Abstract
Tartary buckwheat (Fagopyrum tataricum Gaertn.) is a coarse grain crop rich in flavonoids that are beneficial to human health because they function as anti-inflammatories and provide protection against cardiovascular disease and diabetes. Flavonoid biosynthesis is a complex process, and relatively little is known about the regulatory pathways involved in Tartary buckwheat. Here, we cloned and characterized the FtMYB163 gene from Tartary buckwheat, which encodes a member of the R2R3-MYB transcription factor family. Amino acid sequence and phylogenetic analysis indicate that FtMYB163 is a member of subgroup 7 (SG7) and closely related to FeMYBF1, which regulates flavonol synthesis in common buckwheat (F. esculentum). We demonstrated that FtMYB163 localizes to the nucleus and has transcriptional activity. Expression levels of FtMYB163 in the roots, stems, leaves, flowers, and seeds of F. tataricum were positively correlated with the total flavonoid contents of these tissues. Overexpression of FtMYB163 in transgenic Arabidopsis enhanced the expression of several genes involved in early flavonoid biosynthesis (AtCHS, AtCHI, AtF3H, and AtFLS) and significantly increased the accumulation of several flavonoids, including naringenin chalcone, naringenin-7-O-glucoside, eriodictyol, and eight flavonol compounds. Our findings demonstrate that FtMYB163 positively regulates flavonol biosynthesis by changing the expression of several key genes in flavonoid biosynthetic pathways.
1. Introduction
Tartary buckwheat (Fagopyrum tataricum Gaertn), as an annual herb of the Polygonaceae family, is largely distributed in the Sichuan, Yunnan-Guizhou Plateau, and Tibetan regions of China as well as some other high-altitude, arid mountainous areas [1,2]. Tartary buckwheat is rich in flavonoids, including rutin, quercetin, and other flavonols, and is a potentially important crop for medicine and human health [3]. Flavonoids are one of the largest secondary metabolites in plants, and their content is approximately 1.0~3.0% in Tartary buckwheat, most of which resides in the flowers, leaves, and seeds [4,5]. So far, a total of 93 flavonoids have been identified in Tartary buckwheat seeds, and 32 of them are predicted to play important roles in the resistance to major diseases (cancers/tumors, hypertension, and cardiovascular disease) [6]. In addition, 61 flavonoid components have been absolutely quantified in Tartary buckwheat seeds [7].
Flavonoids encompass a wide range of different phenolic compounds, including anthocyanins, flavonols, flavones, and proanthocyanidins. Different flavonoids have different biological functions, and many of these compounds have antioxidant properties that protect plants from biotic and abiotic stresses [8,9]. Flavonoids also play an important role in plant growth and physiology, including cell wall synthesis [10], root growth and phototropism [11,12], and pollen development [13]. Understanding the regulation of flavonoid biosynthesis and metabolism more fully may enable the composition and concentration of flavonoids to be manipulated to enhance the value of Tartary buckwheat as a commercial crop.
Biosynthetic pathways of flavonoids have been studied in certain monocotyledons [14] and eudicotyledons [15,16,17]. These pathways are regulated by a variety of transcription factors (TFs) from the MYB, bHLH, bZIP, WD40, and WRKY families [18,19,20]. Among these TFs, members of the R2R3-MYB family have been shown to regulate the biosynthesis of flavonols and anthocyanins. R2R3-MYB TFs are classified into more than 20 subgroups (SGs) [21,22], which share similar functions [23]. SG5 to SG7 are mostly involved with flavonoid biosynthesis [24,25]. For instance, the TT2 gene in Arabidopsis regulates the biosynthesis of the procyanidin flavonoids after forming a complex with TT8 (bHLH) and TTG1 (WD40) [26].
