3.1. Qualitative Observations on the Post-Amputation Behavioral
In the first group, after SC transection, the hind limbs of the 25 animals remained rigid and extended backward for one to two days. After this period the limb became flaccid (forceless) but remained extended backward, and could not move up to 11–17 days. Eight lizards died between seven and 20 days post-amputation, probably in relation to the surgical intervention, but 17 animals survived until sacrifice for the histological analysis of the spinal cord. After more than 17 days post-operation the lizards were capable of righting themselves when positioning lying on their back. The latter ability, to turn the body from an inverted position to the normal position, was very difficult or unfeasible at 2–10 days post-lesion. Besides, at 29, 36 and 45 days post-injury, the stepping movements noted in lizards could be induced by stimulating an escaping reaction after tapping on the cage wall or trying to grab the lizards. Apparently, there was no difference in recovery between males and females.
At 29 days post-amputation six animals were sacrificed for the microscopic study: all the animals were able to move their hind limbs, making flexion and extensions when touched. However two of these lizards also used the hind limbs to help actively the stepping movement (good recovery) while the remaining four in part dragged at least one of their hind limbs (modest recovery). Another six animals were sacrificed at 36 days post-amputation for the histological examination of the injured SC. Also in these cases, all the animals showed irregular movements of their legs but in four cases the body movement (stepping) was not linear and some drag of at least one limb was noted (modest recovery) while two individuals moved the legs rapidly helping the forward movement of the body along a horizontal plane (good recovery). At 45 days post-injury, the last five surviving animals were also sacrificed for the histological study. Three animals showed a good recovery but other two cases still dragged at least one limb during the stepping movements (modest recovery). Despite the irregular movements all these lizards at 29, 36 and 45 days post-lesion moved faster in the cage then during the first 17 days post-operation. The lizards from 29, 36 and 45 days post-operation were, however, unable to lift up on their legs when they stood on the vertical wall of the cage, indicating poor muscle strength in their hind limbs after these periods.
In the second group (six lizards) after seven days, the rigid paralysis was evident and the animal drag the stiff hind limbs that remained extended in a posterior direction (
Figure 2A). After 15 days from the lesion no lizard had recovered hind limb movements and two lizards had died, probably for unknown post-operative complications. The four surviving lizards at 36 days post-operation showed a variable degree of hind limb recovery (
Figure 2B). One female and two males showed some recovery of hind limbs folding (flexion and extension) but their movement was irregular and often they dragged at least one limb (modest recovery). The remaining male could make a flexion movement in the two hind limbs, and this helped the animal to move fast along a horizontal plane (good recovery). No convincing vertical movement, capable of lifting these lizards up on the vertical wall of the cage using the hind limbs, was observed in these lizards.
3.2. Cases Utilized for the Histological Observations (1st Group)
The examination of longitudinal sections of the bridge spinal cord at 29, 36 and 45 days post-transection showed similar aspects (
Figure 3). In a case that showed some functional (modest) recovery after 29 days, the neural arc and the vertebral body in the injured vertebrae showed broad areas occupied by cartilage or by a cellular (immature) bone, all signs that indicated a high level of connective, cartilaginous and even bone tissue restoration in lumbar vertebrae (
Figure 3A).
Figure 2.
Examples of two lizards after one week (A) and five weeks (B) post-transection (asterisks indicate the point of transection). In A note the stiff hind limbs directed caudally (arrowheads). Note in B the folded hind limbs (arrowheads) showing a walking movement.
Figure 2.
Examples of two lizards after one week (A) and five weeks (B) post-transection (asterisks indicate the point of transection). In A note the stiff hind limbs directed caudally (arrowheads). Note in B the folded hind limbs (arrowheads) showing a walking movement.
The bridge tissues located between the larger proximal and distal SC stumps contained numerous cells and fibrous bundles that joined the two stumps (
Figure 3A,B). Some of the bundles resembled nerve fascicles containing glial cells, and these bundles were stained dark after the osmium solution utilized to fix the tissue. The fibrous bundles stood out a paler and irregular dense connective tissue, made of oriented or more irregular fibrocytes, and resembling a scar connective tissue (
Figure 3B,C). This connective tissue appeared to occupy most of the space in the neural canal located peripherally to the bridge tissue in which the ependymal canal formed two expanded terminal ampullae, one belonging to the proximal and the other to the distal stump of the spinal cord (
Figure 3C). The two ependymal ampullae were surrounded or in continuity with some of the fibrous nerve bundles of the stumps.
