Spatio-Temporal Extension and Spatial Analyses of Dengue from Rawalpindi, Islamabad and Swat during 2010–2014
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
3. Results
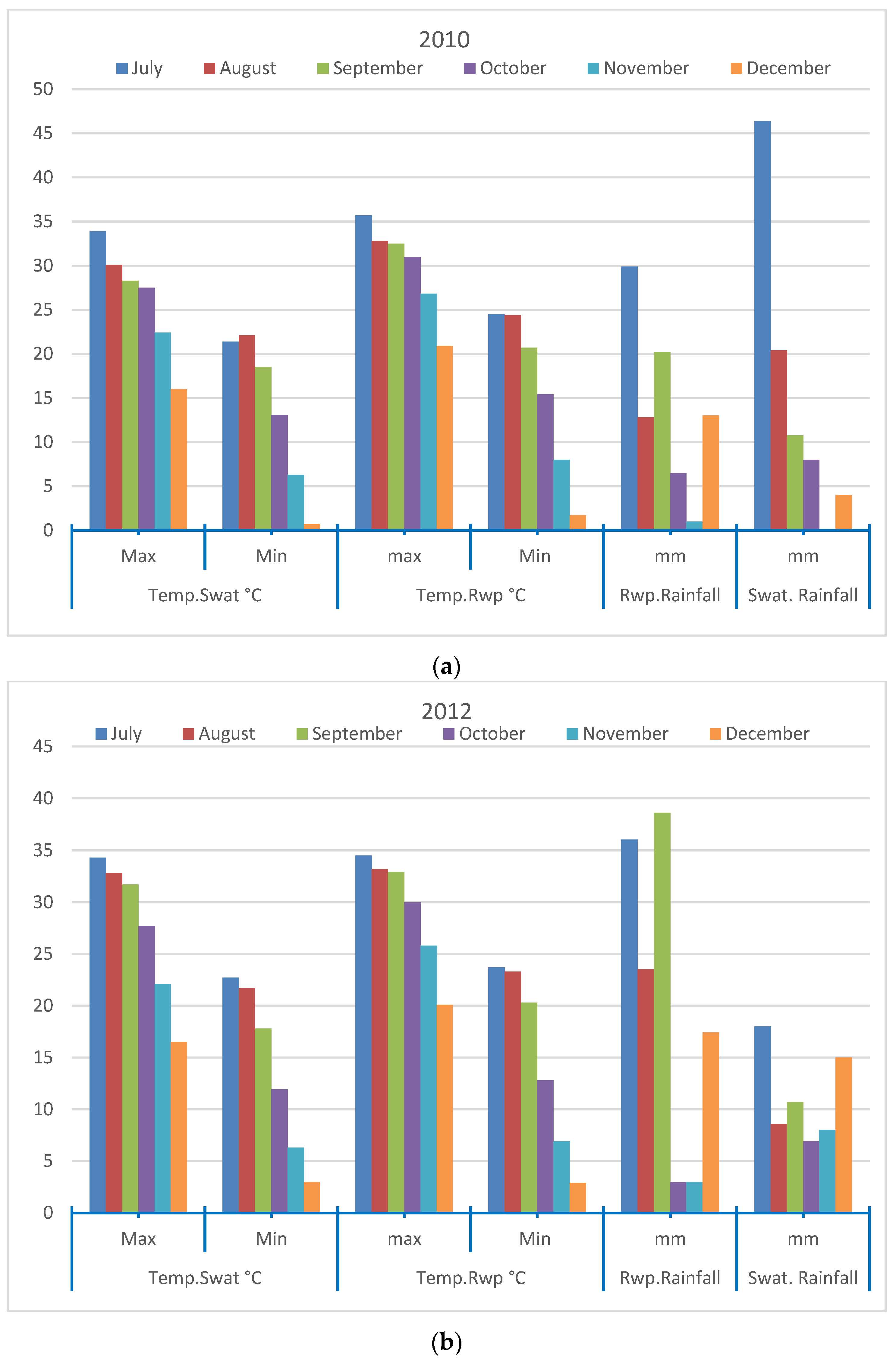
3.1. Relationship between Temperature, Precipitation and Dengue Cases
3.2. Relationship between Elevation, Drainage Patterns, and Dengue Cases
3.3. Impact of LULC on Dengue Transmission
3.4. Point Pattern Analysis of Dengue Transmission
3.5. Spatio-Temporal and Hotspot Analysis of Dengue Transmission
3.6. Regression Analysis and Dengue Transmission
3.7. Overlay Analysis
3.8. TIM Geoprocessing Model
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Halstead, S.B. Dengue virus-mosquito interactions. Rev. Entomol. 2008, 53, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Messer, W.B.; Gubler, D.J.; Harris, E.; Sivananthan, K.; de Silva, A.M. Emergence and global spread of a Dengue serotype 3, subtype III virus. Emerg. Infect. Dis. 2003, 9, 800–809. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Report of the Scientific Working Group Meeting on Dengue; TDR/SWG/08; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- United Nations World Tourism Organization (UNWTO). UNWTO Tourism Highlights; UNWTO: Madrid, Spain, 2006. [Google Scholar]
- Shakoor, T.M.; Ayub, S.; Ayub, Z. Dengue fever: Pakistan’s worst nightmare. WHO South East Asia J. Public Health 2012, 1, 229–231. [Google Scholar]
- Humayoun, M.A.; Waseem, T.; Jawa, A.A.; Hashmi, M.S.; Akram, J. Multiple Dengue serotypes and high frequency of Dengue hemorrhagic fever at two tertiary care hospitals in Lahore during the 2008 Dengue virus outbreak in Punjab, Pakistan. Int. J. Infect. Dis. 2010, 14, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-de-Oliveiraa, R.; Vazeilleb, M.; de Filippisa, A.M.; Faillouxb, A.B. Aedes Aegypti in Brazil, genetically differentiated populations with high susceptibility to Dengue and yellow fever viruses. Trans. R. Soc. Trop. Med. Hyg. 2003, 98, 43–54. [Google Scholar] [CrossRef]
- Rohani, A.; Wong, Y.C.; Zamre, I.; Lee, H.L.; Zurainee, M.N. The effect of extrinsic incubation temperature on development of Dengue serotype 2 and 4 viruses in Aedes Aegypti (L.). Southeast Asian J. Trop. Med. Public Health 2009, 40, 942–950. [Google Scholar] [PubMed]
- Watts, D.M.; Burke, D.S.; Harrison, B.A.; Whitemire, R.E.; Nisalak, A. Effect of temperature on the vector efficiency of Aedes Aegypti for Dengue 2 virus. Am. J. Trop. Med. Hyg. 1987, 36, 143–152. [Google Scholar] [PubMed]
- Scott, T.W.; Amerasinghe, P.H.; Morrison, A.C.; Lorenz, L.H.; Clark, G.G.; Strickman, D. Longitudinal studies of Aedes Aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Blood feeding frequency. J. Med. Entomol. 2000, 37, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Christophers, S.R. Aedes Aegypti the Yellow Fever Mosquito; University Press: Cambridge, UK, 1960. [Google Scholar]