Several R2R3-MYB TFs have been reported to be involved in the regulation of flavonoid biosynthesis in Tartary buckwheat [27,28,29,30]. FtMYB116 specifically regulates rutin accumulation by directly binding to the flavonoid-3′-hydroxylase (F3′H) promoter region [31], while FtMYB1 and FtMYB2 positively regulate the synthesis of procyanidins and anthocyanins [32]. Most of the TF genes mentioned above belong to SG5 R2R3-MYB. Members of SG7 R2R3-MYB have a greater effect on flavonol biosynthesis by regulating the expression of early structural genes [33,34]. Currently, the only member of SG7 R2R3-MYB in Tartary buckwheat, FtMYB6, has been demonstrated to regulate the biosynthesis of flavonols in Tartary buckwheat hairy roots and transgenic tobacco by upregulating the expression of F3H and FLS genes [35]. Our previous study suggested that another SG7 R2R3-MYB gene named FtMYB163 (FtPinG0009153900.01) was also likely to regulate flavonoid biosynthesis in Tartary buckwheat [34]. However, details of FtMYB163 function remain obscure, and it is important to examine the function of this and other members of the SG7 MYB TFs to better understand the biosynthetic pathways of flavonoid synthesis in Tartary buckwheat.
In this study, we analyzed the tissue-specific expression pattern of FtMYB163 and flavonoid content in Tartary buckwheat. We also over-expressed FtMYB163 in transgenic Arabidopsis and investigated the effect on flavonoid concentrations and the expression of relevant endogenous genes. This study provides valuable information for understanding the function and the potential molecular mechanism of FtMYB163 in flavonoid biosynthesis in Tartary buckwheat.
2. Results
2.1. Cloning and Molecular Characteristics of FtMYB163
The complete open reading frame (ORF) of FtMYB163 was isolated from the Tartary buckwheat variety “Heiku013” by PCR using gene-specific primers. The gene was 1119 bp in length and comprised of three exons and two introns (Figure S1, Table S1). It encoded a 372 amino acid protein with a predicted molecular mass of 42.18 kD and a calculated pI of 6.23 (Table S1).
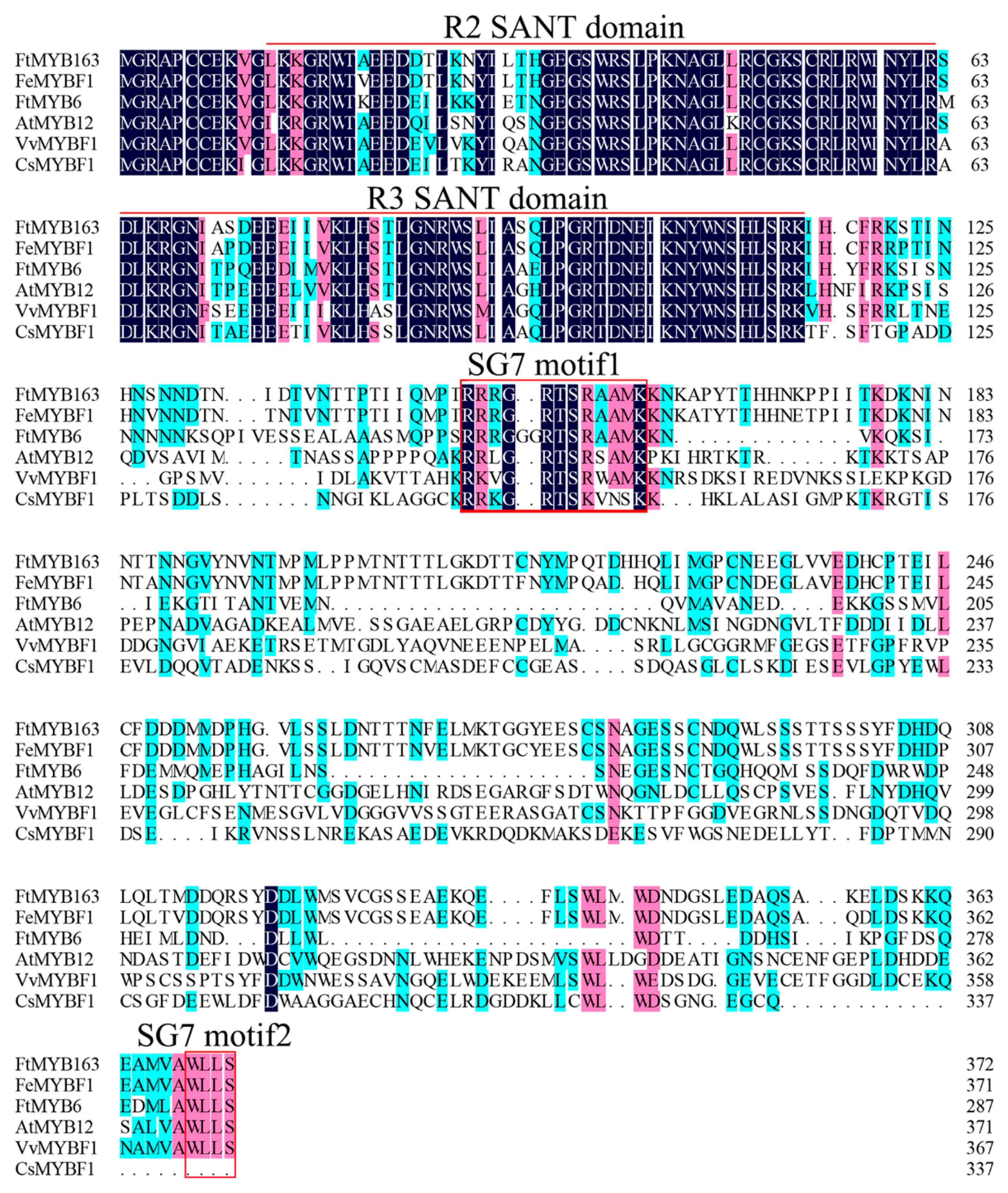
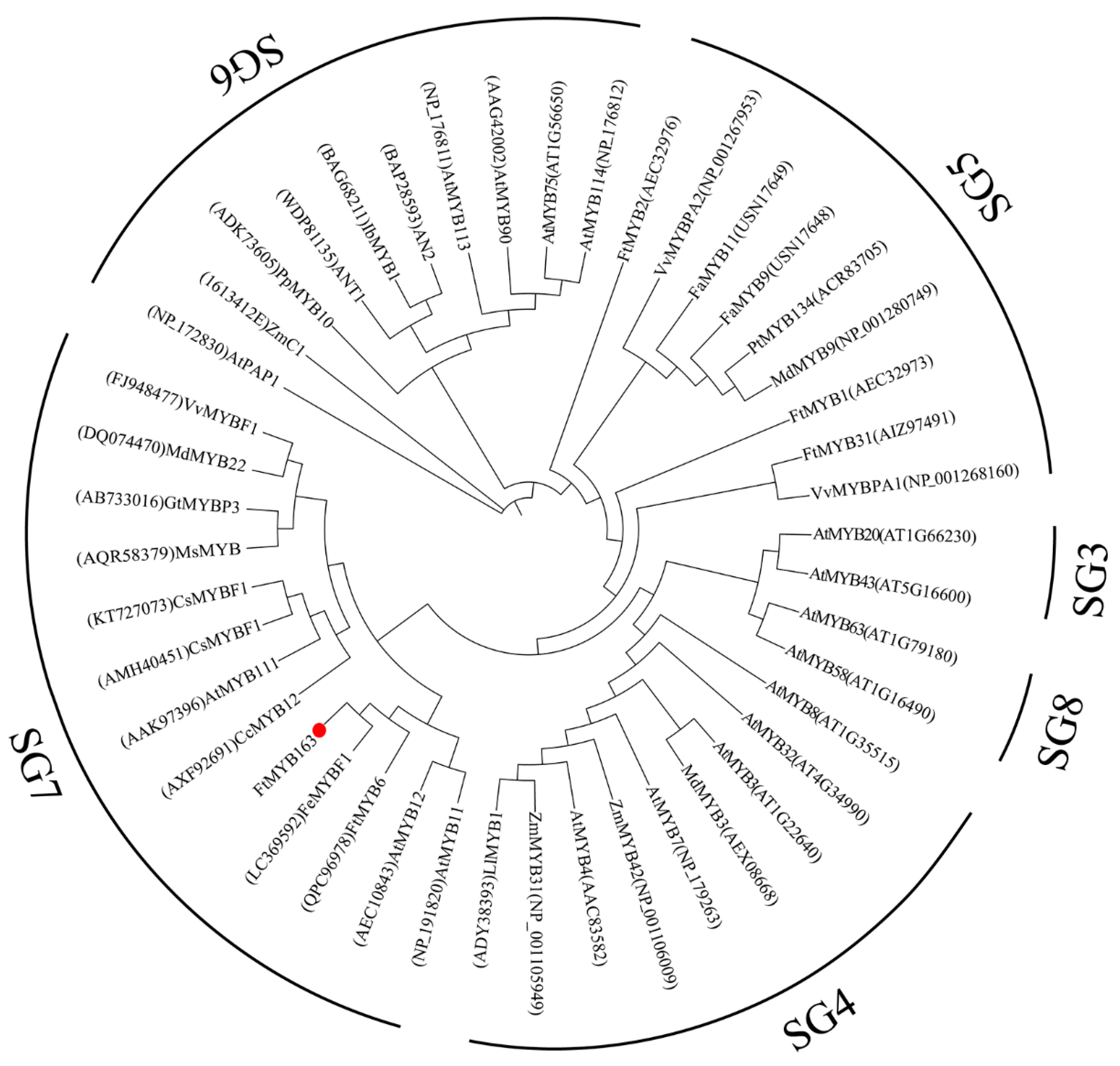
Multiple sequence alignment found that FtMYB163 showed 94.09% amino acid identity with FeMYBF1 (LC369592,) from common buckwheat (F. esculentum); 44.44% with FtMYB6 (QPC96978); and more than 39% identity with AtMYB12 (AEC10843), CsMYBF1 (KT727073), and VvMYBF1 (FJ948477) (Figure 1). The FtMYB163 protein has R2 and R3 SANT domains at the N terminus, which are involved in sequence-specific DNA binding. In addition, the two SG7 motifs were partially conserved in FtMYB163: motif1 [K/R]R/x][R/K]xGRT[S/x][R/G]xx[M/x]K and motif2 [W/x][L/x]LS [35]. Phylogenetic analysis showed that FtMYB163 clustered with FeMYBF1, AtMYB11, AtMYB12, and FtMYB6 (Figure 2), all of which belong to the SG7 subfamily and participate in the regulation of flavonoid metabolism. These results provide the first indication that FtMYB163 is a member of the SG7 R2R3-MYB subgroup and may have a similar function as FtMYB6, regulating flavonol biosynthesis in Tartary buckwheat.

Figure 1.
Multiple sequence alignment of FtMYB163. The transcription factors were FeMYBF1 (LC369592) from common buckwheat, FtMYB6 (QPC96978) from Tartary buckwheat, AtMYB12 (AEC10843) from Arabidopsis, VvMYBF1 (FJ948477) from grape, and CsMYBF1 (KT727073) from citrus. Identical (100%), conservative (75–99%), and blocks (50–74%) of similar amino acid residues are shaded in deep blue, cherry red, and cyan, respectively. The R2/R3 SANT domain and SG7 motif1/2 are indicated in the red line and red box, respectively.
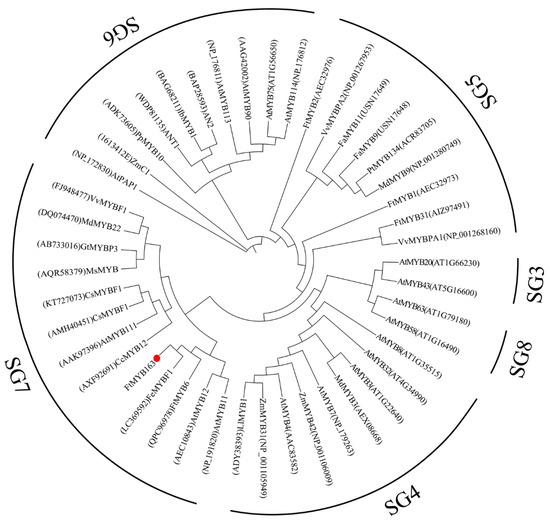
Figure 2.
Phylogenetic analysis of FtMYB163. GeneBack accession numbers are listed as follows: AtMYB58 (NP_173098), AtMYB63 (NP_001321204), LlMYB1 (ADY38393), ZmMYB31 (NP_001105949), ZmMYB42 (NP_001106009), MdMYB3 (AEX08668), AtMYB7 (NP_179263), AtMYB4 (AAC83582), AtMYB8 (NP_849749), AtMYB32 (NP_195225), AtMYB3 (NP_564176), FtMYB2 (AEC32976), FaMYB11 (USN17649), FaMYB9 (USN17648), MdMYB9 (NP_001280749), PtMYB134 (ACR83705), VvMYBPA1 (NP_001268160), VvMYBPA2 (NP_001267953), PpMYB10 (ADK73605), AN2 (BAP28593), ANT1 (WDP81135), IbMYB1 (BAG68211), AtMYB113 (NP_176811), AtMYB114 (NP_176812), GtMYBP3 (AB733016), MdMYB22 (DQ074470), AtMYB111 (AAK97396), AtMYB11 (NP_191820), MsMYB (AQR58379), CcMYB12 (AXF92691), AtMYB90 (AAG42002), AtPAP1 (NP_172830), AtMYB75 (NP_176057), AtMYB43 (NP_197163), AtMYB20 (NP_176797), FtMYB1 (AEC32973), FtMYB31 (AIZ97491). FtMYB163 is highlighted with a red dot.