In another case at 36 days post-lesion with some hind limb movements (modest recovery), terminal ependymal ampullae were also seen in the distal (
Figure 4A,B) and in the proximal (
Figure 4C–E) spinal cord stumps. However, the injured vertebrae were largely dislocated while large part of the vertebral canal appeared obstructed from the proliferation of cartilaginous tissue (
Figure 4C). The partial obstruction of the spinal cord canal was therefore reduced from the broad cartilage regeneration that had occurred to repair part of the injured vertebral bones following SC transection. Few and small threads of nervous aspect were, however, seen crossing the bridge tissue. Despite of this mechanical trap, many neurons in both distal and proximal stumps were present, although their cytoplasm showed clumped Nissl substance, indicative of cell damage (
Figure 4B,E). Numerous vacuolated spaces in the white matter indicated a massive axonal degeneration in both stumps of the transected SC.
Figure 3.
Histological longitudinal section showing the restored spinal cord at 29 days post-lesion. A, general view of the connective tissue and of the nervous bridge located between the two stumps of the spinal cord. Bony fragments of the neural arch (arrowheads) that was cut by the surgical intervention to reach the spinal cord. Arrows point to nervous bundles crossing the bridge. Scale bar, 0.2 mm. B, detail on the glial-connective tissues present in the gap region with sparse bundles of nervous fibers (arrows). Scale bar, 0.1 mm. C, other detail of the lesion spinal cords showing the proximal and distal ependymal dilatations (ampullae) separated by the glia-connective tissues filling the gap (arrows indicate fibrous/nerve fibers; double arrows indicate fibers in continuity with the two ependymal ampullae). Scale bar, 0.1 mm. Legends: bm, bone marrow; de, distal ependymal ampulla; ds, distal spinal cord stump (downstream of the lesion); iv, inter vertebral cartilage; pe, proximal ependymal ampulla (enlarged); ps, proximal spinal cord stump (rostral to the lesion); rc, regenerated cartilage (vertebral repair); rs, regenerated/repaired spinal cord; v, vertebral body.
Figure 3.
Histological longitudinal section showing the restored spinal cord at 29 days post-lesion. A, general view of the connective tissue and of the nervous bridge located between the two stumps of the spinal cord. Bony fragments of the neural arch (arrowheads) that was cut by the surgical intervention to reach the spinal cord. Arrows point to nervous bundles crossing the bridge. Scale bar, 0.2 mm. B, detail on the glial-connective tissues present in the gap region with sparse bundles of nervous fibers (arrows). Scale bar, 0.1 mm. C, other detail of the lesion spinal cords showing the proximal and distal ependymal dilatations (ampullae) separated by the glia-connective tissues filling the gap (arrows indicate fibrous/nerve fibers; double arrows indicate fibers in continuity with the two ependymal ampullae). Scale bar, 0.1 mm. Legends: bm, bone marrow; de, distal ependymal ampulla; ds, distal spinal cord stump (downstream of the lesion); iv, inter vertebral cartilage; pe, proximal ependymal ampulla (enlarged); ps, proximal spinal cord stump (rostral to the lesion); rc, regenerated cartilage (vertebral repair); rs, regenerated/repaired spinal cord; v, vertebral body.
![Jdb 02 00210 g003]()
Figure 4.
