Experimental and Numerical Investigation on Heat Transfer Performance of Water Evaporators with Different Channels and Fin Structures in a Sub-Atmosphere Environment
Abstract
:1. Introduction
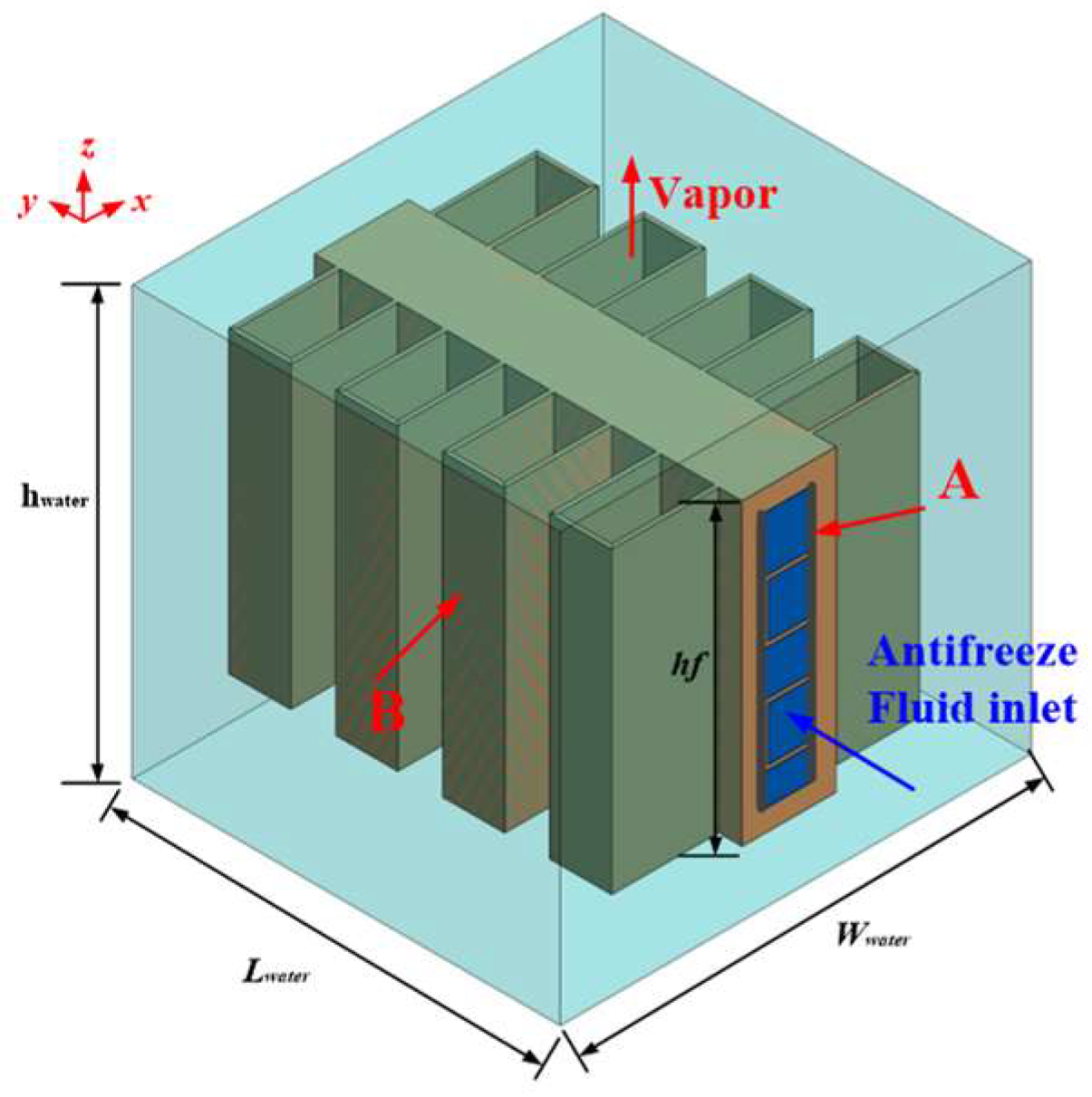
2. Physical Model of Water Evaporators
2.1. Structure Design
2.2. Internal Channels and External Fins
Internal Channels
2.3. External Fins
2.4. Configuration of Water Evaporators
3. Numerical Model
3.1. Governing Equations of Boiling Water Side
- (1)
- Volume fraction
- (2)
- Momentum equation
- (3)
- Energy equation
- (4)
- Rohsenow boiling model
3.2. Governing Equations of Antifreeze Side
- (1)
- Continuity equation for antifreeze region:where, is the velocity vector.
- (2)
- Momentum equation for antifreeze region:where μ is dynamic viscosity, Pa·s; ρ is antifreeze density, kg/m3; p is the pressure, MPa.
- (3)
- Energy equation for antifreeze regionwhere T is the temperature of antifreeze, K; is the thermal conductivity of antifreeze, and W/(m·K); is the specific heat of antifreeze, J/(kg·K).
