Abstract
A mixture of polyvinyl alcohol (PVA) and hydroxylammoniun nitrate (HAN) forms a gummy solid known as a plastisol, which is ionically conducting. When an electrostatic potential of 200 V DC is applied across the plastisol, it ignites. Combustion ceases upon removal of the applied voltage. The products of PVA + HAN combustion are known to include the molecular gases carbon monoxide, carbon dioxide, water, nitrogen, and hydrogen. When the electric field within the plastisol is spatially uniform, combustion occurs preferentially at the anode. The fact that HAN is an ionic conductor suggests that the mechanism of combustion is electrolytic in origin. Consistent with the preference for combustion at the anode and the known gaseous products, we consider two reaction mechanisms. One involves atomic oxygen as the oxidizing agent at the anode and hydroxyl radical as the oxidizing agent at the cathode. The other involves ozone as the oxidizing agent at the anode and hydrogen peroxide as the oxidizing agent at the cathode. Each mechanism is applied to a scenario where the products are rich in the carbon oxides and to a second scenario where the products are poor in the carbon oxides. In the rich case, the heat of the overall reaction is −808.33 kJ per mole of HAN consumed and the electrical energy is converted to thermal energy with an efficiency of 4.2%. In the poor case, the corresponding figures are −567 kJ per mole of HAN and efficiency is 2.9%. The combustion reactions at the electrodes are uniformly exothermic with the exception of the reaction involving hydrogen peroxide at the cathode. When the products are poor in the carbon oxides, this reaction is actually endothermic.
1. Introduction
Miniaturized thrusters are commonly used with light weight spacecraft for the purpose of altitude and attitude control. For years, hydrazine has served as the standard propellant. Recently, however, the popularity of hydrazine has waned due to its environmental effects, toxicity, and its relatively high vapor pressure, properties which serve to increase its cost of handling and storage [1,2,3,4,5,6].
Aqueous solutions of HAN (hydroxylammonium nitrate, (NH3OH+NO3−)) have been considered as a substitute for hydrazine [2]. Although hydroxyl ammonium nitrate in pure form is an unstable [5], it forms a stable solution with water [3,4,5,6,7,8,9,10,11]. Various chemical mechanisms for understanding the combustion of aqueous solutions of HAN have emphasized the role of heat [3,6,10], catalysis [6], and electrolysis [7,8,9,10,11].
In the case of the electrolytic mechanisms, the proposed reactions have included anodic oxidation of water to produce hydrogen ion and molecular oxygen [8,9,10,11], accompanied by the oxidation and dissolution of the metal electrode itself [9,11]. Suggestions for cathode reactions have included the reduction of the hydroxylammonium cation (NH3OH+) to produce hydroxylamine (NH2OH) and molecular hydrogen [8,9,10,11]. It has been further suggested that this might occur in competition with the reduction of water to produce hydroxide ion and molecular hydrogen [8].
The Digital Solid State Propulsion Corporation (DSSP) of Reno, NV (USA), has prepared a HAN based solid state propellant consisting of a mixture of 84% hydroxyl ammonium nitrate and 14% polyvinyl alcohol (PVA), formula [-CH2CH(OH)-]n, where n is a large integer. Boric acid at the level of 2% is added as a polyvinyl alcohol cross linking agent (U.S. Patent 8317952 B2, 27 November 2012 and U.S. Patent 8617327 B1, 31 December 2013). HAN is an ionic liquid at room temperature. The mixture of PVA + HAN forms a gummy solid known as a plastisol, which is ionically conducting. The molecular structures of PVA and HAN are shown in Figure 1.
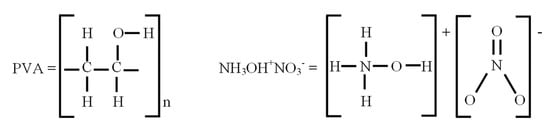
Figure 1.
Molecular structure of polyvinyl alcohol (PVA) on the left and hydroxylamine nitrate (HAN) on the right.
When an external electrostatic potential of 200 V DC is applied across the plastisol, it ignites. Combustion ceases upon removal of the applied voltage. The cessation of combustion before the fuel has been exhausted makes PVA + HAN unique among the solid propellants.
The following aspects of PVA + HAN combustion have been addressed [12,13]:
- (1)
- Upon application of the external voltage, the combustion in PVA + HAN is confined to the electrode surfaces. This observation, plus the fact that HAN is an ionic conductor, strongly suggests that the reactions involved at the electrodes are electrolytic in nature.