Detail of longitudinal sections from an animal with little recovery at 36 days post-lesion. A, distal stump showing the enlargement of the ependymal canal and numerous apparently normal neurons (arrows). Arrowheads indicate vacuolated areas resulting from neuropile/axonal degeneration. Double arrowheads point to the beginning of the bridge tissue. Scale bar, 30 μm. B, cytological detail of interneurons (arrow) and larger nerve cell bodies (arrowhead) containing granulated Nissl material, located in the distal stump of the spinal cord. Scale bar, 10 μm. C, gap region between proximal and distal spinal cord stumps showing that a dislocated vertebra (through the surgical procedure) has largely obliterated the neural canal together the regenerated cartilage. The arrow indicates the neural arch bone. The arrowhead indicates the bone of the vertebral body. Scale bar, 0.1 mm. D, enlargement of the ependymal ampulla formed in the proximal spinal cord stump. Note the numerous vacuolated degenerating axons (arrowheads). Scale bar, 20 μm. E, detail of the proximal stump showing large ventral neurons (arrowhead) and sparse smaller neurons (arrow) containing coarse Nissl bodies, a typical sign of neuron reactivity to axotomy. Scale bar, 10 μm. Legends: bm, bone marrow; dv, displaced vertebra; ds, distal stump of the spinal cord; e, ependyma; mu, muscles; na, neural arch; ps, proximal stump of the spinal cord; rc, regenerated cartilage.
Figure 4.
Detail of longitudinal sections from an animal with little recovery at 36 days post-lesion. A, distal stump showing the enlargement of the ependymal canal and numerous apparently normal neurons (arrows). Arrowheads indicate vacuolated areas resulting from neuropile/axonal degeneration. Double arrowheads point to the beginning of the bridge tissue. Scale bar, 30 μm. B, cytological detail of interneurons (arrow) and larger nerve cell bodies (arrowhead) containing granulated Nissl material, located in the distal stump of the spinal cord. Scale bar, 10 μm. C, gap region between proximal and distal spinal cord stumps showing that a dislocated vertebra (through the surgical procedure) has largely obliterated the neural canal together the regenerated cartilage. The arrow indicates the neural arch bone. The arrowhead indicates the bone of the vertebral body. Scale bar, 0.1 mm. D, enlargement of the ependymal ampulla formed in the proximal spinal cord stump. Note the numerous vacuolated degenerating axons (arrowheads). Scale bar, 20 μm. E, detail of the proximal stump showing large ventral neurons (arrowhead) and sparse smaller neurons (arrow) containing coarse Nissl bodies, a typical sign of neuron reactivity to axotomy. Scale bar, 10 μm. Legends: bm, bone marrow; dv, displaced vertebra; ds, distal stump of the spinal cord; e, ependyma; mu, muscles; na, neural arch; ps, proximal stump of the spinal cord; rc, regenerated cartilage.
![Jdb 02 00210 g004]()
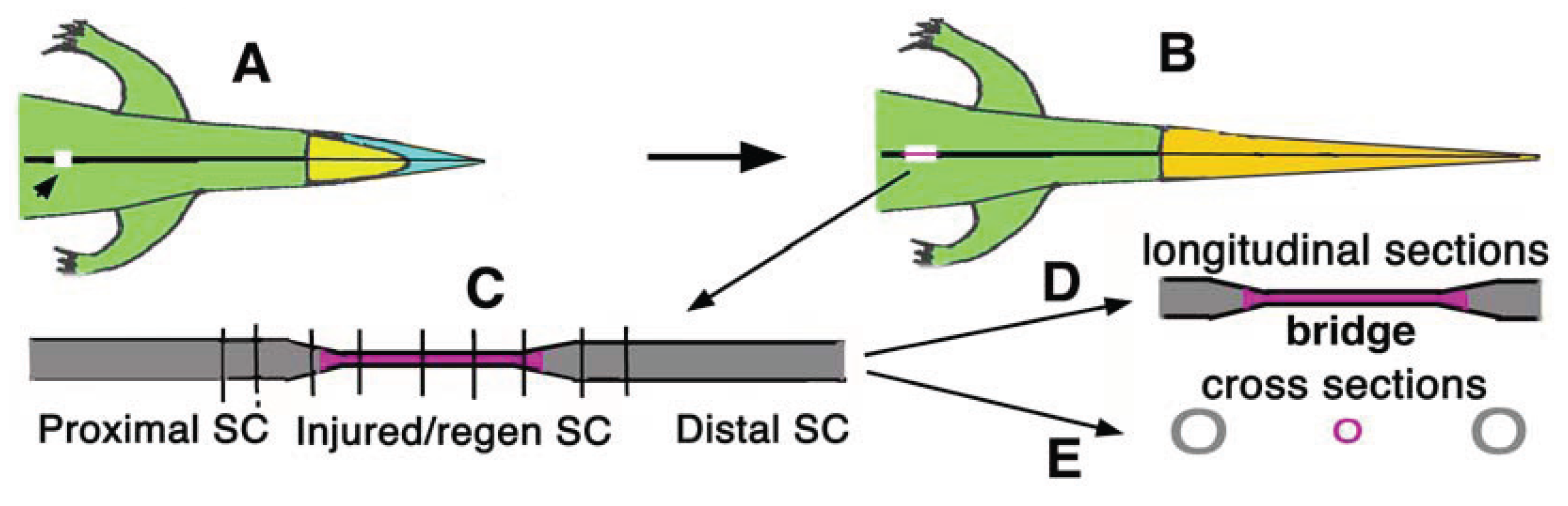
Similar results were observed from the study of sequential cross-sections from the proximal spinal cord stump and across the bridge to reach the distal spinal cord stump. The observations of cross sections produced better information on the degree of white matter degeneration, in terms of distribution of degenerated (vacuolated) fibers in the entire section of the SC. In a case with good recovery at 29 days post-injury, the proximal stump located at about 1–2 mm from the lesion, showed that most axons appeared still myelinated (