2.2. Subcellular Localization and Transcriptional Activity of FtMYB163
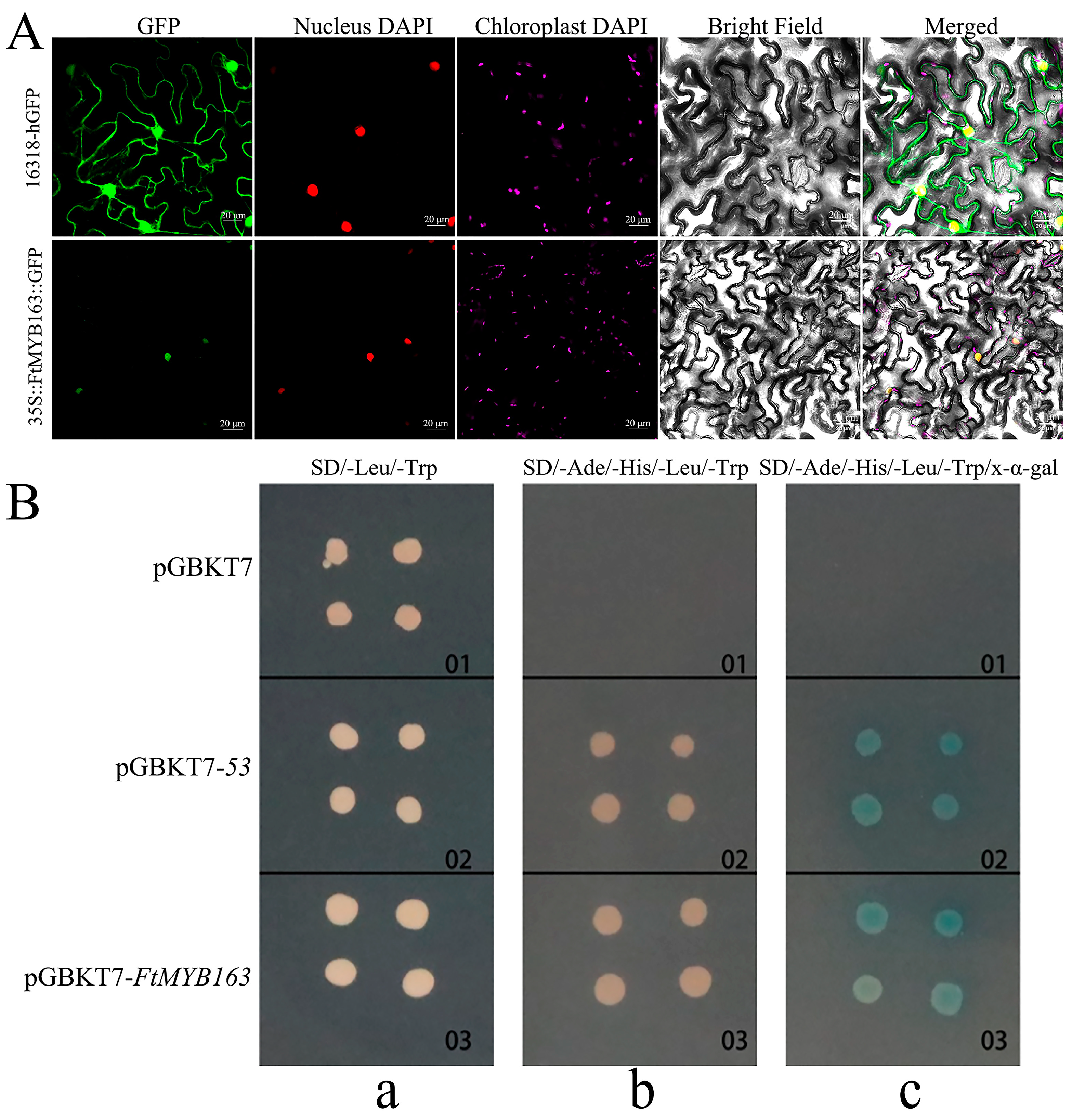
To determine the subcellular localization of the FtMYB163 protein, the GFP reporter gene was fused to the 3′-terminus of the FtMYB163 coding region and ligated into 16318-hGFP plasmid to generate the FtMYB163-GFP vector. The control vectors 16318-hGFP and FtMYB163-GFP were transiently expressed in tobacco leaves. The fluorescence signal from the unfused GFP control (16318-hGFP) was localized to the cytoplasm and the nucleus, whereas the FtMYB163-GFP fusion protein was localized exclusively to the nucleus (Figure 3A).
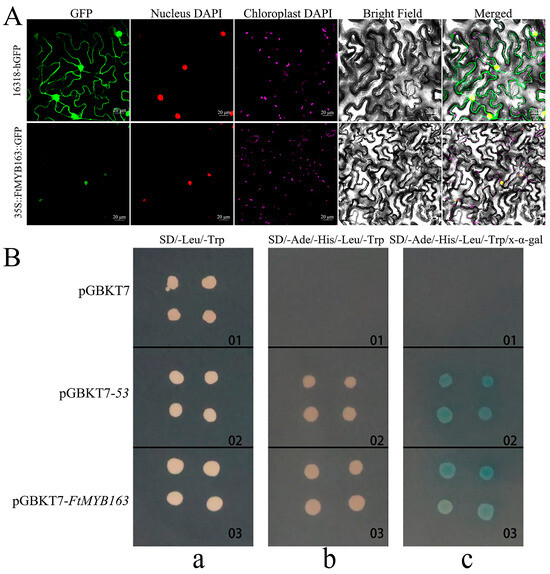
Figure 3.
Subcellular localization and transcription activation activities of FtMYB163. (A) Subcellular localization of FtMYB163-GFP fusion protein in Nicotiana benthamiana leaves. GFP: Green fluorescent protein; DAPI: 4′,6-diamidino-2-phenylindole stain; 16318-hGFP was used as the control. Scale bar: 20 μm. (B) Transcription activation analysis of FtMYB163 in yeast AH109 cells. The transformed cells were plated on an (a) SD/-Leu/-Trp, (b) SD/-Ade/-His/-Leu/-Trp, and (c) SD/-Ade/-His/-Leu/-Trp/x-α-gal medium and incubated in an incubator at 30 °C for 3~5 d. pGBKT7, negative control; pGBKT7-53, positive control.
We demonstrated that FtMYB163 has transcriptional activity using the yeast hybridization system. As shown in Figure 3B, cells transformed with the pGBKT7 (negative control) could only grow on the SD/-Leu/-Trp medium. By contrast, both the pGBKT7-FtMYB163 and pGBKT7-53 (positive control) could grow normally on SD/-Leu/-Trp and SD/-Ade/-His/-Leu/-Trp selection media. When X-α-gal was included in the media, the cells become blue (Figure 3B). These results indicate that FtMYB163 localizes to the nuclear and has transcriptional activity.
2.3. Comparing FtMYB163 Expression and Flavonoid Content in Tartary Buckwheat Tissues
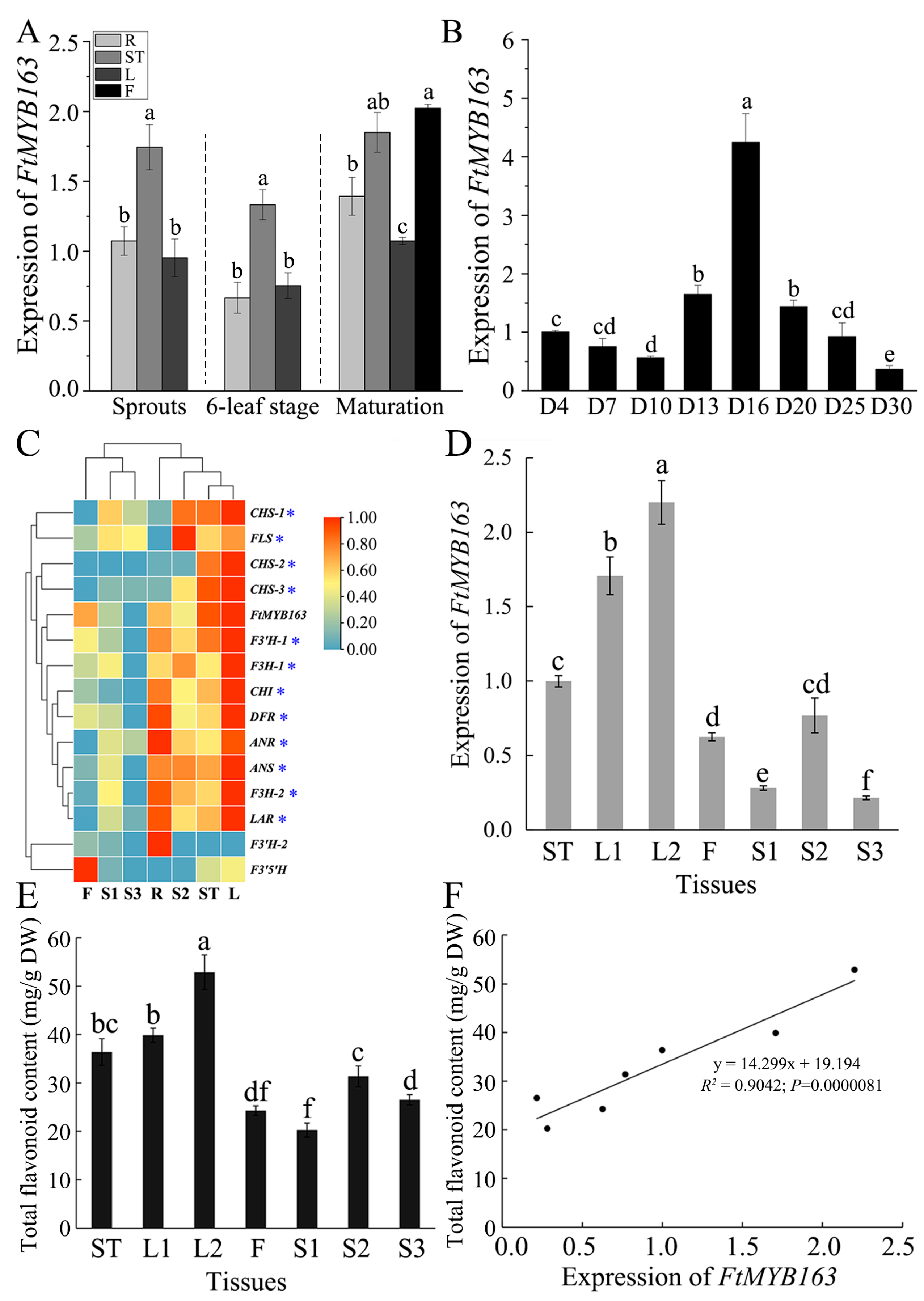
FtMYB163 function was examined by first measuring the expression of FtMYB163 in the roots (R), stems (ST), leaves (L), and flowers (F) at three stages of plant development (spouting, six-leaf stage, and maturation). Expression was also monitored in seed over a 30 d period during seed maturation. As shown in Figure 4A, FtMYB163 expression was detected in all these tissues, with the stems and flowers showing slightly higher expression than the other tissues. Expression of FtMYB163 in developing seed was the highest on day 16 (D16) after grouting, followed by D13 and D20. The lowest level of FtMYB163 expression in seed occurred at D30 and was only 8% of D16 (Figure 4B).