- Tun-Lin, W.; Burkot, T.R.; Kay, B.H. Effects of temperature and larval diet on development rates and survival of the Dengue vector Aedes Aegypti in north Queensland, Australia. Med. Vet. Entomol. 2000, 14, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Barrera, R.; Amador, M.; MacKay, A.J. Population dynamics of Aedes Aegypti and Dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl. Trop. Dis. 2011, 5, 13–78. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I.; Mayers, M.; Burke, D.S.; Vaughn, D.W.; Endy, T.; Anandal, N. Etiology of interepidemic periods of mosquito-borne disease. Proc. Natl. Acad. Sci. USA 2000, 97, 9335–9339. [Google Scholar] [CrossRef] [PubMed]
- Juliano, S.A.; O’Meara, G.F.; Morrill, J.R.; Cutwa, M.M. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia 2002, 130, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.; Peters, T.M.; Greenough, N.C. Over-crowding of mosquito populations, response of larval Aedes aegypti to stress. Environ. Entomol. 1972, 1, 89–93. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Murdie, G.; Yasuno, M.; Tonn, R.J.; Reader, P.M. Studies of the life budget of Aedes Aegypti in Wat Samphaya, Bangkok, Thailand. Bull. World Health Organ. 1972, 46, 211–226. [Google Scholar] [PubMed]
- Kolivras, K.N. Changes in Dengue risk potential in Hawaii, USA, due to climate variability and change. Climate Res. 2010, 42, 1–11. [Google Scholar] [CrossRef]
- Matthew, D.E.; Eric, D.; Irene, C.; Joshua, W.; Cameron, S. Intra and inter-seasonal autoregressive prediction of Dengue outbreaks using local weather and regional climate for a tropical environment in Colombia. Am. J. Trop. Med. Hyg. 2014, 3, 598–610. [Google Scholar]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.W.; Macklin, M.G.; Thomas, C.J. Hydrological and geomorphological controls of malaria transmission. Earth Sci. Rev. 2013, 116, 109–127. [Google Scholar] [CrossRef]
- Yu, H.L.; Yang, S.J.; Yen, H.J.; Christakos, G.A. Spatiotemporal climate-based model of early Dengue fever warning in southern Taiwan. Stoch. Environ. Res. Risk Assess. 2011, 25, 485–494. [Google Scholar] [CrossRef]
- Kim, K.R.; Kwon, T.H.; Kim, Y.-H.; Koo, H.-J.; Choi, B.-C.; Choi, C.-Y. Restoration of an inner-city stream and its impact on air temperature and humidity based on long-term monitoring data. Adv. Atmos. Sci. 2009, 26, 283–292. [Google Scholar] [CrossRef]