- (2)
- If the anode and cathode have the same area, combustion occurs preferentially at the anode. This observation suggests that the oxidizing agent supporting combustion at the anode is stronger than the oxidizing agent supporting combustion at the cathode.
- (3)
- Nonetheless, if the cathode is smaller than the anode, combustion is also observed at the cathode. This suggests a local electric field effect. In particular, the theory of electrostatics predicts that the strength of the electric field in the vicinity of a charged metal electrode increases as the size of the electrode decreases, in which case the value of the field in the vicinity of the electrode may exceed the electric breakdown strength of the plastisol, causing an electric discharge, which enhances the combustion.
- (4)
- The PVA + HAN plastisol fails to ignite in air when exposed to an open flame. This observation suggests that the oxidizing agents supporting combustion at the anode and at the cathode are stronger than molecular oxygen.
- (5)
- The products of PVA + HAN are known to include the molecular gases, carbon monoxide, carbon dioxide, water, hydrogen, and nitrogen. The first two gases necessarily have their origin in PVA. Water and hydrogen can come from either PVA or HAN, whereas nitrogen can have its origin only in HAN.
In the absence of knowledge of the relative amounts of the various products, the stoichiometry of the overall reaction responsible for the production of the combustion gases must remain speculative. Moreover, until some of the reactive intermediates have been identified by spectroscopy or by isotopic labeling, the same uncertainty must be attached to any reaction mechanism that may be proposed in support of the overall combustion reaction.
Nonetheless, in the case of the hydroxyl ammonium ion and nitrate ion in HAN, the oxidation numbers of the atoms, as well as the ionic charges and elemental stoichiometry of the ions, do provide some guidance in the choice of mechanisms. Because HAN is an ionic conductor, application of an external voltage to the propellant will cause the nitrate ion to drift toward the anode and the hydroxylammonium ion to drift toward the cathode. We have proposed that at the anode, the nitrate ion will give up an electron, while at the cathode, the hydroxylammonium ion will gain an electron [12,13]. The transfer of one or more electrons at each electrode is required in order to maintain the electric current in the external circuit. The products of the electrolysis reactions at the two electrodes must be able to support the combustion of PVA. Since combustion is observed preferentially at the anode, the electrolysis product at the anode is presumably a stronger oxidizing agent than the electrolysis product at the cathode.
By experiment, it has been found that 200 V must be applied across the plastisol to cause ignition [12]. If this 200 V drop is assumed to be divided equally between the anode and the cathode, the 100 V applied to each electrode is larger by a factor of at least 50 when compared with the overpotential required to start the typical electrode reaction in aqueous solution. This excess allows us to consider electrode reactions that produce high energy species that are never encountered in aqueous electrochemistry.
Turning now to the consideration of possible electrolysis reactions at the anode, we note first that the nitrogen atom in is in its maximum +5 oxidation state, a state that resists further oxidation. Thus, our attention is drawn to the oxygen atom in the nitrate ion, which is in a −2 oxidation state. It can be oxidized to various higher oxidation state species known to support combustion. Among these are atomic oxygen (oxidation state of oxygen = 0), diatomic oxygen (oxidation state of oxygen = 0), and ozone (oxidation states of the respective oxygen atoms are −1, 0, and +1, averaging to zero). We can rule out diatomic oxygen because PVA + HAN does not ignite upon heating in air. This leaves the two choices: atomic oxygen, which can be produced by the anodic reaction
and ozone, which can be produced by the anodic reaction
In Equations (1) and (2), we have chosen diatomic nitrogen as the other electrolysis product because it is known to be among the ultimate products of the overall PVA + HAN combustion reaction.