Figure 5A). Approaching the bridge tissue, below 1 mm from the lesion area, broad areas of degenerating axons became evident, especially in the ventral and dorsal tracts of the white matter (
Figure 5B,C). Also the central (ependymal) canal became enlarged and the nuclei of ependymal cells were more evident. Although many neurons were still present in the proximal SC stump, the outline of the grey matter became more and more indistinct and irregular in more distal sections approaching the bridge region (
Figure 5C) while the reduced white matter contained numerous small and large degenerating nervous fibers or areas containing degenerated axons (
Figure 5D). The central canal appeared four- to six-folds larger then in the proximal sections more distant from the bridge, with a lumen larger than 40 μm and a partly stratified and hypertrophic ependyma (
Figure 5E). Among elongated ependymal cells, few roundish and basophilic cells (arrowhead in
Figure 5E) or pale cells with a large nucleolus (arrow in
Figure 5E) were seen. Numerous small (glial) cells were seen in the white matter, among the degenerated axons.
In the more central, thinner or irregular part of the bridge spinal cord (about 200 μm thick), neither neurons nor ependyma were seen but instead numerous pale areas, representing axonal and neuropilar tissues, were present among glial cells and degenerating axonal spaces (
Figure 5F,G). This bridge tissue consisted of irregular glial-fibrous connective, resembling scarring connective for the abundance of glial or fibrocyte cells. Progressing distally, beyond the level of transection, the distal spinal cord stump regained, eventually, a similar diameter as in the proximal spinal cord, but few and smaller motorneurons were seen in the ventral grey horns, suggesting that most of these cells had degenerated after transection (
Figure 5H).
In the five cases sacrificed 45 days post-operation, histological features similar to those described at 29 and 36 days post-transection were observed. In an animal with a good recovery, numerous myelinated axons disappeared while they were replaced by vacuolated spaces (
Figure 6A,B). Besides, the presence of stained clumps in the cytoplasm (chromatolysis) in many neurons of the grey matter indicated cell damage. The enlargement of the central canal was also noted in the proximal spinal cord approaching the bridge, while the grey matter became less clearly defined and vacuolated axons appeared quite numerous in the ventral, lateral and dorsal columns of the white matter (
Figure 6C). In cross sections of the proximal bridge area, the anatomical structure of the spinal cord was lost, the ependymal canal was much enlarged while few neurons were seen in the irregular grey matter and numerous small (glial) cells were present in the white matter (
Figure 6D). The bridge was often surrounded by a scarring connective that occupied a large part of the vertebral canal and that internally contacted the nervous tissue and externally the dura meninges or the periosteum of the vertebrae (
Figure 6E). Progressing distally toward the medial region of the bridge, although the central canal appeared narrow (
Figure 6E), the ependymal wall was still stratified, featuring denser (basophilic) or paler cells among the elongated ependymal cells (
Figure 6F).