- Patz, J.; Martens, W.; Focks, D.; Jetten, T. Dengue fever epidemic potential as projected by general circulation models of global climate change. Environ. Health Perspect. 1998, 106, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Muttitanon, W.; Kongthong, P.; Kongthong, C.; Yoksan, S.; Nitatpattana, N.; Gonzalez, J.P.; Barbazan, P. Spatial and temporal dynamics of DHF epidemics, Nakhon Pathom Province, Thailand (1997–2001). Dengue Bull. 2004, 28, 35–43. [Google Scholar]
- Smolinski, M.S.; Hamburg, M.A.; Lederberg, J. Addressing the threats: Conclusions and recommendations. In Microbial Threats to Health: Emergence, Detection, and Response; National Academy Press: Washington, DC, USA, 2003; pp. 56–67. [Google Scholar]
- Nazri, C.D.; Hashim, A.; Rodziah, I.; Hassan, A.; Yazid, A. Utilization of geoinformation tools for Dengue control management strategy: A case study in Seberang Prai, Penang Malaysia. Int. J. Remote Sens. Appl. 2013, 3, 11–17. [Google Scholar]
- National Institute of Population Studies (NIPS). Pakistan Demographics and Health Survey 2012–13; NIPS: Islamabad, Pakistan, 2013. [Google Scholar]
- Pakistan Poverty Alleviation Fund (PPAF). Situation Analysis & Baseline Surveys for Poverty Reduction through Rural Development in KP, FATA & Balochistan; PPAF: Islamabad, Pakistan, 2015. [Google Scholar]
- Pakistan Bureau of Statistics. Demographics Indicators-1998 Census. Available online: http://www.pbs.gov.pk/content/demographic-indicators-1998-census (accessed on 16 September 2015).
- Zareen, S.; Mursalin, M.S. Managing dengue fever by using the one health approach and electronic surveillance. Online J. Public Health Inform. 2015, 1, 209–218. [Google Scholar] [CrossRef]
- The News International. Dengue Fever Outbreak Hits Rawalpindi. Available online: http://www.thenews.com.pk/print/60163-dengue-fever-outbreak-hits-rawalpindi (accessed on 3 September 2015).
- DAWN. 245 Dengue Patients Still in Pindi Hospitals. Available online: http://www.dawn.com/news/1212742 (accessed on 16 September 2015).
- The Nation. Dengue Sets Alarm Bells in Rawalpindi. Available online: http://nation.com.pk/islamabad/01-Oct-2015/dengue-sets-alarm-bells-in-rawalpindi (accessed on 16 September 2015).
- IRIN. Pakistan’s Swat Valley Hit by Dengue. Available online: http://www.irinnews.org/report/98900/pakistan-s-swat-valley-hit-by-dengue (accessed on 16 September 2015).
- PMD. Rainfall (mm) statement for the month of December-2015. Available online: http://www.pmd.gov.pk/FFD/index_files/daily/rainfalldec15.htm (accessed on 16 September 2015).
- Vettek, M.; Brink, A.; Donnay, F.; Simonetty, D.; Desclee, B. Land cover change monitoring using Landsat MSS/TM satellite image data over West Africa between 1975 and 1990. Remote Sens. 2014, 6, 658–676. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, R.S. Assessing the Quality of ASTER DEMs for Hydrological Applications. Available online: http://www.ipcbee.com/vol8/9-S030.pdf (accessed on 16 September 2015).