At the cathode, the nitrogen atom in the hydroxyl ammonium ion is in a −1 oxidation state, whereas the oxygen atom is in a −2 oxidation state. Further reduction of the oxygen atom is impossible, while further reduction of the nitrogen atom to its −2 or −3 states would require reaction with hydrogen to produce or , which are not among the ultimate products of combustion. Moreover, none of the many known nitrogen oxides are among the ultimate products of combustion. Even if they were produced, they would be unable to support combustion at the cathode because the oxygen atoms that they contain are all in the lowest −2 oxidation state. We thus turn our attention to the hydrogen atom, which is in the +1 state. The hydrogen oxidation state can be reduced to the zero by the formation of diatomic hydrogen. This choice recommends itself because diatomic hydrogen is one of the ultimate products of the overall combustion reaction. On the basis of these considerations, we suggest the reduction reaction at the cathode produces diatomic hydrogen accompanied either by hydroxyl radical according to the reaction
or by hydrogen peroxide according to the reaction
Both hydroxyl radical (oxygen oxidation state = −1) in Equation (3) and hydrogen peroxide (oxygen oxidation state = −1) in Equation (4) are capable of supporting the combustion of PVA. We have chosen diatomic nitrogen as the third product of the cathodic reduction reaction because no other nitrogen containing compound is known to be among the ultimate products of the PVA + HAN combustion.
With these observations at hand, we shall compare the combustion results obtained from two proposed mechanisms: (1) an anodic oxygen + cathodic hydroxyl radical mechanism, where the agent supporting combustion at the anode is atomic oxygen, while the agent supporting combustion at the cathode is hydroxyl radical, and (2) an anodic ozone + cathodic hydrogen peroxide mechanism, where the agent supporting combustion at the anode is ozone, while the agent supporting combustion at the cathode is hydrogen peroxide. The two mechanisms will be applied first to a case that is weakly exothermic and in which the product distribution is rich in diatomic hydrogen, and then for comparison to a second case that is strongly exothermic and in which the product distribution is poor in diatomic hydrogen. With the stoichiometry fixed in each case, it is possible to make valid comparisons of how the overall heat generation is partitioned among the assumed electrolysis, anodic combustion, and cathodic combustion reactions.
These comparisons depend upon a knowledge of the heat contents of the various chemical species involved. These heat contents can be calculated with reference to a table of standard bond energies. As there is no absolute zero for energy, the zero-energy state for the standard bond energy table consists of an atom of each chemical element in complete isolation [14]. Calculation of the heat content of a given species begins with a count of the various types of bonds in a molecule of the species; then by reference to the table of standard bond energies, the heat content of a mole of that species is computed by adding the various bond energies [14]. The results are summarized in Table 1. It should be noted that Table 1 lists the heat contents in the form of , where the heat content of a species, , is understood to be a negative number. The exception is atomic oxygen, which has zero heat content.

Table 1.
Heat contents, −ΔH(X), of the oxidizing agents and the products of combustion, generic designation, “X”, when formed from isolated atoms of the chemical elements. The heat content of atomic oxygen is zero, ΔH(O) = 0.
Perusal of Table 1 indicates that the absolute values of the heat contents of the various product species are in the order . As there is no experimental evidence regarding the prevalence of one of these gases over the others among the products, we are limited in our choice of anodic and cathodic combustion reactions only by the principle of mass conservation. This flexibility allows us to compare the performance of the propellant in both low energy and high energy scenarios. We stress, however, that the total heat released by the propellant depends solely on the energy difference between the initial reactants and the final products and is independent of the mechanism leading from one to the other. This observation rests upon the fact that the enthalpy is a thermodynamic state function.
2. Case A: A Low Energy Scenario Where the Product Distribution Is Rich in Molecular Hydrogen
2.1. Chemistry of the Overall Reaction
We have chosen the stoichiometric equation for the overall reaction in this case to be
where the stoichiometric coefficients are all integers, and their values favor the production of the low energy product, . According to Equation (5), the mole percentages of the various products in the effluent gas stream are: (5.9%), (29.4%), (15.7%), (9.8%), and (39.2%). The high energy components, which are those other than hydrogen, account for 60.8% of the total. Hydrogen accounts for 39.2%.
As will be shown below, the stoichiometry in Equation (5) is validated by forming a linear combination of the stoichiometries of the overall electrolysis reaction, the anodic combustion reaction, and the cathodic combustion reaction. The stoichiometric coefficients in Equation (5) have the smallest magnitudes consistent with the absence of a common factor.