In more distal regions, past the transection level, the collected serial sections progressively intercepted the distal stump of the spinal cord where the nervous tissue regained the normal size and showed the typical organization in a distinct white and grey matter, although the distal stump remained thinner when compared to the proximal stump of the transected spinal cord. The ependymal canal became initially larger again (corresponding to an ependymal dilatation or ampulla) and sparse vacuolated areas were apparent in the surrounding white matter (
Figure 6G). In the distal stump of the spinal cord, however, rare large motorneurons were seen in the ventral grey horn or they were absent. The central canal of the distal SC past the bridge appeared reduced like in the normal, proximal stump of the spinal cord (
Figure 6H).
Figure 5.
Representative proximal-distal cross-sections of lesion spinal cord at 29 days post-injury. A, proximal spinal cord showing the characteristics H-shapes of the grey matter surrounded by numerous myelinated axons in the white matter. Scale bar, 0.1 mm. B, a more caudal section closer to the transected region but still in the proximal SC stump shows numerous vacuolated axons (arrows) in the white matter. The arrowhead indicates the ependyma. Scale bar, 0.1 mm. C, a further caudal section shows the presence of large vacuolated areas (arrows) previously occupied by axons. The arrowhead indicates the ependyma. Scale bar, 0.1 mm. D, section of the reduced SC in the bridge region with the enlarged ependymal canal (arrowhead) while the distinction between grey (very reduced) and white matter appears irregular. Numerous degenerated axons are seen (arrows) and the SC is occupied by numerous glial cells. Scale bar, 0.1 mm. E, detail of the stratified ependymal canal with indicated pale cells (arrows) among ependymal cells. Another cells with basophilic cytoplasm is seen nearby the ependyma (arrowhead). Scale bar, 15 μm. F, section across the bridge region between the two SC stumps formed by a scarring glial tissue (arrow), sparse nerve bundles and degenerating axons (arrowheads). Scale bar, 0.1 mm. G, more caudal part of the bridge showing the presence of bundles of nerves (arrows) within the surrounding dense connective (scar). Scale bar, 0.1 mm. H, distal spinal cord with the typical H-shaped grey matter surrounded by numerous degenerated axons in the white matter (arrows). The ependymal camnal (arrowhead) appear reduced as in the proximal stump. Scale bar, 0.1 mm. Legends: bv, blood vessel; e, ependyma; gm, grey matter; mx, meninx; n, neuron; na, neural arch; rc, regenerated cartilage; sc, spinal cord; sco, scarring connective-meninx; sn, small neuron; v, vertebra; vc, vertebral canal; wm, white matter.
Figure 5.
Representative proximal-distal cross-sections of lesion spinal cord at 29 days post-injury. A, proximal spinal cord showing the characteristics H-shapes of the grey matter surrounded by numerous myelinated axons in the white matter. Scale bar, 0.1 mm. B, a more caudal section closer to the transected region but still in the proximal SC stump shows numerous vacuolated axons (arrows) in the white matter. The arrowhead indicates the ependyma. Scale bar, 0.1 mm. C, a further caudal section shows the presence of large vacuolated areas (arrows) previously occupied by axons. The arrowhead indicates the ependyma. Scale bar, 0.1 mm. D, section of the reduced SC in the bridge region with the enlarged ependymal canal (arrowhead) while the distinction between grey (very reduced) and white matter appears irregular. Numerous degenerated axons are seen (arrows) and the SC is occupied by numerous glial cells. Scale bar, 0.1 mm. E, detail of the stratified ependymal canal with indicated pale cells (arrows) among ependymal cells. Another cells with basophilic cytoplasm is seen nearby the ependyma (arrowhead). Scale bar, 15 μm. F, section across the bridge region between the two SC stumps formed by a scarring glial tissue (arrow), sparse nerve bundles and degenerating axons (arrowheads). Scale bar, 0.1 mm. G, more caudal part of the bridge showing the presence of bundles of nerves (arrows) within the surrounding dense connective (scar). Scale bar, 0.1 mm. H, distal spinal cord with the typical H-shaped grey matter surrounded by numerous degenerated axons in the white matter (arrows). The ependymal camnal (arrowhead) appear reduced as in the proximal stump. Scale bar, 0.1 mm. Legends: bv, blood vessel; e, ependyma; gm, grey matter; mx, meninx; n, neuron; na, neural arch; rc, regenerated cartilage; sc, spinal cord; sco, scarring connective-meninx; sn, small neuron; v, vertebra; vc, vertebral canal; wm, white matter.