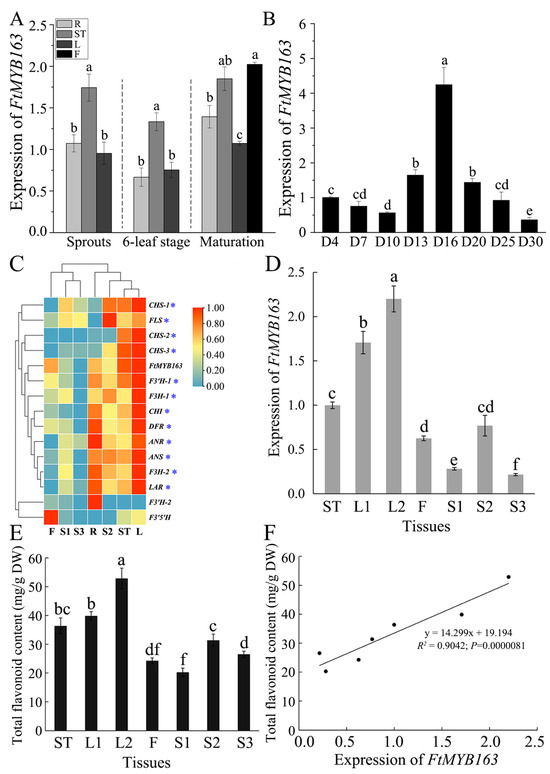
Figure 4.
Expression pattern of FtMYB163 and the total flavonoid content in Tartary buckwheat. The expression of FtMYB163 in (A) R (roots), ST (stems), L (leaves), and F (flowers) at sprouts, six-leaf stage, and maturation stage and (B) in seeds at different developmental stages. (C) Gene expression clustering heat map of FtMYB163 and Tartary buckwheat flavonoid biosynthesis structure. (D) FtMYB163 expression analysis and (E) the total flavonoid content detection in each tissue at the corresponding period (as in (C)). (F) The correlation between the FtMYB163 expression in different tissues and the content of total flavonoids. D4~D30, the seeds 4~30 days after flowering; L1, top one leaf of adult plants; L2, top three leaves of adult plants; S1, seeds before grouting; S2, seeds at filling stage; S3, mature seeds. Blue asterisks indicated the structural genes co-expressed with FtMYB163 during the three seed development periods. The expression levels were evaluated by the 2−ΔΔCT method, and FtActin7 was used as a reference gene. The values are represented as mean ± SD (n = 5) and marked with different letters to indicate statistically significant differences at p < 0.05 (Tukey’s test).
To investigate the function of FtMYB163 further, we compared the expression of FtMYB163 with 14 other genes involved in flavonoid biosynthesis in different tissues of Tartary buckwheat. Initially, we used the publicly available transcriptome data for Tartary buckwheat tissues [34,36], specifically the roots (R), stems (ST), leaves (L), flowers (F), and seeds. The seed data covered three development stages: before grouting (S1), at the filling stage (S2), and mature seed (S3). The database showed that the expression of FtMYB163 and most of the other genes was the highest in the leaves, while the lowest expression was in the S3 seed. The cluster analysis indicated that the expression pattern of FtMYB163 was similar to 12 of the 14 genes of the flavonoid biosynthesis-related genes, including DRF involved in anthocyanin biosynthesis. The two genes not showing the same general pattern were F3′H-2 and F3′5′H (Figure 4C).
We confirmed the FtMYB163 expression pattern using RT-PCR to measure different Tartary buckwheat tissues, including the stem (ST), the top leaf of adult plants (L1), the top three leaves of adult plants (L2), flowers (F) and three stages of seed development (S1, S2, and S3, as above). The results again showed that FtMYB163 expression was highly expressed in leaves and relatively low in seeds (Figure 4D).
We then used HPLC to determine how FtMYB163 expression levels compared with the total flavonoid contents in those same tissues. Flavonoid contents were greatest in the L2 and L1 leaves (52.8 and 39.9 mg/g DW respectively) and lowest in the S1 (20.5 mg/g DW). Contents in the stem and flowers were 36.8 and 24.3 mg/g DW, respectively (Figure 4E). A highly significant positive correlation occurred between FtMYB163 expression and total flavonoid content in these tissues (R2 = 0.9042) (Figure 4F). These results indicate that FtMYB163 expression influences the synthesis of flavonoids in Tartary buckwheat.
2.4. Overexpression of FtMYB163 in Arabidopsis Increases Flavonoid Accumulation
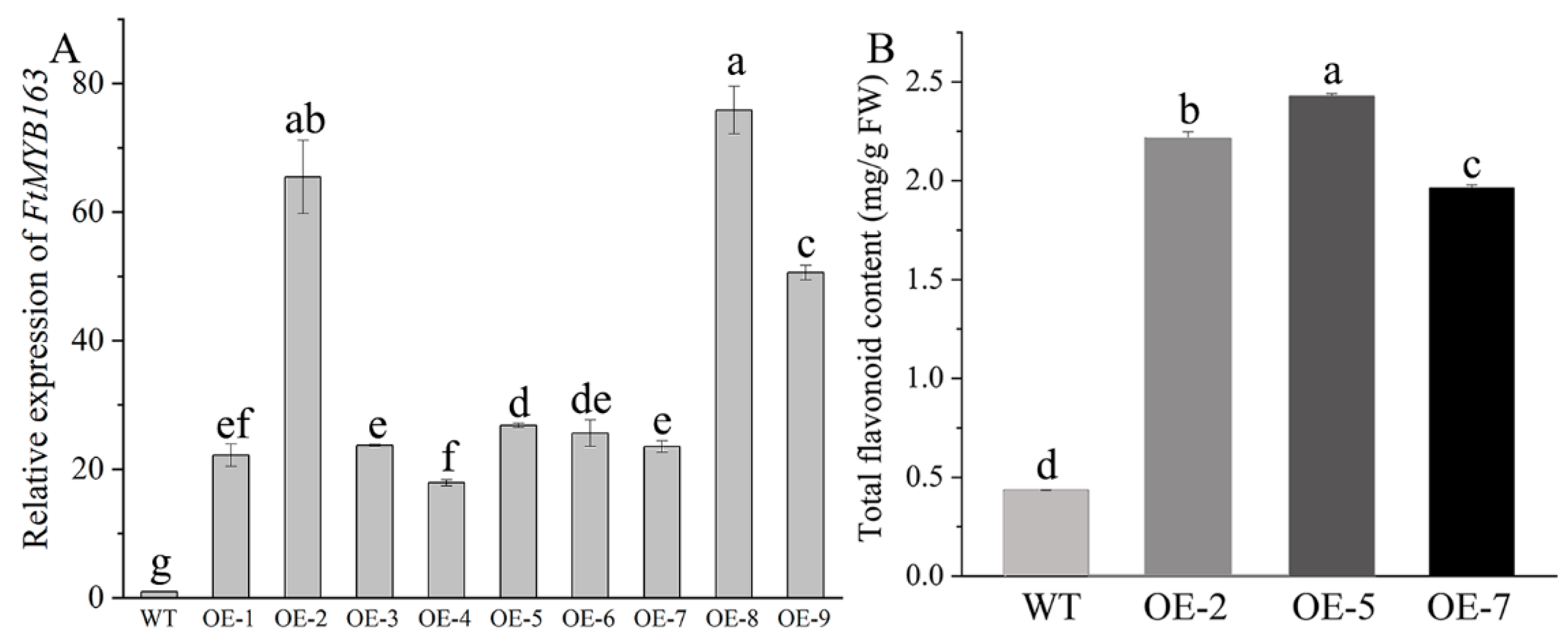
To validate the function of FtMYB163 further, the complete FtMYB163 ORF was expressed in the Arabidopsis thaliana Columbia-0 (Col-0) using the CaMV35S promoter (Figure S2). Nine independent homozygous T3 lines showing high FtMYB163 expression were generated and three (OE-2, OE-5, and OE-7) were selected for further analyses (Figure 5A). Total flavonoid contents in the leaves of the transgenic and wild-type (WT) plants are shown in Figure 5B. Transgenic line OE-5 exhibited the highest accumulation of total flavonoids (2.43 mg/g FW), followed by OE-2 (2.21 mg/g FW) and OE-7 (1.96 mg/g FW), and all three were more than five-fold greater than the total flavonoid content in WT plants (0.4 mg/g FW). These results demonstrate that overexpressing FtMYB163 in Arabidopsis significantly increased the total flavonoid content.