2.2. Anodic Oxygen + Cathodic Hydroxyl Radical Mechanism
2.2.1. Electrolysis Reaction
In this section, we reprise our previously introduced anodic oxygen + cathodic hydroxyl radical mechanism [12,13]. In order to account for the observed preferential combustion at the anode, we assume as in Equation (1) that the oxidizing agent supporting combustion at the anode is atomic oxygen, . At the cathode, the oxidizing agent supporting combustion is assumed to be the hydroxyl radical, , as mentioned in Equation (3). In electrolysis, the ion of the HAN electrolyte drifts toward the anode, where it gives up an electron to the electrode and gets oxidized according to Equation (1), which for the sake of completeness we copy below:
The ion of the HAN electrolyte drifts toward the cathode, where it picks up an electron from the electrode and gets reduced according to Equation (3), which we repeat below:
The sum of Equations (6) and (7) determines the stoichiometry of the overall electrolysis reaction, namely,
The enthalpy change, , associated with the electrolytic reaction in Equation (8) is
where the data assembled in Table 1 have been used to evaluate the heat contents of the various species involved. As two moles of HAN are consumed by the reaction in Equation (8), the enthalpy in Equation (9) can be placed upon a molar basis by forming the ratio
Notice that this molar heat, , is positive, which means that the electrolysis process consumes energy from the external voltage source.
2.2.2. Combustion at the Anode Produces
A monomeric unit of the PVA polymer has the stoichiometric formula . The oxygen atom produced by the electrolytic oxidation in Equation (6) is capable of supporting the combustion of PVA at the anode. The balanced anodic combustion equation consistent with the proposed products is
The enthalpy change, , associated with the anodic reaction in Equation (11) can be calculated as
The consumption of 15 moles of atomic oxygen in Equation (11) is responsible for the heat computed in Equation (12). According to Equation (8), two moles of HAN produce six moles of atomic oxygen. Hence, the anodic combustion heat released per mole of HAN consumed is given by the ratio
2.2.3. Combustion at the Cathode Produces
Among the products in Equation (7), the hydroxyl radical is responsible for combustion of PVA at the cathode. The balanced cathodic combustion equation consistent with the proposed products is
The enthalpy change, , associated with this reaction is
According to Equation (14), the heat calculated in Equation (15) results from the consumption of two moles of hydroxyl radical. Equation (8) indicates that two moles of hydroxyl radical are produced by the electrolysis of two moles of HAN. The heat released at the cathode by PVA combustion per mole of HAN is thus given by
2.2.4. Overall Reaction Heat
The stoichiometry of the overall reaction of PVA with HAN in Equation (5) is reproduced by forming the linear combination, (5/2) Equation (8) + Equation (11) + (5/2) Equation (14). Per mole of HAN, the heat released by the reaction in Equation (5) can be evaluated by adding the heats calculated in Equations (10), (13) and (16). The result is
As can be seen from this result and Table 2, more than half the heat released by combustion is required just to maintain electrolysis. Although the electrolysis reaction is quite endothermic, the combined exothermicity of the anodic and cathodic combustion reactions more than compensates, making the overall reaction exothermic as is necessary if PVA + HAN is to serve as a propellant. The stoichiometry in Equation (8) produces and . The stoichiometries in Equations (11) and (14) combine to produce the remaining gases, , , , plus additional . Thus, the three reactions account for all of the known gaseous products.
2.3. The Anodic Ozone + Cathodic Hydrogen Peroxide Mechanism
2.3.1. Electrolysis Reaction
So far, it has been assumed that the applied voltage is sufficient to release atomic oxygen at the anode and hydroxyl radical at the cathode. It may very well be that the anodic and cathodic electrolytic reactions produce less reactive species, however. To examine the effects of this possibility, we will assume, as an example, that the oxidizing species produced by electrolysis at the anode is ozone, , while the oxidizing species produced by electrolysis at the cathode is hydrogen peroxide, .
The electrolysis half reaction at the anode is that in Equation (2), which we repeat below:
The electrolysis half reaction at the cathode is assumed to be that in Equation (4), which is
The sum of Equations (18) and (19) gives the overall electrolysis reaction in the form
The heat associated with the reaction in Equation (20) is given by
According to Equation (20), this heat is associated with the electrolysis of two moles of HAN. Hence, per mole of HAN the electrolysis enthalpy is
which represents a substantial reduction in the electrolytic endothermicity when compared with that predicted by Equation (10).