![Jdb 02 00210 g005a]()
![Jdb 02 00210 g005b]()
3.3. Cases Utilized for the Dil Tract-Tracing Study (2nd Group)
After 15 days from the lesion, no lizard had recovered hind limb movements and two lizards died; possibly as the result of the surgical intervention. The four survival lizards at twenty-six days showed a variable degree of recovery of hind limb movement. One female (case #1) and two males (cases #3 and #4) showed some recovery (modest recovery) of hind limb folding (flexion and extension), but one male (case #2) could make a flexion movement in both hind limbs that helped the stepping movements (good recovery). The animals were all sacrificed at the 36th days post-lesion, and the operated area was removed and fixed as previously indicated for the application of the Dil fluorescent tracer.
The observations of the extracted spinal cord in the four cases, before the Dil application, showed that case #1, with a modest recovery of hind limb motility, was discontinuous in the dorsal part of the SC so that about half of the SC was actually continuous between the two stumps. This bridge probably represented regenerated tissue, since the transection was completed. Case #2, also featuring a limited recovery, showed the presence of a thin tissue bridge formed at 36 days post-transection, likely regenerated, present between the two stumps. The other two cases with a relative recovery of the limb mobility (modest and good recovery) showed that the SC was still largely discontinuous between the two stumps, aside the more ventral part of the SC where a thin bridge tissue, likely regenerated, was present.
Figure 6.
Representative proximal distal cross sections of spinal cord 36 days post-lesion. A, proximal spinal cord has the normal H-shape of the grey matter and numerous axons in the white matter are myelinated. Scale bar, 0.1 mm. B, a more caudal section closer to the bridge region showing a reduction in diameter and the presence of numerous degenerated axons (arrows). The arrowheads point to neurons within the grey matter. Scale bar, 20 μm. C, a more caudal section of the proximal stump spinal cord shows a further reduction of the white matter, flattening and vacuolization (arrows) while numerous cells are present in the grey matter (arrowheads). Scale bar, 0.1 mm. D, more caudal section at the level of the ependymal ampulla that appears surrounded by few neurons and nerve fibers of the white matter. Some vacuoles (arrow) indicate degenerated areas. Scale bar, 0.1 mm. E, further caudal section in the bridge region showing a narrow spinal cord with a reduced ependymal canal (arrowhead) while the irregular white matter contains numerous glial cells and fewer neurons. Abundant scar tissue is seen between the spinal cord and the vertebral bone, probably derived from the surgical incision made to reach the spinal cord to perform the transection. Scale bar, 0.1 mm. F, close-up on the stratified ependymal epithelium present in the bridge region. Around the reduced central canal numerous stratified cells from the ependyma are seen, including dark round cells (arrowheads), pale cells (double arrowheads) representing neural or glial cells. Scale bar, 25 μm. G, caudal section of the spinal cord stump after the bridge, showing the ependymal canal still enlarged (arrowhead) surrounded by a larger number of neurons and white matter not yet distinct into grey and white matter. Arrows indicate the larger degenerated axons. Scale bar, 0.1 mm. H, caudal most level of the distal SC stump that shows increased diameter. Arrowheads indicate the grey matter with numerous neurons, but small or large (arrow) vacuolated areas are present. Scale bar, 0.1 mm. Legends: c, central canal; e, ependyma; gm, grey matter; mx, meninx; sc, spinal cord; sco, scarring connective-meninge; v, vertebral bone; vc, vertebral canal; wm, white matter.
Figure 6.