3.3. Boundary Conditions and Initial Conditions
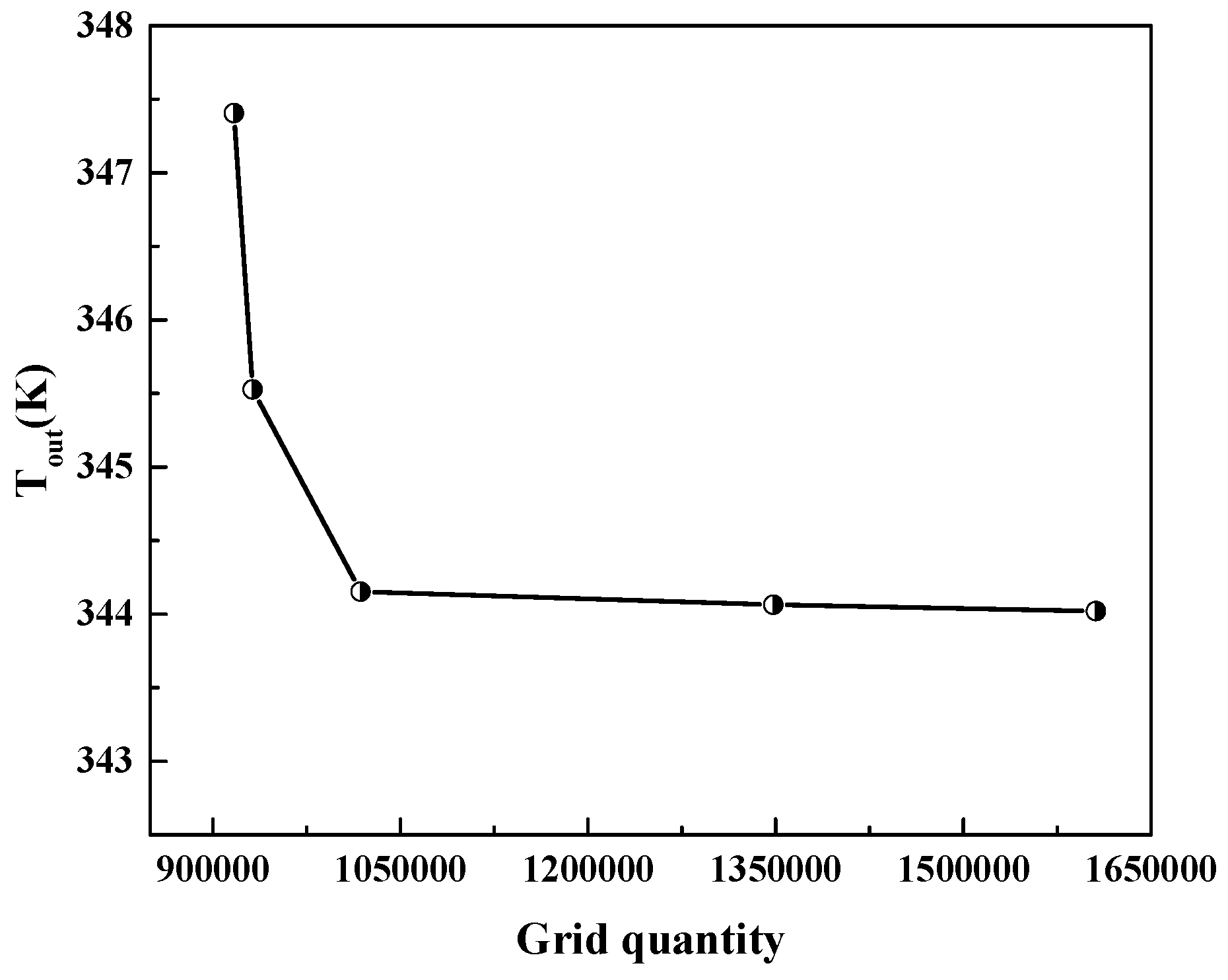
3.4. Grid Independence
4. Experimental Methodology
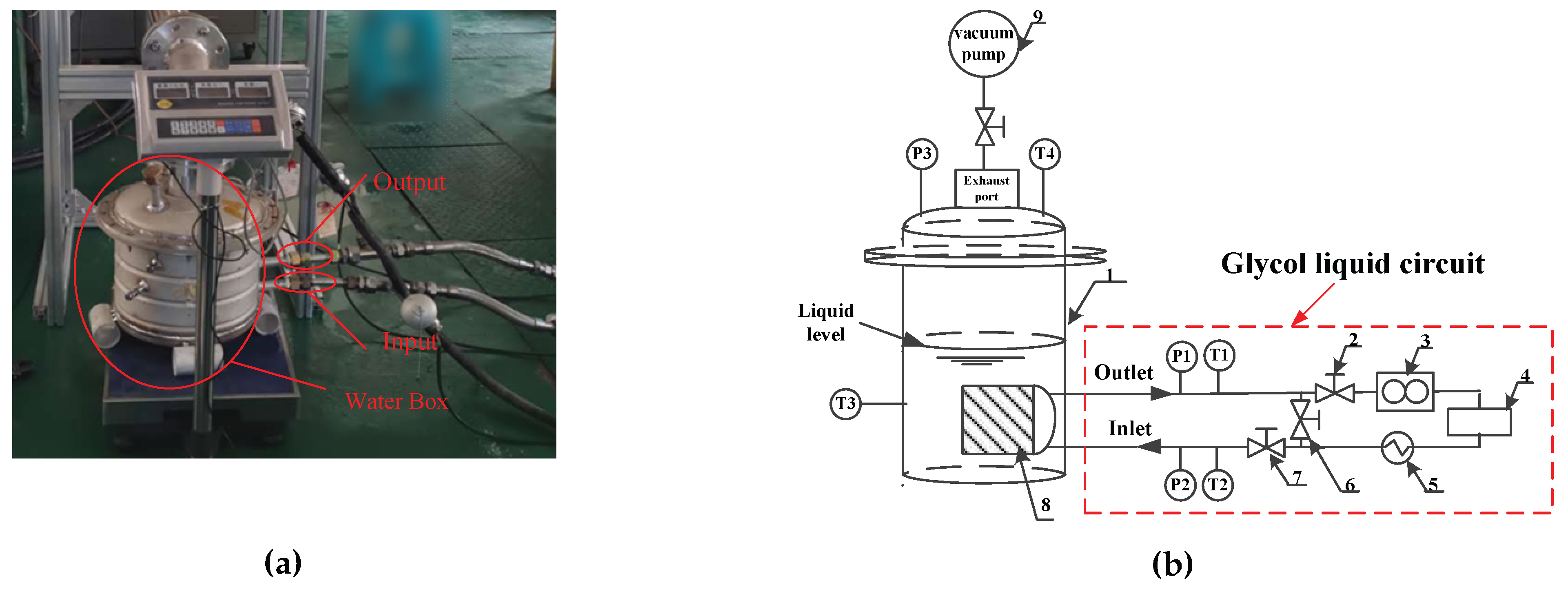
4.1. Experimental Procedure