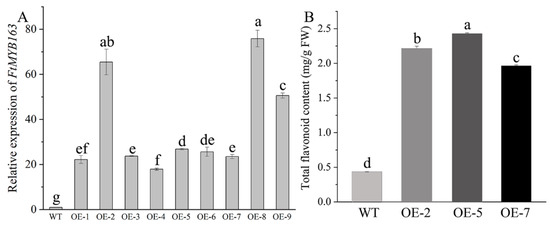
Figure 5.
Characterization of T3 transgenic Arabidopsis lines expressing FtMYB163. (A) Relative expression levels of FtMYB163 in independent homozygous T3 lines using qRT-PCR. AtACT2 was used as a reference gene. Data show the mean ± SD of three biological replicates. Different letters indicate significant differences at p < 0.05 (Student’s t-test). (B) Total flavonoid content of WT Arabidopsis and three transgenic T3 lines expressing FtMYB163 (OE-2, OE-5, and OE-7). Values are mean ± SD (n = 10), and different letters indicate significant differences at p < 0.01 (Tukey’s test).
We then determined which individual flavonoids showed the greatest changes in the leaves of the transgenic Arabidopsis lines. Analysis with LC-MS/MS detected 18 flavonoids that were indicatively higher in the transgenic lines compared with the WT. These included two chalcones, three dihydroflavones, one dihydroflavone glycoside, two dihydroflavonols, two flavones, and eight flavonols (Table 1). The five flavonoids showing the greatest changes were naringenin 7-O-glucoside, eriodictyol, naringenin chalcone, isorhamnetin-3-O-neohespeidoside, and quercetin. The content of these was at least 50-fold greater in one or more of the transgenic lines compared with the WT (Table 1). The flavonols quercetin and kaempferol were 55-fold and 43-fold greater than the WT, respectively. Notably, no changes were detected in the content of proanthocyanidins or anthocyanins. These results suggest that FTMYB163 expression in Arabidopsis alters the biosynthesis of some flavonoids but not anthocyanins.

Table 1.
The fold change in flavonoid content between WT and transgenic Arabidopsis.
2.5. Expression Analysis of Key Enzyme Genes of Flavonoid Synthesis
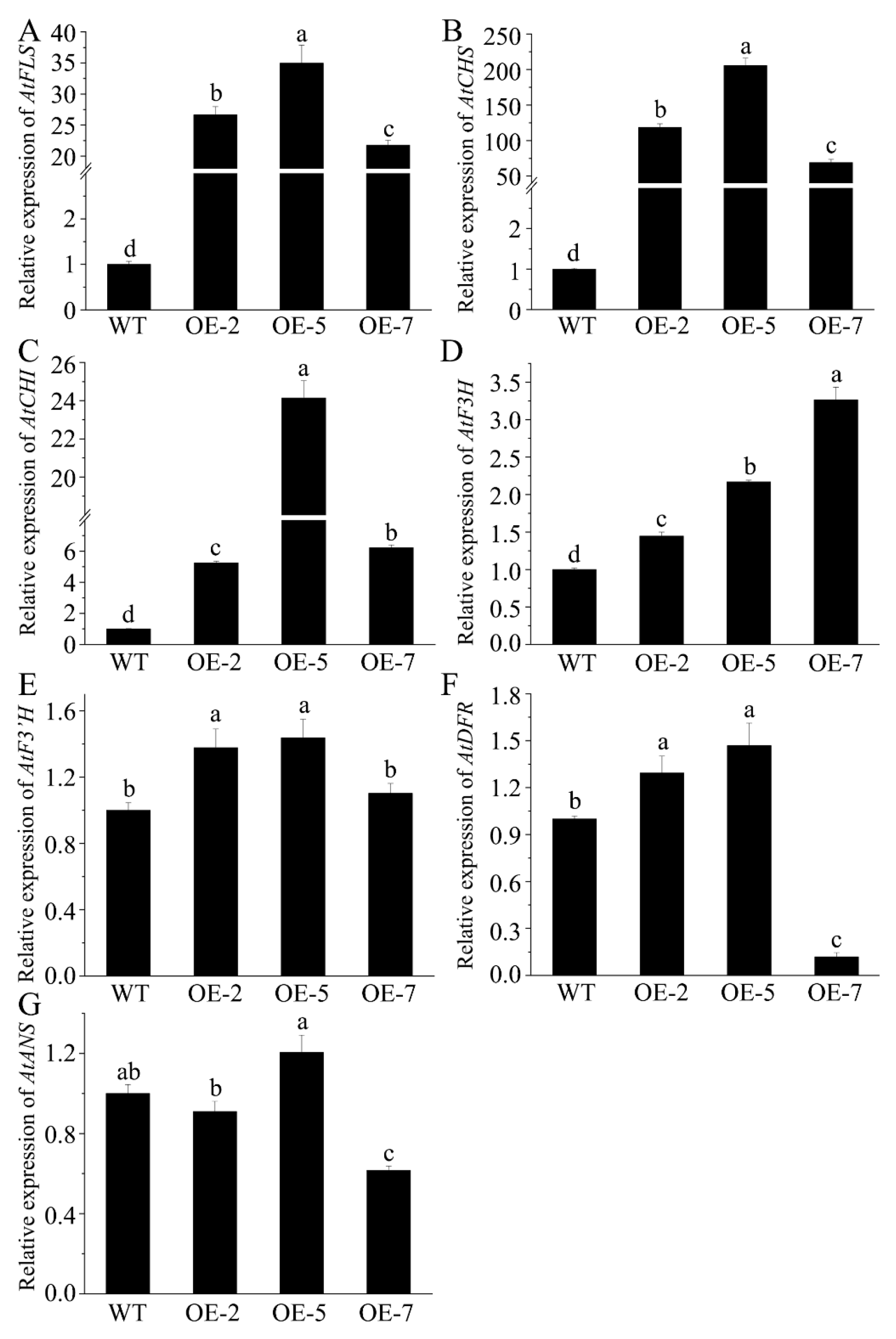
We investigated how FtMYB163 expression in Arabidopsis affected the expression of endogenous Arabidopsis genes in the flavonoid synthesis pathway. The expression of AtCHS, AtCHI, AtF3H, AtF3′H, AtFLS, AtDFR, and AtANS was measured in the leaves of WT and transgenic lines. As shown in Figure 6, FtMYB163 expression induced the greatest changes in AtFLS, AtCHS, and AtCHI expression, with average increases among the three transgenic lines of 28-fold, 130-fold, and 12-fold (p < 0.01), respectively, compared with the WT (Figure 6A–C). Smaller average increases of 2.3-fold were measured for AtF3H expression (Figure 6D), while much smaller and variable responses were detected for AtF3′H, AtDFR, and AtANS (Figure 6E–G). These results indicated that FtMYB163 enhanced the biosynthesis of flavonoids, including flavonols, by altering the expression genes in the flavonoid synthesis pathway.
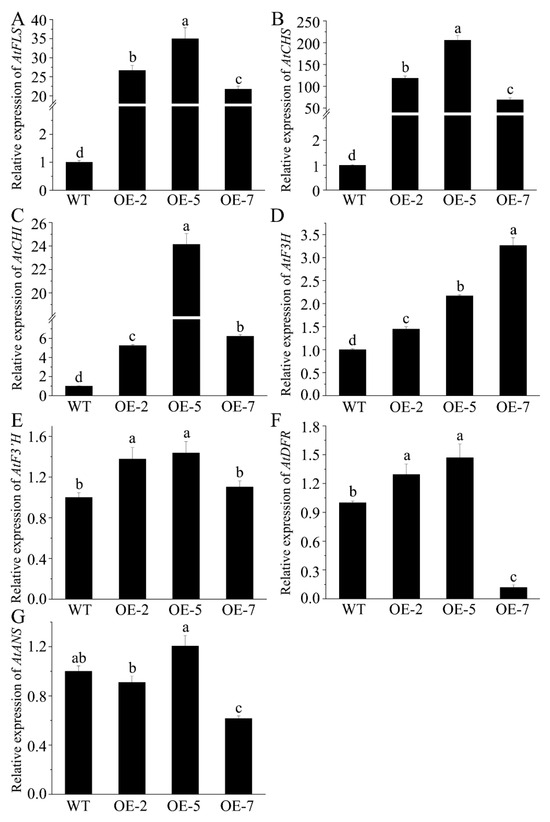
Figure 6.