Representative proximal distal cross sections of spinal cord 36 days post-lesion. A, proximal spinal cord has the normal H-shape of the grey matter and numerous axons in the white matter are myelinated. Scale bar, 0.1 mm. B, a more caudal section closer to the bridge region showing a reduction in diameter and the presence of numerous degenerated axons (arrows). The arrowheads point to neurons within the grey matter. Scale bar, 20 μm. C, a more caudal section of the proximal stump spinal cord shows a further reduction of the white matter, flattening and vacuolization (arrows) while numerous cells are present in the grey matter (arrowheads). Scale bar, 0.1 mm. D, more caudal section at the level of the ependymal ampulla that appears surrounded by few neurons and nerve fibers of the white matter. Some vacuoles (arrow) indicate degenerated areas. Scale bar, 0.1 mm. E, further caudal section in the bridge region showing a narrow spinal cord with a reduced ependymal canal (arrowhead) while the irregular white matter contains numerous glial cells and fewer neurons. Abundant scar tissue is seen between the spinal cord and the vertebral bone, probably derived from the surgical incision made to reach the spinal cord to perform the transection. Scale bar, 0.1 mm. F, close-up on the stratified ependymal epithelium present in the bridge region. Around the reduced central canal numerous stratified cells from the ependyma are seen, including dark round cells (arrowheads), pale cells (double arrowheads) representing neural or glial cells. Scale bar, 25 μm. G, caudal section of the spinal cord stump after the bridge, showing the ependymal canal still enlarged (arrowhead) surrounded by a larger number of neurons and white matter not yet distinct into grey and white matter. Arrows indicate the larger degenerated axons. Scale bar, 0.1 mm. H, caudal most level of the distal SC stump that shows increased diameter. Arrowheads indicate the grey matter with numerous neurons, but small or large (arrow) vacuolated areas are present. Scale bar, 0.1 mm. Legends: c, central canal; e, ependyma; gm, grey matter; mx, meninx; sc, spinal cord; sco, scarring connective-meninge; v, vertebral bone; vc, vertebral canal; wm, white matter.
![Jdb 02 00210 g006a]()
![Jdb 02 00210 g006b]()
The analysis of fluorescent-Dil tracer distribution in case #1 showed that the tracer was mainly applied and adsorbed in the dorsal-lateral right side and in the ventral-medial right side of the distal spinal cord, localized caudally to the bridge tissue (
Figure 7A). This area included mainly white matter and the grey matter of the right dorsal horn. This labeled area became more diffuse moving toward the narrower bridge region where some intensely labeled fibers were seen in the central part of the SC (
Figure 7B–D). After moving 2–3 mm rostral to reach the proximal spinal cord stump, numerous fluorescent fibers were seen, and they were mainly localized in the medial ventral part of the white matter (
Figure 7E). Sparse small or large neurons present in various areas of the grey matter of the proximal spinal cord were also retrograde-labeled with the fluorescent Dil (
Figure 7E–H). No fluorescent labeled, large neurons in the ventral grey were seen.
The microscopic analysis of distal-proximal cross-sections from case # 2 showed a more diffuse Dil-fluorescence over most grey and white matter in the distal spinal cord (the region of application of the tracer), although a much higher fluorescent area was present in the left dorsal white matter (
Figure 8A,B). Moving proximally toward the transected region, the collected cross-sections showed few labeled fibers present in the narrow bridge spinal cord, some especially intense in the dorsal part (
Figure 8C). In more proximally collected sections, sparse fluorescent neurons were observed in the grey matter of the proximal stump of the spinal cord, and also in the proximal spinal cord located at 5–7 mm form the point of application of Dil (
Figure 8D–G). These small to elongated neurons were mainly present in the intermediate grey matter, and no Dil-labeled motor-neurons were seen.
Also the other two cases showed similar features, indicating the presence of Dil-labeled fibers within the bridge. The study on the distribution of the retrograde Dil-labeled neurons in the grey matter and of the axons in the white matter of the proximal spinal cord stump in all four cases is summarized in
Figure 9.
Figure 7.
Representative proximal-distal cross-sections of the spinal cord in case 1 experiment (thin bridge tissues with modest recovery) after Dil application. A, section close to the point of Dil application (arrows on most labeled areas). The arrowhead points the position of the central ependymal canal. gm, grey matter. Scale bar, 0.2 mm B, more rostral section within the caudal SC stump (the arrow shows the region with higher tracer-load). Asterisks indicate missing areas of the SC (artifact derived from the vibratome sectioning). Scale bar, 0.15 mm. C, further rostral region preceding the bridge between proximal and distal SC stumps. The arrow indicates the most labeled area. The asterisk indicates some missing tissue due to sectioning. Scale bar, 0.1 mm. D, narrow diameter of the bridge region showing sparse labeled axons (arrowhead). Scale bar, 0.1 mm. E, rostral spinal cord past the bridge area showing a large labeled region in the white matter (arrow) and some cells (arrowheads) in the grey matter. gm, grey matter. Scale bar, 0.15 mm. F, rostralmost region of the SC with labeling in the white matter (arrow) and inside cell bodies (arrowheads) in the grey matter (gm). Scale bar, 0.15 mm. G, detail of a labeled neuron and its likely axonal elongation. Scale bar, 10 μm. H, other two small neurons present in the intermediate region of the grey matter. Scale bar, 20 μm.