2.3.2. Combustion at the Anode Produces
For the purposes of comparison, we will continue to assume as in Section 2.2.2 above that the combustion of PVA at the anode produces , , and . The balanced anodic combustion equation consistent with the proposed products is
The heat released by this reaction is
The heat calculated in Equation (24) is released upon consumption of five moles of ozone. According to Equation (20), two moles of ozone are produced upon electrolysis of two moles of HAN. Hence, the molar enthalpy of combustion at the anode is
2.3.3. Combustion at the Cathode Produces
For the purposes of comparison, we will continue to assume as in Section 2.2.3 above that combustion at the cathode produces and . The balanced cathodic combustion equation consistent with the proposed products is
The heat associated with this reaction is
According to Equation (26), the heat calculated in Equation (27) requires the consumption of one mole of hydrogen peroxide. According to Equation (20), the production of one mole of hydrogen peroxide is associated with the consumption of two moles of HAN. Hence,
The results calculated in Equations (27) and (28) are endothermic! As such, the cathode reaction does not represent combustion. This extreme result provides a reason why combustion is observed preferentially at the anode. If combustion is to be observed at the cathode, it must be due to some competing process such as electrical discharge [13].
2.3.4. Overall Reaction Heat
The stoichiometry of the overall reaction in Equation (5) is obtained by forming the linear combination of Equation (20) + Equation (23) + Equation (26). The heat associated with this overall reaction is calculated as the sum of Equations (22), (25) and (28). Hence,
Because the two mechanisms considered in Section 2.1 and Section 2.2 result in the same overall stoichiometry, the heats calculated in Equations (17) and (29) agree. The results calculated in Section 2 are summarized in Table 2.
3. Case B: A High Energy Scenario Where the Product Distribution Is Poor in Molecular Hydrogen
3.1. Chemistry of the Overall Reaction
We have chosen the stoichiometric equation for the overall reaction in this case to be
where the stoichiometric coefficients have been chosen in order to favor the production of the higher energy products , , , and . As will be shown below, the stoichiometry in Equation (30) is obtained by forming a linear combination of the stoichiometries of the overall electrolysis reaction, the anodic combustion reaction, and the cathodic combustion reaction. The stoichiometric coefficients in Equation (30) have the smallest integer magnitudes consistent with the absence of a common factor.
The mole percentages of the various gaseous products are: (19.8%), (5.5%) (33.5%), (16.5%), and (24.7%). The high energy components, which are those other than hydrogen, account for 75.3% of the total. The low energy product, hydrogen, accounts for just 24.7%.
3.2. The Anodic Oxygen + Cathodic Hydroxyl Radical Mechanism
3.2.1. Electrolysis Reaction
The electrolysis reaction in this mechanism is taken to be the same as in Equation (8).
3.2.2. Combustion at the Anode Produces and
As in Section 2.2.2, atomic oxygen supports combustion at the anode. Now, however, the products of combustion are assumed to be and . The balanced anodic combustion equation consistent with the proposed products is
The heat released in this reaction is
By reference to Equations (8) and (31), the heat released per mole of HAN by combustion of PVA at the anode is
3.2.3. Combustion at the Cathode Produces and
As before, the hydroxyl radical supports combustion at the cathode. This time, however, the products of combustion are assumed to be and . The balanced cathodic combustion equation consistent with the proposed products is
The heat released in this reaction is
According to the stoichiometry in Equations (8) and (34), the heat released per mole of HAN due to combustion at the cathode is
3.2.4. Overall Reaction Heat
The stoichiometry of the overall reaction in Equation (30) is obtained by making the linear combination 15 Equation (8) + 18 Equation (31) + 5 Equation (34). The heat released by the reaction in Equation (30) per mole of HAN is given by the sum of the heats calculated in Equations (10), (33) and (36). The result is
which is summarized in Table 2.
3.3. The Anodic Ozone + Cathodic Hydrogen Peroxide Mechanism
3.3.1. Electrolysis Reaction
The electrolysis reaction remains the same as described in Equation (20).
3.3.2. Combustion at the Anode Produces and
In this mechanism, the oxidizing agent at the anode is ozone. The balanced anodic combustion equation consistent with the proposed products is
The heat associated with this reaction is
By virtue of Equations (20) and (38), the heat released by combustion at anode per mole of HAN consumed is
3.3.3. Combustion at the Cathode Produces and
The oxidizing agent at the cathode is hydrogen peroxide. The balanced cathodic combustion equation consistent with the proposed products is
The heat associated with this reaction is
By virtue of Equations (20) and (41) the heat released per mole of HAN due to combustion at the cathode is
3.3.4. Overall Reaction Heat
The stoichiometry of the overall reaction in Equation (30) is obtained by forming the linear combination of Equation (20) + Equation (38) + Equation (41). The heat associated with this overall reaction is the sum of Equations (22), (40) and (43). The result is
Since Equation (30) was adopted as the overall stoichiometry in Section 3.2 and Section 3.3, the enthalpies calculated in Equations (37) and (44) also coincide. The results are summarized in Table 2.
