERESA project: From space research to ground tele-echography. Ind. Robot. Int. J. 2003, 30, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Nouaille, L.; Smith-Guérin, N.; Poisson, G.; Arbeille, P. Optimization of a 4 dof tele-echography robot. In Proceedings of the 2010 IEEE/RSJ International Conference on Intelligent Robots and Systems, Taipei, Taiwan, 18–22 October 2010; pp. 3501–3506. [Google Scholar]
- Avgousti, S.; Panayides, A.S.; Christoforou, E.G.; Argyrou, A.; Jossif, A.; Masouras, P.; Novales, C.; Vieyres, P. Medical telerobotics and the remote ultrasonography paradigm over 4g wireless networks. In Proceedings of the 2018 IEEE 20th International Conference on e-Health Networking, Applications and Services (Healthcom), Ostrava, Czech Republic, 17–20 September 2018; pp. 1–6. [Google Scholar]
- Ito, K.; Sugano, S.; Iwata, H. Portable and attachable tele-echography robot system: FASTele. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 487–490. [Google Scholar]
- Vilchis, A.; Troccaz, J.; Cinquin, P.; Masuda, K.; Pellissier, F. A new robot architecture for tele-echography. IEEE Trans. Robot. Autom. 2003, 19, 922–926. [Google Scholar] [CrossRef]
- Chatelain, P.; Krupa, A.; Navab, N. Optimization of ultrasound image quality via visual servoing. In Proceedings of the 2015 IEEE International Conference on Robotics and Automation (ICRA), Seattle, WA, USA, 26–30 May 2015; pp. 5997–6002. [Google Scholar]
- Mathur, B.; Topiwala, A.; Schaffer, S.; Kam, M.; Saeidi, H.; Fleiter, T.; Krieger, A. A semi-autonomous robotic system for remote trauma assessment. In Proceedings of the 2019 IEEE 19th International Conference on Bioinformatics and Bioengineering (BIBE), Athens, Greece, 28–30 October 2019; pp. 649–656. [Google Scholar]
- Mathiassen, K.; Fjellin, J.E.; Glette, K.; Hol, P.K.; Elle, O.J. An ultrasound robotic system using the commercial robot UR5. Front. Robot. AI 2016, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Fang, T.Y.; Zhang, H.K.; Finocchi, R.; Taylor, R.H.; Boctor, E.M. Force-assisted ultrasound imaging system through dual force sensing and admittance robot control. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Finocchi, R.; Aalamifar, F.; Fang, T.Y.; Taylor, R.H.; Boctor, E.M. Co-robotic ultrasound imaging: A cooperative force control approach. In Proceedings of the Medical Imaging 2017: Image-Guided Procedures, Robotic Interventions, and Modeling, Orlando, FL, USA, 11–16 February 2017. [Google Scholar]