Figure 7.
Representative proximal-distal cross-sections of the spinal cord in case 1 experiment (thin bridge tissues with modest recovery) after Dil application. A, section close to the point of Dil application (arrows on most labeled areas). The arrowhead points the position of the central ependymal canal. gm, grey matter. Scale bar, 0.2 mm B, more rostral section within the caudal SC stump (the arrow shows the region with higher tracer-load). Asterisks indicate missing areas of the SC (artifact derived from the vibratome sectioning). Scale bar, 0.15 mm. C, further rostral region preceding the bridge between proximal and distal SC stumps. The arrow indicates the most labeled area. The asterisk indicates some missing tissue due to sectioning. Scale bar, 0.1 mm. D, narrow diameter of the bridge region showing sparse labeled axons (arrowhead). Scale bar, 0.1 mm. E, rostral spinal cord past the bridge area showing a large labeled region in the white matter (arrow) and some cells (arrowheads) in the grey matter. gm, grey matter. Scale bar, 0.15 mm. F, rostralmost region of the SC with labeling in the white matter (arrow) and inside cell bodies (arrowheads) in the grey matter (gm). Scale bar, 0.15 mm. G, detail of a labeled neuron and its likely axonal elongation. Scale bar, 10 μm. H, other two small neurons present in the intermediate region of the grey matter. Scale bar, 20 μm.
![Jdb 02 00210 g007]()
Figure 8.
Proximal-distal representative cross-sections of the SC in case 2 (completely transected with poor recovery) after Dil application. A, caudalmost area located near the point of application of the tracer (the arrow points to the region that has incorporated most of the tracer). The asterisk indicates that some tissue is missing due to the sectioning. The arrowhead points to the central canal. gm, grey matter. Scale bar, 0.1 mm. B, more rostral section of the SC near the bridge region (the arrow indicates the most labeled area). Asterisks point to regions with missing tissue due to the sectioning. gm, grey matter. Scale bar, 0.1 mm. C, narrow spinal cord in the bridge area. The arrowheads indicate few axons containing higher levels of the tracer. Scale bar, 0.1 mm. D, cross section of the rostral spinal cord stump past the bridge region showing some labeled neurons (arrowheads) in the grey matter (gm). Scale bar, 0.15 mm. E, a rostral most section showing most labeled neurons (arrowheads) in the medial-upper part of the grey matter (gm). Scale bar, 0.15 mm. F, detail on a labeled neuron in the medial ventral grey matter. Scale bar, 10 μm. G, detail of labeled neurons localized in intermediate grey matter area. Scale bar, 10 μm.
Figure 8.
Proximal-distal representative cross-sections of the SC in case 2 (completely transected with poor recovery) after Dil application. A, caudalmost area located near the point of application of the tracer (the arrow points to the region that has incorporated most of the tracer). The asterisk indicates that some tissue is missing due to the sectioning. The arrowhead points to the central canal. gm, grey matter. Scale bar, 0.1 mm. B, more rostral section of the SC near the bridge region (the arrow indicates the most labeled area). Asterisks point to regions with missing tissue due to the sectioning. gm, grey matter. Scale bar, 0.1 mm. C, narrow spinal cord in the bridge area. The arrowheads indicate few axons containing higher levels of the tracer. Scale bar, 0.1 mm. D, cross section of the rostral spinal cord stump past the bridge region showing some labeled neurons (arrowheads) in the grey matter (gm). Scale bar, 0.15 mm. E, a rostral most section showing most labeled neurons (arrowheads) in the medial-upper part of the grey matter (gm). Scale bar, 0.15 mm. F, detail on a labeled neuron in the medial ventral grey matter. Scale bar, 10 μm. G, detail of labeled neurons localized in intermediate grey matter area. Scale bar, 10 μm.
![Jdb 02 00210 g008]()