4.2. Experimental Uncertainty Analysis
4.3. Experimental Validation
5. Analysis of Simulation Results
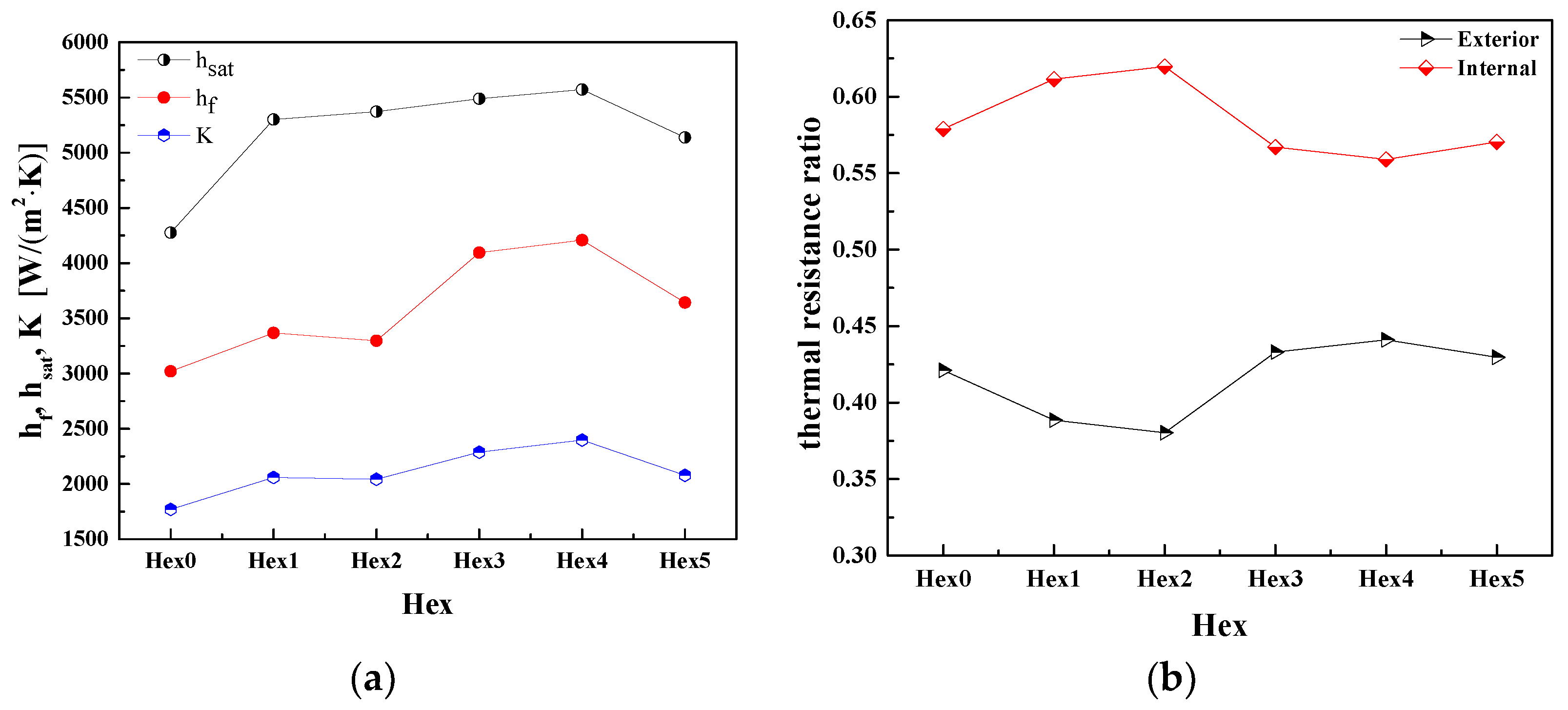
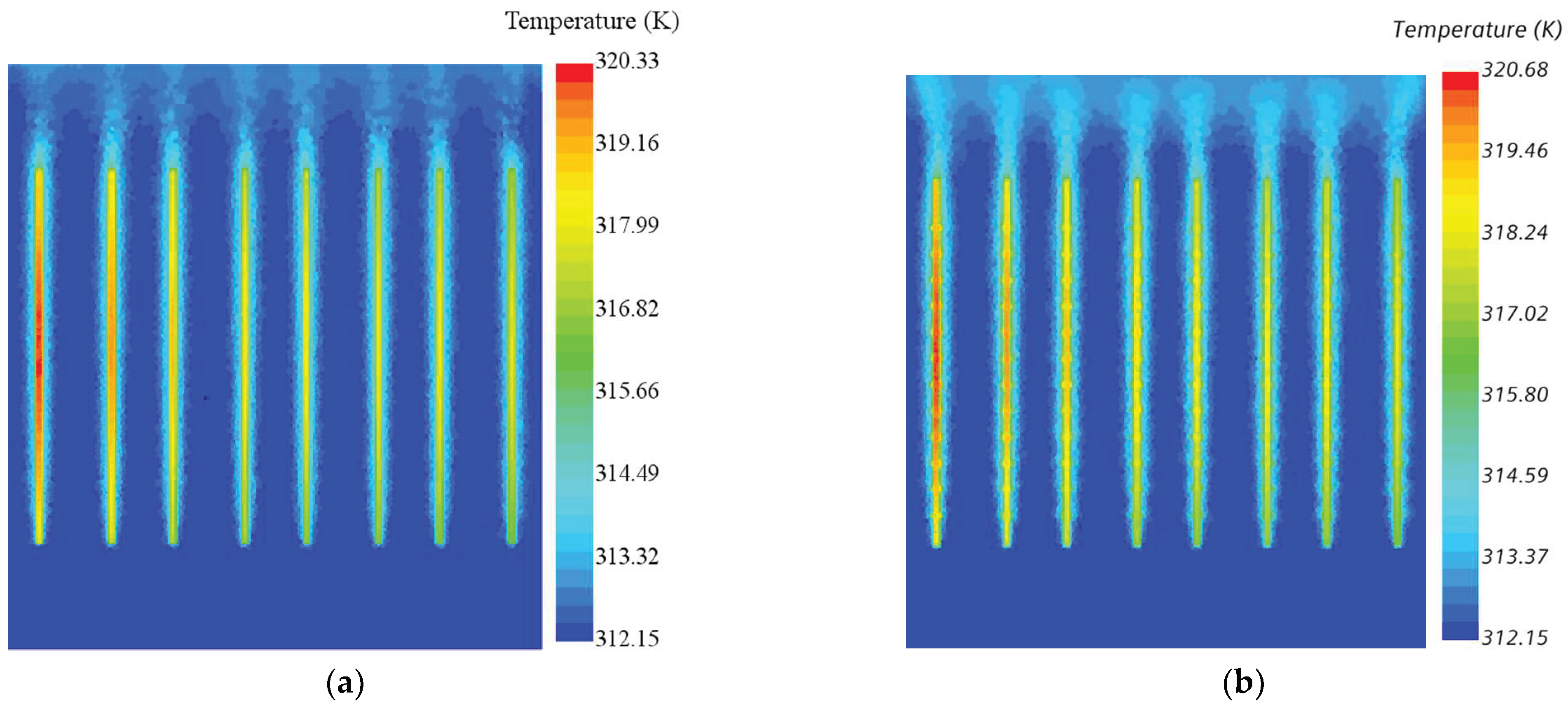
5.1. Heat Transfer Coefficient and Thermal Resistance