4. Performance Characteristics
4.1. Energy Efficiency
According to the reactions in Equations (6) and (7) as well as Equations (18) and (19), the consumption of one mole of HAN by electrolysis requires the passage of one mole of electrons or what is the same thing, one Faraday, , of charge. Thus, a current of 96,485 C/mol HAN per second = 96,485 A/mol HAN produces a chemical power of W/mol HAN. The ratio of the absolute value of the chemical power produced to the electric current consumed in electrolysis can be expressed in the units of Watts per Amp and serves to define the electrolytic conversion fraction, , where
The total combustion heat data calculated in Section 2 and Section 3 and summarized in Table 2 are rendered in the form of in Table 3. The energy efficiency of the propellant can be expressed as the ratio of the total power produced by combustion to the total power delivered by the external voltage. Since the applied voltage is 200 V, and the Volt equals a Watt per Ampere, the overall electrical efficiency, , as a percentage can be written as

Table 3.
Electrical conversion factor, fe; overall energy efficiency, E; and specific mass flow rate, dm/dt. Last column gives the mole percent hydrogen for the corresponding case.
The electrical efficiency, , has been calculated for the corresponding values of and is listed in Table 3.
4.2. Mass Transfer Rate
The catalog of gases released with their stoichiometric formulas and approximate formula weights in parentheses are as follows: , , , , and . Let the numbers of moles of these gases released by one of the overall combustion reactions be: , , , , and , respectively. If the release of these mole numbers requires the consumption of moles of HAN, and if Coulombs of charge must be passed to produce one mole of HAN, then the specific mass flow rate, , in g/s per Ampere of current can be defined by
For example, in the case of Equation (5), , , , , , , and . These mole numbers, as well as those corresponding to Equation (30), are summarized in Table 4. The mass flow rates associated with the scenarios considered in Section 2 and Section 3, respectively, are summarized in Table 3.

Table 4.
Product mole numbers and HAN mole numbers for Case A (see Equation (5)) and Case B (see Equation (30)). The last column gives the mole % hydrogen consistent with the mole numbers of products.
5. Discussion
On the basis of the entries for in Table 2, it is clear that the electrolysis mechanism producing oxidizing species is more endothermic than the electrolysis mechanism producing oxidizing species . For a given mechanism, however, the heats of combustion at the corresponding electrodes, and , are always more exothermic in Case B (high energy products) as compared with Case A (low energy products). Indeed, in Case A, the enthalpy of cathodic combustion, , when is the oxidizing agent, is endothermic, thus ruling out the possibility of combustion altogether. Should combustion be observed experimentally at the cathode in Case A with as the oxidizing agent, the reaction at the cathode will have to be supplemented by some non-electrolytic mechanism, such as electric discharge [13].
According to Table 2, the overall reaction enthalpy, , is always more exothermic in Case B (rich in high energy products) than in Case A (poor in high energy products). As a consequence, the values of the electrolytic conversion factor, , and the energy efficiency, , shown in Table 3 are larger in Case B than in Case A. By contrast, the reverse conclusion holds when considering the specific mass transfer rate, , which is larger in Case A as compared with Case B. This is a consequence of how HAN functions to convert the mass of PVA into gaseous products. By reference to Table 4, one can multiply the mole numbers in the first line by the corresponding molar masses to find that in Case A, 175.2 g of products are carried off per mole of HAN consumed. By contrast, the same calculation applied to the second line reveals that in Case B, 129.7 g are carried off per mole of HAN consumed.
Chemical reactions involving gases and solids are known to be caused by heat [15], electrolysis [16], electric discharge plasmas [17,18], and light absorption [19]. Glasscock et al. [20] point out that PVA + HAN can burn under three different scenarios:
- (1)
- Upon application of a steady eternal potential. This is termed the pyroelectric mode [20]. Above, we have made the case that this mode involves a combination of electrolytic [16] and thermal reactions [15].
- (2)
- Upon application of an electric discharge to the surface of the plastisol. This is termed the ablation fed plasma mode [20]. This mode presumably involves a combination of plasma chemical reactions [17,18] and thermal [15] reactions.