- Blaes, X.; Waldner, F. Review of the existing apps for field data collection. Sigma 2013, 1, 1–8. [Google Scholar]
- Chainey, S.; Tompson, L.; Uhlig, S. The Utility of Hotspot Mapping for Predicting Spatial Patterns of Crime. Secur. J. 2008, 21, 4–28. [Google Scholar]
- Delmelle, E.M.; Zhu, H.; Tang, W.; Casas, I. A web-based geospatial toolkit for the monitoring of Dengue fever. Appl. Geogr. 2014, 52, 144–152. [Google Scholar] [CrossRef]
- Yang, K.; Li, W.; Sun, L.; Huang, Y.; Zhang, J.F.; Wu, F.; De-Rong; Hang; Steinmann, P.; Liang, Y.S. Spatio-temporal analysis to identify determinants of Oncomelania hupensisinfection with Schistosoma japonicumin Jiangsu province, China. Parasit. Vectors 2013, 6, 138–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.; Domschke, C. GIS Methods for Identifying Wintering Marine Bird Hotspots in the U.S. Salish Sea; University of Washington: Seattle, WA, USA, 2014. [Google Scholar]
- Eisen, L.; Eisen, R.J. Using Geographic information systems and decision support systems for the prediction, prevention, and control of vector-borne diseases. Annu. Rev. Entomol. 2011, 56, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Nazri1, C.D.; Rodziah, I.; Hashim, A. Distribution pattern of a Dengue fever outbreak using GIS. J. Environ. Health Res. 2009, 9, 89–96. [Google Scholar]
- Hagenlocher, M.; Delmelle, E.; Casas, I.; Kienberger, S. Assessing socioeconomic vulnerability to Dengue fever in Cali, Colombia: Statistical vs. expert-based modeling. Int. J. Health Geogr. 2013, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wena, T.H.; Linb, M.H.; Fangc, C.T. Population movement and vector-borne diseases transmission: Differentiating spatial-temporal diffusion patterns of commuting and non-commuting dengue cases. Ann. Assoc. Am. Geogr. 2012, 102, 1026–1037. [Google Scholar] [CrossRef]
- Manteghi, G.; Limit, H.B.; Remaz, D. Water bodies an urban microclimate: A review. Mod. Appl. Sci. 2015, 9, 1–12. [Google Scholar] [CrossRef]









| Year | Swat (Population) | Rawalpindi (Population) | Islamabad (Population) |
|---|---|---|---|
| 1998 | 1,257,602 | 3,363,911 | 529,180 |
| 2013 * | 2,137,000 | 4,786,392 | 919,000 |
| Year | Rawalpindi (Cases) | Islamabad (Cases) | Swat (Cases) |
|---|---|---|---|
| 2010 | 0 | 0 | 0 |
| 2011 | 666 | 201 | 0 |
| 2013 | 784 | 180 | 6376 |
| 2014 | 1118 | 254 | 306 |
| Variable | R2 | Adjusted R2 | Distribution |
|---|---|---|---|
| indoor | 0.54 | 0.39 | Normal |
| outdoor | 0.28 | 0.12 | Normal |
| Indoor-outdoor | 0.60 | 0.30 | Normal |
| Number | Raster Layer | Threat Index | |
|---|---|---|---|
| 1 | Parks | Playground/play land | 1 |
| Small park | 1 | ||
| Small park with trees | 2 | ||
| Public park | 3 | ||
| 2 | Urbanization Type | Planned | 1 |
| Unplanned | 3 | ||
| 3 | Houses Types | Independent | 1 |
| Mixed | 2 | ||
| Interconnected | 3 | ||
| 4 | House Density Index | Low | 1 |
| Medium | 2 | ||
| High | 3 | ||
| 5 | Graveyards | Old (High) | 3 |
| Medium (Medium) | 2 | ||
| New (Low) | 1 | ||
| 6 | Water bodies | Nala/River | 2 |
| Pond/stagnant water | 3 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fareed, N.; Ghaffar, A.; Malik, T.S. Spatio-Temporal Extension and Spatial Analyses of Dengue from Rawalpindi, Islamabad and Swat during 2010–2014. Climate 2016, 4, 23. https://doi.org/10.3390/cli4020023
Fareed N, Ghaffar A, Malik TS. Spatio-Temporal Extension and Spatial Analyses of Dengue from Rawalpindi, Islamabad and Swat during 2010–2014. Climate. 2016; 4(2):23. https://doi.org/10.3390/cli4020023
Chicago/Turabian StyleFareed, Nadeem, Abdul Ghaffar, and Tahir S. Malik. 2016. "Spatio-Temporal Extension and Spatial Analyses of Dengue from Rawalpindi, Islamabad and Swat during 2010–2014" Climate 4, no. 2: 23. https://doi.org/10.3390/cli4020023
APA StyleFareed, N., Ghaffar, A., & Malik, T. S. (2016). Spatio-Temporal Extension and Spatial Analyses of Dengue from Rawalpindi, Islamabad and Swat during 2010–2014. Climate, 4(2), 23. https://doi.org/10.3390/cli4020023