5.2. Velocity Distribution of Antifreeze Side
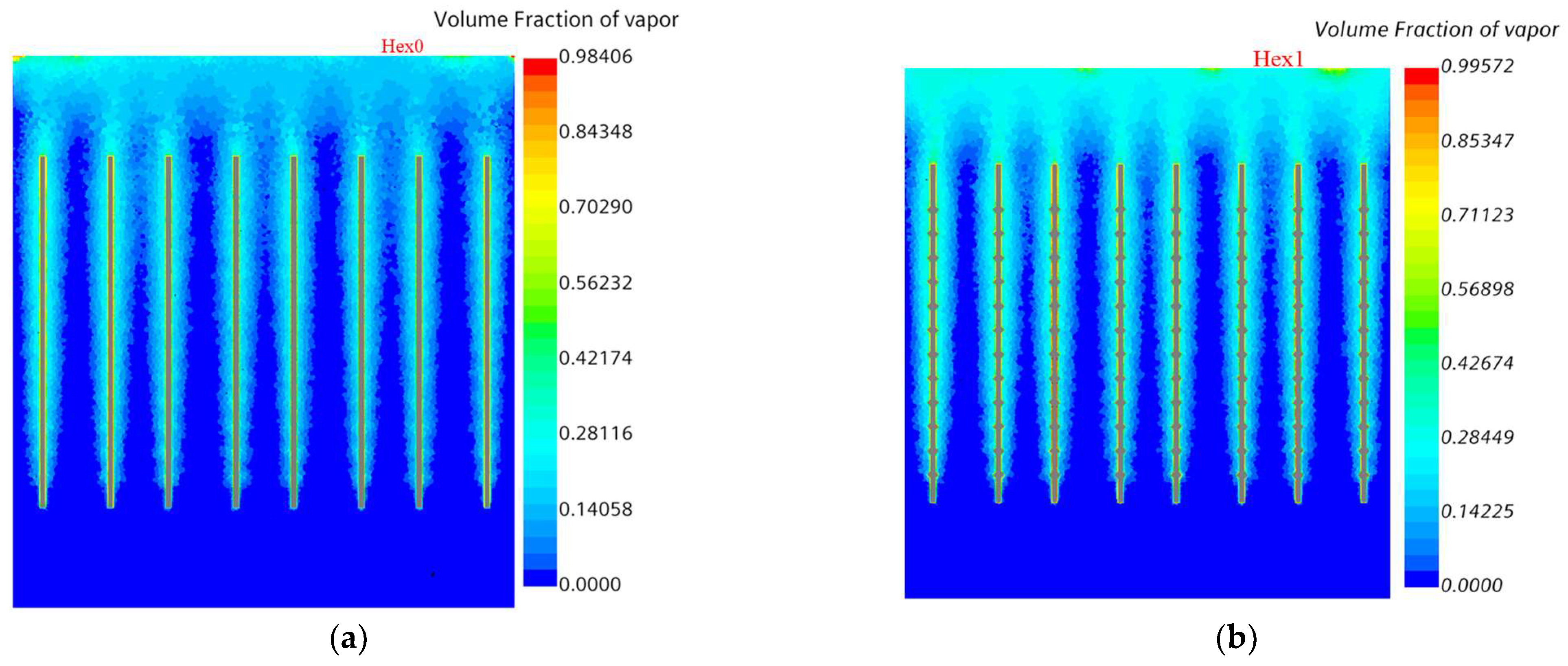
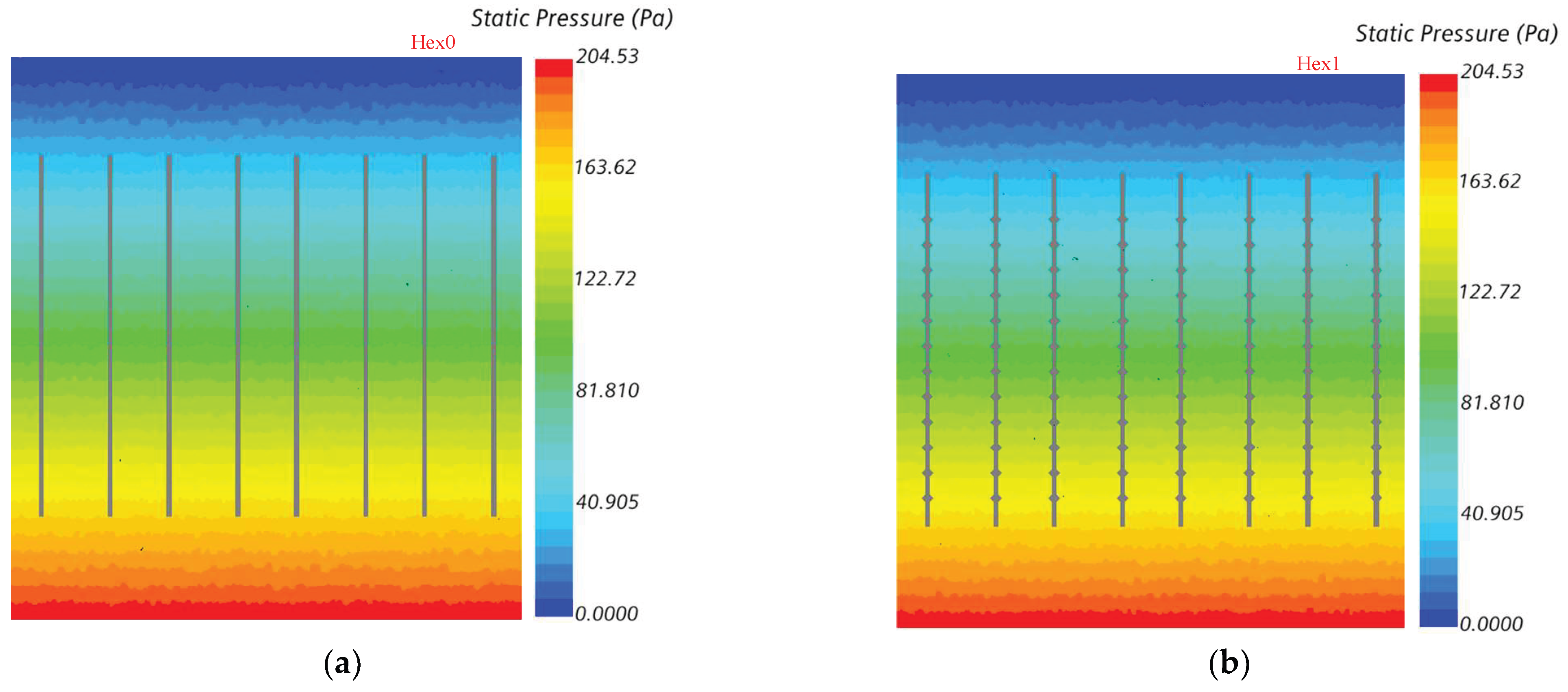
5.3. Volume Fraction and Static Pressure at Boiling Water Side
5.4. Optimal Combination Structure for Water Evaporator
6. Conclusions
- (1)
- The comparison of experimental and simulation results shows that the built numerical models can satisfy the accuracy requirements of sub-atmospheric boiling study in the water evaporator.