- (3)
- Under sufficient external pressure, the pyroelectric mode becomes self-sustaining. This is termed the continuous decomposition mode [20]. This mode is presumably thermal in nature [15].
It should be remarked, however, that when a flame exists, there arises the possibility of photochemical reactions [19]. All three of the scenarios mentioned above may in fact be supplemented in part by photochemical processes.
Glasscock et al. [20] have performed extensive statistical thermodynamic calculations of the distribution of products to be expected upon heating a mixture of nitric acid, hydroxylamine, acetaldehyde, and ammonium chloride—components chosen to model the elemental composition of the DSSP formulation of PVA + HAN. Their mechanism begins with the dissociation of hydroxyl ammonium nitrate into hydroxylamine and nitric acid. This reaction can be stimulated, for example, by the heat delivered to the plastisol in the ablation fed plasma mode. As a model for PVA, they adopt acetaldehyde , a substance that has the same elemental composition as PVA. They include ammonium nitrate because it is known to be a minor component of the DSSP formulation. In summary, their starting mixture consists of , , , and .
In the electrochemical mechanism discussed above, electrolysis takes the place of plasma ablation in establishing the composition of the starting mixture. The starting mixture consists of PVA plus electrolysis products, which in the anodic oxygen + cathodic hydroxyl mechanism are , , , and , and which in the anodic ozone + cathodic hydrogen peroxide mechanism are , , , and . In both cases, PVA is modeled by the diradical [See Figure 1].
In the calculations of Glasscock et al. [20], the composition of the mixture at an operating temperature of 700 K is found by minimizing the Gibbs free energy, which presumably corresponds to chemical equilibrium. In the electrochemical model discussed above, the system is considered to be in steady state with electric power flowing in and heat and mass flowing out. The calculated quantities are referred to the standard thermodynamic reference temperature at 298 K. To compare the values of these quantities to the values appropriate to an operating temperature of 700 K, it would be necessary to calculate the difference in the heat required to warm the reactants from 298 K to 700 K and then cool the products from 700 K to 298 K. As a consequence, our estimates of and will differ from those appropriate to the actual operating temperature of the propellant.
6. Conclusions
The principal conclusions that can be drawn from the electrolytic model are the following:
- (1)
- The proposed mechanisms account for the appearance of carbon monoxide, carbon dioxide, water, molecular nitrogen, and molecular hydrogen among the products.
- (2)
- The heat of oxidation of PVA at the cathode can be endothermic. According to Table 2, this occurs in Case A, where the cathodic oxidizing species, , is weak and the products of combustion are poor in the oxides.
- (3)
Author Contributions
The thermochemical calculations are the work of the first author. The funding for this research was obtained by the second author. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Propulsion Research Center of the University of Alabama in Huntsville, Huntsville, AL, USA.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank the University of Alabama in Huntsville for support of this resarch.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PVA | polyvinyl alcohol |
| HAN | hydroxylammonium nitrate |
Nomenclauture
| specific mass transfer rate | |
| electrical efficiency | |
| electrolytic conversion fraction | |
| number of moles of species “X” | |
| heat content of species “X” | |
| electrolytic enthalpy | |
| heat of combustion at the anode | |
| heat of combustion at the cathode | |
| total reaction enthalpy | |
| electrolytic enthalpy per mole of HAN | |
| anodic combustion enthalpy per mole of HAN | |
| cathodic combustion enthalpy per mole of HAN | |
| total reaction enthalpy per mole of HAN |