- Geng, C.; Xie, Q.; Chen, L.; Li, A.; Qin, B. Study and Analysis of a Remote Robot-assisted Ultrasound Imaging System. In Proceedings of the 2020 IEEE 4th Information Technology, Networking, Electronic and Automation Control Conference (ITNEC), Chongqing, China, 12–14 June 2020; Volume 1, pp. 389–393. [Google Scholar]
- Sandoval, J.; Laribi, M.A.; Zeghloul, S.; Arsicault, M.; Guilhem, J.M. Cobot with Prismatic Compliant Joint Intended for Doppler Sonography. Robotics 2020, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, J.T.; Rafatzand, K.; Zhang, H.K. Feasibility of robot-assisted ultrasound imaging with force feedback for assessment of thyroid diseases. In Proceedings of the Medical Imaging 2020: Image-Guided Procedures, Robotic Interventions, and Modeling, Houston, TX, USA, 15–20 February 2020. [Google Scholar]
- Kebria, P.M.; Al-Wais, S.; Abdi, H.; Nahavandi, S. Kinematic and dynamic modelling of UR5 manipulator. In Proceedings of the 2016 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Budapest, Hungary, 9–12 October 2016; pp. 004229–004234. [Google Scholar]
- Patel, S.; Sobh, T. Manipulator performance measures-a comprehensive literature survey. J. Intell. Robot. Syst. 2015, 77, 547–570. [Google Scholar] [CrossRef] [Green Version]
- Kelaiaia, R.; Company, O.; Zaatri, A. Multiobjective optimization of a linear Delta parallel robot. Mech. Mach. Theory 2012, 50, 159–178. [Google Scholar] [CrossRef]
- Bicchi, A.; Melchiorri, C.; Balluchi, D. On the mobility and manipulability of general multiple limb robots. IEEE Trans. Robot. Autom. 1995, 11, 215–228. [Google Scholar] [CrossRef]
- Bicchi, A.; Prattichizzo, D. Manipulability of cooperating robots with passive joints. In Proceedings of the 1998 IEEE International Conference on Robotics and Automation (Cat. No. 98CH36146), Leuven, Belgium, 20–20 May 1998; Volume 2, pp. 1038–1044. [Google Scholar]