- (2)
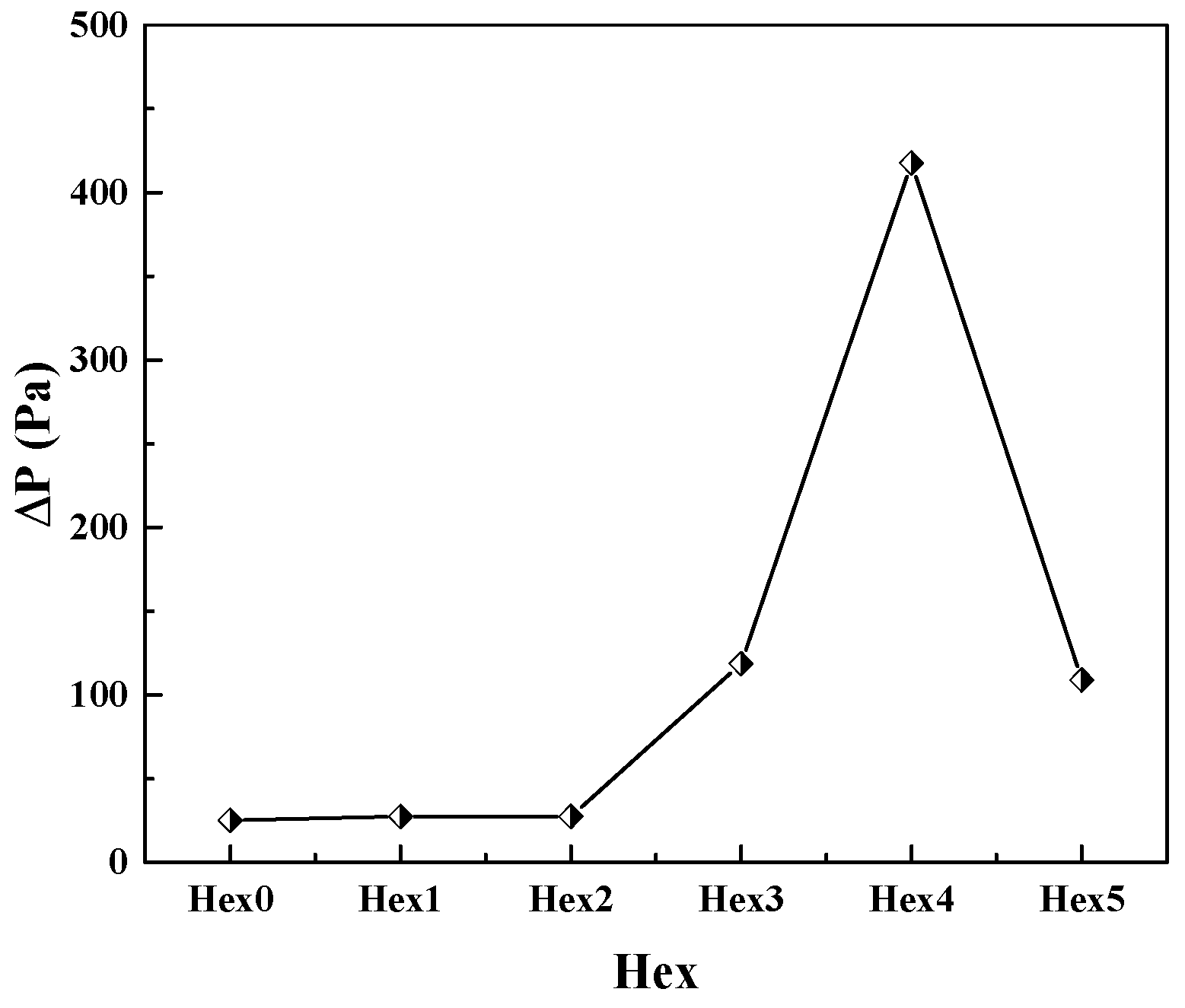
- The heat transfer performances of five internal channel structures are compared. It is concluded that the change of channel continuity has little effect on the heat transfer performance. The height reduction of the channel wave is beneficial for improving the heat exchange performance, but it will increase the pressure drop accordingly. The pressure drop increases about two times with a height reduction of one half. The change of the internal channel shape can lead to a change in the heat exchange characteristics.