Assessing how the expression of FtMYB163 in Arabidopsis leaves affects the expression of endogenous genes in the flavonoid synthesis pathway. The relative expression of Arabidopsis genes (A) flavonol synthase (AtFLS), (B) chalcone synthase (AtCHS), (C) chalcone isomerase (AtCHI), (D) flavonoid 3-hydroxylase (AtF3H), (E) flavonoid 3′-hydroxylase (AtF3′H), (F) dihydroflavonol 4-reductase (AtDFR), and (G) anthocyanidin reductase (AtANS) were measured in WT and transgenic lines (OE-2, OE-5, and OE-7). Gene expression in the WT was set to 1.0 to provide fold changes in expression. The AtACT2 gene was used as a reference. Values represent mean ± SD (n = 5), and different letters indicate significant differences (p < 0.01) (Tukey’s test).
3. Discussion
Flavonoids are important secondary metabolites that benefit plants and human health. Flavonoids are synthesized in plants by the flavonoid biosynthetic pathway, which consists of early flavonoid biosynthesis and late flavonoid synthesis. The early flavonoid biosynthesis pathway mainly depends on the activity of the enzymes CHS, CHI, F3H, F3′H and FLS, which ultimately form flavonols and glycosides [37]. This study investigated the underlying mechanisms of flavonoid biosynthesis in different tissues of developing Tartary buckwheat. We isolated the FtMYB163 gene, which encodes a predicted protein, with the conserved R2R3 domain and both SG7 motif1 and SG7 motif2 domains. Phylogenetic analysis supported FtMYB163 belonging to the SG7 subgroup of R2R3-MYB transcription factors. These include FeMYBF1 from common buckwheat, FtMYB6 from Tartary buckwheat, and AtMYB12 from Arabidopsis (Figure 1 and Figure 2). Previous studies have demonstrated that this group of MYB TFs regulates the synthesis of flavonols [22,38,39]. For example, flavonol biosynthesis in Arabidopsis is spatiotemporally regulated by the SG7 MYBs, including MYB11, MYB12, and MYB111 [12]. Yao et al. [35] expressed FtMYB6 in Arabidopsis and found that transcript levels and contents of rutin (a flavonol glycoside) were concentrated in the roots, stems, leaves, and flowers of the transgenic plants. Overexpression of MtMYB134 in both Arabidopsis and hairy roots of Medicago truncatula enhanced the biosynthesis of various flavonol derivatives by promoting the early flavonol biosynthesis genes CHS and FLS [39]. Also, FtMYB31 expression in transgenic tobacco promoted flavonol biosynthesis by inducing the expression of CHS, F3H, and FLS genes, and the expression of FtMYB6 in hairy roots and transgenic tobacco significantly increased the accumulation of rutin and other flavonols by inducing the expression of F3H and FLS1 [30,35]. Similar findings have been reported in grape [38], potato [40], common buckwheat [37], Chrysanthemum morifolium [41], and Paeonia qiui [33]. Therefore, we speculated that FtMYB163 had a similar function to other genes in the SG7 subgroup.
We demonstrated that FtMYB163 expression in different tissues of Tartary buckwheat was positively correlated to the total flavonoid content in those tissues (Figure 4D–F), which is consistent with our preliminary observations [34]. The expression profile of FtMYB163 in different tissues of Tartary buckwheat was similar to the expression of several structural genes, CHS, CHI, F3H, F3′H, and FLS, involved with flavonoid synthesis (Figure 4C), which supports the conclusion that FtMYB163 is involved with the regulation of flavonoid metabolism.
Some MYB TFs can also suppress the expression of genes in this pathway, thereby affecting flavonoid accumulation in other ways [38,42]. Our study found that expression of FtMYB163 in Arabidopsis enhanced the total flavonoid content of the transgenic lines compared with the WT (Figure 5). In particular, the contents of two flavone and eight flavonol compounds in the transgenic lines were significantly greater than in the WT (Table 1). Furthermore, the relative expression levels of AtCHS, AtCHI, AtFLS, and AtF3H genes were consistently greater in all transgenic lines, whereas AtDFR, AtF3′H, and AtANS expression levels were either unchanged, much smaller, or sometimes reduced (e.g., AtDFR and AtANS expression in OE-7) (Figure 6). Interestingly, the FLS and DFR enzymes usually impact different flavonoid pathways: FLS for the synthesis of colorless flavonols and DFR for the synthesis of colored anthocyanidins [43]. The strong induction of AtFLS in all transgenic Arabidopsis lines is consistent with the accumulation of eight flavonol compounds in those plants. By contrast, the changes in AtDFR expression were much smaller and even suppressed in line OE-7 (Figure 6F,G), which is consistent with there being no detectable changes to the contents of proanthocyanidin and anthocyanin in those lines. These results indicated that FtMYB163 increased the accumulation of the total flavonoid and promoted flavonol biosynthesis by regulating the expression of CHS, CHI, FLS, and F3H.
Although similar to FtMYB6, overexpression of FtMYB163 could promote the content of flavonoid and flavonol in transgenic plants (Figure 5B and Table 1), the expression of FtMYB6 and the flavonol accumulation was involved in the light environment [35]. In addition, FtMYB6 and FtMYB116 regulated flavonoid synthesis by binding to the promoters of structural genes FtF3H, FtFLS1, or FtF3′H [31,35]. Therefore, whether FtMYB163 could directly bind to the promoter of the above-mentioned structural genes to regulate flavonol biosynthesis, and whether the expression of FtMYB163 is affected by environmental factors, such as light, need to be investigated further. Meanwhile, flavonoids can enhance the resistance to abiotic stresses in plants, so we also could further explore the relationship between FtMYB163 and plant stress resistance.
4. Materials and Methods
4.1. Plant Materials and Culture Conditions
Seed of the Tartary buckwheat variety “Heiku013” was provided by the Guizhou Normal University (Guiyang, China). Seeds were sown and grown in small pots in a growth chamber under controlled conditions: 16/8 h light/dark cycle at 25 °C, 70% relative humidity, and ~150 μmol m−2 s−1 light intensity. After growing to the filling stage, different tissues, including roots (R), stems (ST), leaves (L), flowers (F), and seeds (S), were collected, frozen in liquid nitrogen, and then stored at −80 °C for later extraction of total RNA. Tobacco (Nicotiana benthamiana) was used for examining subcellular localization of FtMYB163 and stable transformations were generated in Arabidopsis using the same methods described previously [44,45].
4.2. Gene Cloning and Sequencing
Based on the reference sequence of “Pinku No.1” cDNA, the FtMYB163 gene was amplified by specific primers (Table S2). Gene and predicted protein structures were analyzed online using GSDS 2.0 (https://gsds.gao-lab.org/ (accessed on 5 July 2023)) and ExPASy server (https://web.expasy.org/protparam/ (accessed on 5 July 2023)). Amino acid sequences of other related MYB transcription factors (TFs) were collected from NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 5 July 2023)), and the phylogenetic analysis was carried out using the Neighbor-Joining (NJ) method of MEGA 11 software. The multiple sequence alignment of chosen amino acids with high identity and similarity was performed with DNAMAN 7.0 software (LynnonBiosoft, San Ramon, CA, USA).
4.3. RNA Extraction and Gene Expression Analysis
Total RNA was extracted using the Omega E.Z.N.A Plant RNA Kit (Omega Bio-Tek, Norcross, GA, USA). The quantitative RT-PCR (qRT-PCR) was performed using NovoStart® SYBR q-PCR SuperMix Plus (Novoprotein, Suzhou, China). FtActin7 and AtActin2 were used as the Tartary buckwheat and Arabidopsis internal reference genes, respectively. The relative expression levels were calculated with the 2−ΔΔCT method [46]. All analyses were performed for a minimum of three biological replicates per sample. The primers used for qRT-PCR are listed in Table S2.
4.4. Subcellular Localization
The FtMYB163 sequence without terminator sequence was amplified by PCR using primers housing Sal I and BamH I restriction sites. The ClonExpress® II One Step Cloning kit (Vazyme, Nanjing, China) was used to insert this fragment into the 16318-hGFP plasmid to generate the FtMYB163-GFP vector, where the CaMV35S promoter drives expression of the transgene. Subcellular localization was determined by transiently expressing translational fusions of FtMYB163 with GFP in Nicotiana benthamiana leaves according to the method described before [47].
4.5. Transcriptional Activation of FtMYB163
To verify the transcriptional activation of FtMYB163, the full-length CDS of FtMYB163 was inserted into the pGBKT7 to construct the pGBKT7-FtMYB163 recombinant plasmid. The empty pGBKT7 vector (negative control), pGBKT7-FtMYB163 vector, and pGBKT7-53 vector (positive control) were introduced into the yeast strain AH109 and grown on SD/-Leu/-Trp and SD/-Ade/-His/-Leu/-Trp selective plates. Positive cells were subsequently grown on SD/-Ade/-His/-Leu/-Trp plates containing X-gal. All plates were incubated in the dark at 28 °C for three to five days and photographed. All primers are listed in Table S2.