- Chiu, S.L. Kinematic characterization of manipulators: An approach to defining optimality. In Proceedings of the 1988 IEEE International Conference on Robotics and Automation, Leuven, Belgium, 20–20 May 1988; pp. 828–833. [Google Scholar]
- Nakamura, Y.; Hanafusa, H. Inverse Kinematic Solutions With Singularity Robustness for Robot Manipulator Control. J. Dyn. Syst. Meas. Control. 1986, 108, 163–171. [Google Scholar] [CrossRef]
- Corke, P. Robotics, Vision and Control: Fundamental Algorithms in MATLAB® Second, Completely Revised; Springer: Berlin/Heidelberg, Germany, 2017; Volume 118. [Google Scholar]
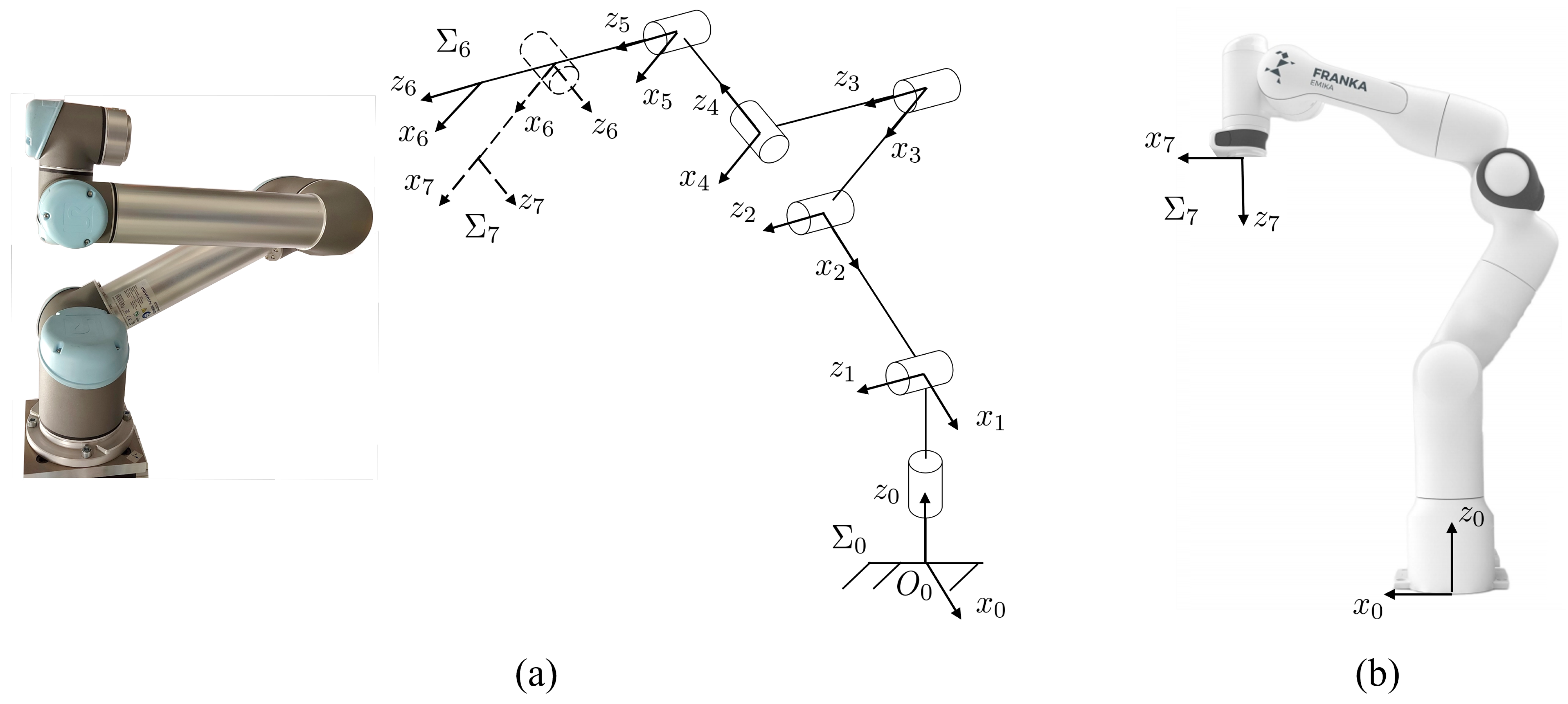


| UR5 | ||||
|---|---|---|---|---|
| link | ||||
| 1 | 0 | 0.0895 | ||
| 2 | −0.4250 | 0 | 0 | |
| 3 | −0.3922 | 0 | 0 | |
| 4 | 0 | 0.1091 | ||
| 5 | 0 | 0.0946 | ||
| 6 * | 0 | 0 | 0.0823 | |
| 6 ** | 0 | |||
| 7 | 0 | 0 | 0 | |
| Panda | ||||
| link | ||||
| 1 | 0 | 0 | 0.333 | |
| 2 | 0 | 0 | ||
| 3 | 0 | 0.316 | ||
| 4 | 0.0825 | 0 | ||
| 5 | −0.0825 | 0.384 | ||
| 6 | 0 | 0 | ||
| 7 | 0.088 | 0.107 | ||
| Robot | [m] | [m] | [m] | [m] | [m] | [m] | [deg] | [] | |
|---|---|---|---|---|---|---|---|---|---|
| UR5 | 0.55 | −0.23 | 0.11 | 0.017 | −0.019 | 0.161 | −65.9 | 5.56 | 8.54 |
| UR5 + 1 | 0.73 | −0.33 | 0.20 | 0.037 | 0.005 | 0.123 | - | 1.97 | 3.23 |
| Panda | 0.39 | −0.17 | 0.31 | 0.023 | 0.071 | 0.146 | - | 5.28 | 14.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippeschi, A.; Griffa, P.; Avizzano, C.A. Kinematic Optimization for the Design of a Collaborative Robot End-Effector for Tele-Echography. Robotics 2021, 10, 8. https://doi.org/10.3390/robotics10010008
Filippeschi A, Griffa P, Avizzano CA. Kinematic Optimization for the Design of a Collaborative Robot End-Effector for Tele-Echography. Robotics. 2021; 10(1):8. https://doi.org/10.3390/robotics10010008
Chicago/Turabian StyleFilippeschi, Alessandro, Pietro Griffa, and Carlo Alberto Avizzano. 2021. "Kinematic Optimization for the Design of a Collaborative Robot End-Effector for Tele-Echography" Robotics 10, no. 1: 8. https://doi.org/10.3390/robotics10010008
APA StyleFilippeschi, A., Griffa, P., & Avizzano, C. A. (2021). Kinematic Optimization for the Design of a Collaborative Robot End-Effector for Tele-Echography. Robotics, 10(1), 8. https://doi.org/10.3390/robotics10010008