- (3)
- The heat transfer performances of the two external fin structures (B1 and B2) are analyzed. It is concluded that adding ribs to the fins is conducive to enhancing the heat transfer performance, which can improve the boiling heat transfer performance by about 13.31%. Meanwhile, the influence of water static pressure cannot be ignored in study of heat transfer performance in a sub-atmospheric environment.
- (4)
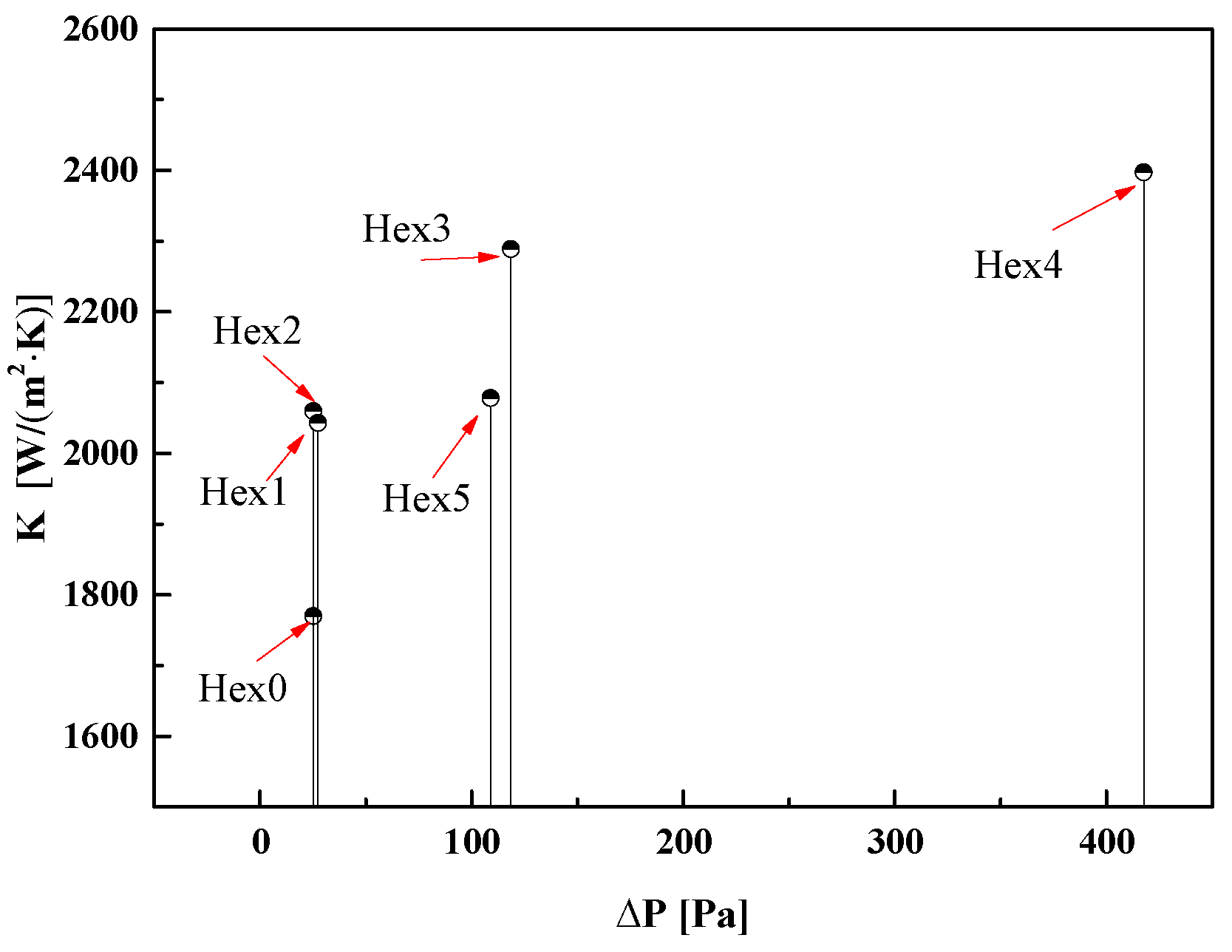
- Comparing the heat transfer coefficients of different structural water evaporators, the heat transfer coefficient of Hex4 is 1.35 times, 1.16 times, 1.17 times and 1.15 times larger than the ones of Hex0, Hex1, Hex2 and Hex5, respectively. For the aircraft, the best design of a water evaporator is Hex4.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | heat exchange area, m2 |
| Cpl | specific heat of liquid phase, J/(kg·K) |
| Cqw | empirical coefficient |
| E | specific energy, J/kg |
| F | momentum source term |
| g | gravity, m/s2 |
| h | height, mm |
| hf | convective heat transfer coefficient, W/(m2·K) |
| hsat | boiling heat transfer coefficient, W/(m2·K) |
| hw | height of water evaporator, mm |
| hwater | height of simulated water, mm |
| K | heat transfer coefficient, W/(m2·K) |
| keff | thermal conductivity, W/(m·K) |
| l | dispersion, mm |
| l1 | wave distance, mm |
| lc | length water evaporator |
| lwater | length of water region |
| mass flow rate, kg/s | |
| np | Prandtl index |
| p | pressure, MPa |
| Prl | Prandtl number |
| q | heat flux, W/m2 |
| Q | heat, W |
| Sαi | source phase, kg/(m3) |
| Sh | heat source, J/(m3) |
| T | temperature, K |
| W | width, mm |
| Greek Symbols | |
| λ | thermal conductivity, W/(m·K) |
| μ | dynamic viscosity, Pa·s |
| α | volume fraction of phase |
| ρ | density, kg/m3 |
| δ | thickness, mm |
References
- Glass, D. Ceramic Matrix Composite (CMC) Thermal Protection Systems (TPS) and Hot Structures for Hypersonic Vehicles. In Proceedings of the 15th AIAA International Space Planes and Hypersonic Systems and Technologies Conference, Dayton, OH, USA, 28 April–1 May 2008. [Google Scholar]
- Jackson, T.A.; Eklund, D.R.; Fink, A.J. High speed propulsion: Performance advantage of advanced materials. J. Mater. Sci. 2004, 39, 5905–5913. [Google Scholar] [CrossRef]
- Esser, B.; Barcena, J.; Kuhn, M.; Okan, A.; Haynes, L.; Gianella, S.; Ortona, A.; Liedtke, V.; Francesconi, D.; Tanno, H. Innovative Thermal Management Concepts and Material Solutions for Future Space Vehicles. J. Spacecr. Rocket. 2015, 53, 1051–1060. [Google Scholar] [CrossRef] [Green Version]
- Kellermann, H.; Lüdemann, M.; Pohl, M.; Hornung, M. Design and Optimization of Ram Air-Based Thermal Management Systems for Hybrid-Electric Aircraft. Aerospace 2021, 8, 3. [Google Scholar] [CrossRef]