4.6. Stable Transformation of Arabidopsis
FtMYB163 was inserted into the intermediate vector pDONR22 by the Gateway technique (Thermo Fisher Scientific, Waltham, MA, USA). A specific primer with an attB site was designed to construct the pK7WG2D-35S-FtMYB163 vector. The vector was transformed into Arabidopsis ecotype Columbia-0 (Col-0) via the floral dip method mediated by Agrobacterium tumefacien strain GV3101 [48]. The first generation (T1) seeds of transgenic plants were collected and screened in a 1/2 MS medium with 50 mg/L kanamycin and confirmed by PCR. qRT-PCR was used to select transgenic Arabidopsis plants with high FtMYB163 expression. Independent homozygous T3 seeds were collected and used for all following experiments. Primer sequences are listed in Table S2.
4.7. Determination of the Flavonoid Content
The following tissues of “Heiku013” Tartary buckwheat were collected for analyses: Stem, Leaf 1 (top leaf of adult plants), Leaf 2 (top three leaves of adult plants), Seed 1 (seeds before grouting), Seed 2 (seed at filling stage), and Seed 3 (mature seed). Total flavonoid content was determined with the AlCl3 method [30]. The standard curve of rutin used was y = 0.002x + 0.0539 (R2 = 0.9999). Briefly, dry sample powder (0.1 g) was dissolved in 3 mL of 70% methanol solution (MS) and shaken for 4 h at 65 °C protected from light at 160 ± 10 r/min. The sample solution was ultrasonically extracted at 15% output power for 10 min and then centrifuged at 8000 rpm/min for 10 min. The supernatant was collected and filtered through a 0.45 μm microporous membrane, dissolved in 70% MS to 5 mL, and shaken well. After this, the standard rutin solution (1.0 mg/mL) was pipetted at 0.0 mL, 0.5 mL, 1.0 mL, 1.5 mL, and 3.5 mL into different 10-mL volumetric flasks. Then, 2 mL 0.1 mol/L AlCl3 and 3 mL 1 mol/L CH3COOK were added. Subsequently, 70% MS was used to bring the volume to 10 mL, and it was stewed at room temperature for 30 min. The absorbance at 420 nm was measured, and a standard curve was established based on the absorbance value. Finally, the total flavonoid content was calculated based on the absorbance values of the tested samples and the standard curve.
Flavonoid content in transgenic Arabidopsis thaliana plants was determined by gas chromatography-mass spectrometry (GC-MS). Briefly, the frozen samples were freeze-dried, ground for 1.5 min in a grinder under 30 Hz frequency. Then, 20 mg of the ground sample powder was dissolved in 10 μL of a 4000 nM internal standard mixed working solution and 500 μL of 70% methanol extract. The samples were then subjected to an ultrasonic treatment for 30 min and centrifuged at 12,000 r/min at 4 °C. The supernatant was collected and filtered with a 0.22 μm microporous membrane. The final solution (metabolites) was detected by Ultra Performance Liquid Chromatography (UPLC) and Tandem Mass Spectrometry (MS/MS), and the structure of the metabolites was analyzed using the Metware Database. Subsequently, the quantitative analysis of metabolites was measured by the Multiple Reaction Monitoring (MRM) mode of triple quadrupole mass spectrometry.
The UPLC collection conditions included the chromatographic column: Waters T3 C18 column 1.8 μm, 2.1 mm × 100 mm, mobile phases A (ultrapure water added with 0.05% formic acid) and B (acetonitrile with 0.05% formic acid), 0.35 mL/min flow rate, 40 °C column temperature, 2 μL injection volume, and elution gradient. Mobile phase A/B was 90:10 (v/v), 80:20, 30:70, 5:95, 5:95, 90:10, and 90:10 at 0 min, 1 min, 9 min, 12.5 min, 13.5 min, 13.6 min, and 15 min, respectively. The MS/MS collection conditions included electrospray ionization (ESI, 550 °C), mass spectrum voltage (5500 V under positive ion mode and −4500 V under negative ion mode), and curtain gas (CUR, 35 psi). Each ion pair was scanned based on declustering potential (DP) and collision energy (CE) optimization in the Q-Trap 6500+ (PerkinElmer, Hopkinton, MA, USA).
4.8. Statistical Analysis and Reproducibility
Each experiment included at least five samples and was repeated thrice to confirm reproducibility. Origin software version 2021 (OriginLab Corporation, Northampton, MA, USA) was used to plot the figures. The statistical analysis of the data was conducted using one-way ANOVA, including Tukey’s test, and was performed using the SPSS 21.0 software (IBM, Chicago, IL, USA).
5. Conclusions
We conclude that FtMYB163 encodes an SG7 R2R3-MYB transcription factor. Measurements in Tartary buckwheat and transgenic Arabidopsis suggest that expression of this gene regulates the synthesis of flavonoids, especially flavonols, by inducing the expression of the early structural genes CHS, CHI, F3H, and FLS. This study lays the foundation for future attempts to improve the nutritional value of Tartary buckwheat.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13192704/s1, Figure S1: Amplification and gene structure analysis of FtMYB163; Figure S2: Screening and detection of transgenic Arabidopsis lines; Table S1: Basic information of FtMYB163; Table S2: The primers used in this study.
Author Contributions
H.D. analyzed the data and drafted the manuscript; H.L. designed the experiment; J.K. and X.S. carried out the experiments; L.T., Q.Y., C.W. and A.W. contributed with consultation on the manuscript; P.R.R. advised on data interpretation and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32260461, 32460468), the Open Project Program of Panxi Crops Research and Utilization Key Laboratory of Sichuan Province (SZ21ZZ05), and the Science and Technology Foundation of Guizhou Province (QianKe-HeJiChu-ZK [2021]ZhongDian035).
Data Availability Statement
Data are available on request to the corresponding author’s email with appropriate justification.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, C.; Kobayashi, K.; Yoshida, Y.; Ohasawa, R. Genetic analyses of agronomic traits in Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.). Breed. Sci. 2012, 62, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Bai, X.; Zhao, W.; Xiang, D.; Wan, Y.; Yan, J.; Zou, L.; Zhao, G. De dovo assembly and analysis of Tartary buckwheat (Fagopyrum tataricum Garetn.) transcriptome discloses key regulators involved in salt-stress response. Genes 2017, 8, 255. [Google Scholar] [CrossRef] [PubMed]
- Fabjan, N.; Rode, J.; Kosir, I.J.; Wang, Z.; Kreft, I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Xiao, C.L. Determination of total flavones on Fagopyrum Gaertn of variety organs. Food Sci. 2004, 25, 264–266. (In Chinese) [Google Scholar]
- Qin, P.Y. Quality Evaluation of Chinese Main Buckwheat Cultivars and Effect of Processing on the Components and Health-Relevant Functionality of Buckwheat. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2012. [Google Scholar]
- Li, H.Y.; Lv, Q.Y.; Liu, A.K.; Wang, J.R.; Sun, X.Q.; Deng, J.; Chen, Q.F.; Wu, Q. Comparative metabolomics study of Tartary (Fagopyrum tataricum (L.) Gaertn) and common (Fagopyrum esculentum Moench) buckwheat seeds. Food Chem. 2022, 371, 131125. [Google Scholar] [CrossRef]
- Ke, J.; Ran, B.; Sun, P.Y.; Cheng, Y.Z.; Chen, Q.F.; Li, H.Y. An evaluation of the absolute content of flavonoids and the identification of their relationship with the flavonoid biosynthesis genes in Tartary buckwheat seeds. Agronomy 2023, 13, 3006. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.J.; Zhang, F.J.; Zhang, G.Z.; Jiang, X.Y.; Yu, H.M.; Hou, B.K. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 2016, 89, 85–103. [Google Scholar] [CrossRef]
- Zhang, P.; Du, H.; Wang, J.; Pu, Y.; Yang, C.; Yan, R.; Yang, H.; Cheng, H.; Yu, D. Multiplex CRISPR/Cas9-mediated metabolic engineering increases soya bean isoflavone content and resistance to soya bean mosaic virus. Plant Biotechnol. J. 2019, 18, 1384–1395. [Google Scholar] [CrossRef]
- Saffer, A.M.; Irish, V.F. Flavonol rhamnosylation indirectly modifies the cell wall defects of RHAMNOSE BIOSYNTHESIS1 mutants by altering rhamnose flux. Plant J. 2018, 94, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Fernie, A.R. Specialized metabolites of the flavonol class mediate root phototropism and growth. Mol. Plant 2016, 9, 1554–1555. [Google Scholar] [CrossRef]