References
- Yetter, R.A.; Yang, V.; Wu, M.-H.; Wang, Y.; Milius, D.; Aksay, I.A.; Dryer, F.L. Combustion Issues and Approaches for Chemical Microthrusters. Int. J. Energ. Mater. 2007, 6, 393–424. [Google Scholar] [CrossRef]
- Gohardani, A.S.; Stanojev, J.; Demaire, A.; Anflo, K.; Persson, M.; Wignborg, N.; Nilsson, C. Green Space Propulsion: Opportunities and Prospects. Prog. Aerosp. Sci. 2014, 71, 128–149. [Google Scholar] [CrossRef]
- Amroussee, R.; Katsimi, P.; Itouyama, N.; Azuma, N.; Kagawa, H.; Hatai, K.; Ikeda, H.; Hori, K. New HAN-Based Mixtures for Reaction Control System and Low Toxic Spacecraft Propulsion System: Thermal Decomposition and Possible Thruster Applications. Combust. Flame 2015, 162, 2686–2692. [Google Scholar] [CrossRef]
- Amroussee, R.; Katsumi, T.; Azuma, N.; Hori, K. Hydroxylammonium Nitrate (HAN)—Based Green Propellant as Alternative Energy Resource for Potential Hydrazine Substitution: From Lab Scale to Pilot Plant Scale-Up. Combust. Flame 2017, 176, 334–348. [Google Scholar] [CrossRef]
- Kidd, F.G., III; Taylor, N.R.; Lemmer, K.M. Decomposition of Hydroxylammonium Nitrate in a Low Pressure Flowing Capillary System. J. Mol. Liq. 2018, 262, 396–404. [Google Scholar] [CrossRef]
- Amrousee, R.; Hori, K.; Fetimi, W.; Farhat, K. HAN and AND as Liquid Ionic Monopropellants: Thermal and Catalytic Decomposition Processes. Appl. Catal. B Environ. 2012, 127, 121–128. [Google Scholar] [CrossRef]
- Risha, G.A.; Yetter, R.A.; Yang, V. Electrolytic-Induced Decomposition and Ignition of HAN-Based Liquid Monopropellants. Int. J. Energetic Mater. Chem. Propuls. 2007, 6, 575–588. [Google Scholar] [CrossRef]
- Wu, M.-H.; Yetter, R.A. A Novel Electrolytic Ignition Monopropellant Microthruster Based on Low Temperature Co-Fired Ceramic Tape Technology. Lab Chip 2009, 9, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.S.; Chin, J.; Chik, T.F.W.K. Role of Electrodes in Ambient Electrolytic Decomposition of Hydroxylammonium Nitrate (HAN) Solutions. Propuls. Power Res. 2013, 2, 194–200. [Google Scholar] [CrossRef]
- Khare, P.; Yang, V.; Meng, H.; Risha, G.A.; Yetter, R.A. Thermal and Electrolytic Decomposition and Ignition of HAN—Water Solutions. Combust. Sci. Technol. 2015, 187, 1065–1078. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheah, K.H.; Koh, K.S.; Chin, J.; Chik, T.F.W.K. Parametric Studies of Electrolytic Decomposition of Hydroxylammonium Nitrate (HAN) Energetic Ionic Liquid in Microractor Using Image Processing Technique. Chem. Eng. J. 2016, 296, 19–27. [Google Scholar] [CrossRef]
- Baird, J.K.; Lang, J.R.; Hiatt, A.T.; Frederick, R.A., Jr. Electrolytic Combustion in the Polyvinyl Alcohol + Hydroxylammonium Nitrate Solid Propellant. J. Propuls. Power 2017, 33, 1589–1590. [Google Scholar] [CrossRef]
- Baird, J.K.; Huang, S.; Frederick, R.A., Jr. Space Charge Limited Conduction in Polyvinyl Alcohol + Hydroxylammonium Nitrate Solid Propellant. J. Propuls. Power 2020, 36, 479–484. [Google Scholar] [CrossRef]
- Birk, J.P. Chemistry; Houghton Mifflin, Co.: Palo Alto, CA, USA, 1994; p. 292. [Google Scholar]
- Pilling, M.J.; Smith, I.W.M. (Eds.) Modern Gas Kinetics: Theory Experiment and Application; Blackwell Scientific Publishers: Oxford, UK, 1987. [Google Scholar]
- Bruce, P.G. (Ed.) Solid State Electrochemistry; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Baird, J.K.; Lee, N.; Miller, G. The G-Value in Plasma and Radiation Chemistry. J. Appl. Phys. 1990, 68, 3661–3668. [Google Scholar] [CrossRef]
- Miller, G.P.; Baird, J.K. Radiofrequency Plasma Decomposition of Ammonia: A Comparison with Radiation Chemistry Using the G-Value. J. Phys. Chem. 1993, 97, 10984–10988. [Google Scholar] [CrossRef]
- Wayne, R.P. Principles and Applications of Photochemistry; Oxford Science Publications: Oxford, UK, 1988. [Google Scholar]
- Glasscock, M.S.; Drew, P.D.; Rovey, J.L.; Polzin, K.A. Thermodynamic Properties of Hydroxylammonium Nitrate—Based Electric Solid Propellants. J. Thermodyn. Heat Transf. 2020, 34, 522–529. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).