- Tan, H.; Man, C.; Xie, Y.; Yan, J.; Chu, J.; Huang, J. A crucial role of GA-regulated flavonol biosynthesis in root growth of Arabidopsis. Mol. Plant 2019, 12, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Yang, J.; Abhinandan, K.; Nie, Y.; Li, X.; Li, Y.; Samuel, M.A. Flavonoids and ROS play opposing roles in mediating pollination in ornamental kale (Brassica oleracea var. acephala). Mol. Plant 2017, 10, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chai, C.L.; Morohashi, K.; Grotewold, E.; Snook, M.; Chopra, S. Expression of flavonoid 3’-hydroxylase is controlled by P1, the regulator of 3-deoxyflavonoid biosynthesis in maize. BMC Plant Biol. 2012, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Broun, P. Transcriptional control of flavonoid biosynthesis: A complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr. Opin. Plant Biol. 2005, 8, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Duan, B.; Yuan, B.; Chen, Z.; Yu, D.; Ke, L.; Zhou, W.; Liu, H.; Sun, Y. Flavanone and flavonoid hydroxylase genes regulate fiber color formation in naturally colored cotton. Crop J. 2023, 11, 766–773. [Google Scholar] [CrossRef]
- Bogs, J.; Ebadi, A.; McDavid, D.; Robinson, S.P. Identification of the flavonoid hydroxylases from grapevine and their regulation during fruit development. Plant Physiol. 2006, 140, 279–291. [Google Scholar] [CrossRef]
- Weerawanich, K.; Halbwirth, H.; Sirikantaramas, S. A novel MYB transcription factor from durian (Durio zibethinus), DzMYB1, regulates flavonoid biosynthesis in fruit pulp. Sci. Hortic. 2024, 333, 113246. [Google Scholar] [CrossRef]
- Tu, M.; Fang, J.; Zhao, R.; Liu, X.; Yin, W.; Wang, Y.; Wang, X.; Wang, X.; Fang, Y. CRISPR/Cas9-mediated mutagenesis of VvbZIP36 promotes anthocyanin accumulation in grapevine (Vitis vinifera). Hortic. Res. 2022, 1, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, W.; Zhang, T.; Jiang, S.; Xu, H.; Wang, Y.; Zhang, Z.; Wang, C.; Chen, X. Transcriptomic analysis of red-fleshed apples reveals the novel role of MdWRKY11 in flavonoid and anthocyanin biosynthesis. J. Agric. Food Chem. 2018, 66, 7076–7086. [Google Scholar] [CrossRef]
- Ito, M. Conservation and diversification of three-repeat Myb transcription factors in plants. J. Plant Res. 2005, 118, 61–69. [Google Scholar] [CrossRef]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Z.; Maximova, S.N.; Payne, M.J.; Guiltinan, M.J. Tc-MYBPA is an Arabidopsis TT2-like transcription factor and functions in the regulation of proanthocyanidin synthesis in Theobroma cacao. BMC Plant Biol. 2015, 15, 160. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Nesi, N.; Jond, C.; Debeaujon, I.; Caboche, M.; Lepiniec, L. The Arabidopsis TT2 gene encodes and R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell 2001, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhao, H.; Yao, P.; Li, Q.; Huang, Y.; Li, C.; Chen, H.; Wu, Q. An R2R3-MYB transcription factor FtMYB15 involved in the synthesis of anthocyanin and proanthocyanidins from Tartary buckwheat. J. Plant Growth Regul. 2017, 37, 76–84. [Google Scholar] [CrossRef]
- Wang, S.; Luo, X.P.; Yao, Y.J.; Yang, J.J.; Chen, Y.; Wu, Q.; Wu, Q. Characterization of an R2R3-MYB transcription factor involved in the synthesis of proanthocyanidins from Tartary buckwheat. Acta Bot. Boreali-Occident. Sin. 2019, 39, 1911–1918. (In Chinese) [Google Scholar]
- Yu, Y.; Liao, X.L.; Xiao, Y.H.; Zhao, H.X. Transcription factors gene FtMYB56 cloned from Fagopyrum tataricum, and its effects on flavonoid biosynthesis. Genom. Appl. Biol. 2020, 39, 4664–4671. (In Chinese) [Google Scholar]
- Sun, Z.; Linghu, B.; Hou, S.; Liu, R.; Wang, L.; Hao, Y.; Han, Y.; Zhou, M.; Liu, L.; Li, H. Tartary buckwheat FtMYB31 gene encoding an R2R3-MYB transcription factor enhances flavonoid accumulation in Tobacco. J. Plant Growth Regul. 2019, 39, 564–574. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.L.; Huang, C.H.; Wen, D.; Lu, J.N.; Chen, S.; Zhang, T.Y.; Shi, Y.H.; Xue, J.P.; Ma, W.; et al. The light-induced transcription factor FtMYB116 promotes accumulation of rutin in Fagopyrum tataricum. Plant Cell Environ. 2019, 42, 1240–1351. [Google Scholar] [CrossRef]
- Bai, Y.C.; Li, C.L.; Zhang, J.W.; Li, S.J.; Luo, X.P.; Yao, H.P.; Chen, H.; Zhao, H.X.; Park, S.U.; Wu, Q. Characterization of two tartary buckwheat R2R3-MYB transcription factors and their regulation of proanthocyanidin biosynthesis. Physiol. Plant 2014, 152, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, J.J.; Wang, Q.Y.; Zhang, M.; Zhi, H.; Bai, Z.Z.; Zhang, Y.L.; Luo, J.R. The Paeonia qiui R2R3-MYB transcription factor PqMYBF1 positively regulates flavonol accumulation. Plants 2023, 12, 1427. [Google Scholar] [CrossRef]
- Li, H.; Lv, Q.; Ma, C.; Qu, J.; Cai, F.; Deng, J.; Huang, J.; Ran, P.; Shi, T.; Chen, Q. Metabolite profiling and transcriptome analyses provide insights into the flavonoid biosynthesis in the developing seed of Tartary buckwheat (Fagopyrum tataricum). J. Agric. Food Chem. 2019, 67, 11262–11276. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Huang, Y.; Dong, Q.; Wan, M.; Wang, A.; Chen, Y.; Li, C.; Wu, Q.; Chen, H.; Zhao, H. FtMYB6, a light-induced SG7 R2R3-MYB transcription factor, promotes flavonol biosynthesis in Tartary buckwheat (Fagopyrum tataricum). J. Agric. Food Chem. 2020, 68, 13685–13696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The Tartary buckwheat genome provides insights into rutin biosynthesis and abiotic stress tolerance. Mol. Plant 2017, 10, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Oshima, Y.; Mitsuda, N.; Sakamoto, S.; Nishiba, Y.; Walker, A.R.; Ohme-Takagi, M.; Robinson, S.P.; Yasui, Y.; Mori, M.; et al. Buckwheat R2R3 MYB transcription factor FeMYBF1 regulates flavonol biosynthesis. Plant Sci. 2018, 274, 466–475. [Google Scholar] [CrossRef]
- Czemmel, S.; Stracke, R.; Weisshaar, B.; Cordon, N.; Harris, N.N.; Walker, A.R.; Robinson, S.P.; Bogs, J. The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berries. Plant Physiol. 2009, 151, 1513–1530. [Google Scholar] [CrossRef]
- Naik, J.; Rajput, R.; Pucker, B.; Stracke, R.; Pandey, A. The R2R3-MYB transcription factor MtMYB134 orchestrates flavonol biosynthesis in Medicago truncatula. Plant Mol. Biol. 2021, 106, 157–172. [Google Scholar] [CrossRef]
- Lin, S.; Singh, R.K.; Moehninsi; Navarre, D.A. R2R3-MYB transcription factors, StmiR858 and sucrose mediate potato flavonol biosynthesis. Hortic. Res. 2021, 8, 25. [Google Scholar] [CrossRef]
- Yang, F.; Wang, T.; Guo, Q.S.; Zou, Q.J.; Yu, S.Y. The CmMYB3 transcription factors isolated from the Chrysanthemum morifolium regulate flavonol biosynthesis in Arabidopsis thaliana. Plant Cell Rep. 2023, 42, 791–803. [Google Scholar] [CrossRef]
- Aharoni, A.; Vos, C.; Wein, M.; Sun, Z.; O’Connell, A.P. The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J. 2001, 28, 319–332. [Google Scholar] [CrossRef]
- Davies, K.M.; Schwinn, K.E.; Deroles, S.C.; Manson, D.G.; Lewis, D.H.; Bloor, S.J.; Bradley, J.M. Enhancing anthocyanin production by altering competition for substrate between flavonol synthase and dihydroflavonol 4-reductase. Euphytica 2003, 131, 259–268. [Google Scholar] [CrossRef]
- Yao, P.F.; Li, C.L.; Zhao, X.R.; Li, M.F.; Zhao, H.X.; Guo, J.Y.; Cai, Y.; Chen, H.; Wu, Q. Overexpression of a Tartary buckwheat gene, FtbHLH3, enhances drought/oxidative stress tolerance in transgenic Arabidopsis. Front. Plant Sci. 2017, 8, 625. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Huang, R.N.; Hong, L.H.; Xu, J.X.; Hong, Y.G.; Wu, Y.H.; Chen, W.W. The transcription factor NAC102 confers cadmium tolerance by regulating WAKL11 expression and cell wall pectin metabolism in Arabidopsis. J. Integr. Plant Biol. 2023, 65, 2262–2278. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Maron, L.G.; Piñeros, M.A.; Guimarães, C.T.; Magalhaes, J.V.; Pleiman, J.K.; Mao, C.; Shaff, J.; Belicuas, S.N.J.; Kochian, L.V. Two functionally distinct members of the MATE (multi-drug and toxic compound extrusion) family of transporters potentially underlie two major aluminum tolerance QTLs in maize. Plant J. 2010, 61, 728–740. [Google Scholar] [CrossRef]
- Colugh, S.J.; Bent, A.F. Floral dip: A simplifed method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. Cell. Mol. Biol. 1998, 16, 735. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).