- Wang, R.; Dong, S.; Jiang, H.; Li, P.; Zhang, H. Parameter-Matching Algorithm and Optimization of Integrated Thermal Management System of Aircraft. Aerospace 2022, 9, 104. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Colin, S.; Peles, Y.; Garimella, S.; Pease, R.F.; Brandner, J.J.; Tuckerman, D.B. Heat transfer in microchannels-2012 status and research needs. J. Heat Transf. 2013, 135, 942–955. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.Y.; Liu, M.; Wu, H.; Zheng, Y.; Zhuang, J. Numerical Study on Low-Temperature Region as Heat Sink and Its Heat Dissipation Capacity for Hypersonic Vehicle. Aerospace 2021, 8, 238. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhang, Z.Q.; Gao, F. Fuel temperature analysis of advanced fighter aircraft during supersonic cruise. J. Aerosp. Power 2010, 2, 0258-06. [Google Scholar]
- Das, A.; Das, P.; Saha, P. Some investigations on the enhancement of boiling heat transfer from planer surface embedded with continuous open tunnels. Exp. Therm. Fluid Sci. 2010, 34, 1422–1431. [Google Scholar] [CrossRef]
- Chan, M.A.; Yap, C.R.; Ng, K.C. Pool Boiling Heat Transfer of Water on Finned Surfaces at Near Vacuum Pressures. J. Heat Transf. 2010, 132, 031501. [Google Scholar] [CrossRef]
- Magnini, M.; Matar, O. Numerical study of the impact of the channel shape on microchannel boiling heat transfer. Int. J. Heat Mass Transf. 2020, 150, 119322. [Google Scholar] [CrossRef]
- Webb, R.L. Odyssey of the Enhanced Surface. ASME J. Heat Transf. 2004, 126, 1051–1059. [Google Scholar] [CrossRef]
- McGillis, W.R.; Fitch, J.S.; Hamburgen, W.R.; Carey, V.P. Pool Boiling Enhancement Techniques for Water at Sub-atmosphere. In WRL Research Report 90/9; Western Research Laboratory: Palo Alto, CA, USA, 1990. [Google Scholar]
- Wen, M.-Y.; Ho, C.-Y. Pool boiling heat transfer of deionized and degassed water in vertical/horizontal V-shaped geometries. Heat Mass Transf. 2003, 39, 729–736. [Google Scholar] [CrossRef]
- Han, J.; Zhang, Y. High performance heat transfer ducts with parallel broken and V-shaped broken ribs. Int. J. Heat Mass Transf. 1992, 35, 513–523. [Google Scholar] [CrossRef]
- Pastuszko, R.; Kaniowski, R.; Wójcik, T.M. Comparison of pool boiling performance for plain micro-fins and micro-fins with a porous layer. Appl. Therm. Eng. 2020, 166, 114658. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, X.; Xu, N.; Wang, D.; Yang, J.; Chu, H. Effect of T-shaped micro-fins on pool boiling heat transfer performance of surfaces. Exp. Therm. Fluid Sci. 2022, 136, 110663. [Google Scholar] [CrossRef]
- Giraud, F.; Rulliere, R.; Toublanc, C.; Clausse, M.; Bonjour, J. Experimental evidence of a new regime for boiling of water at sub-atmospheric pressure. Exp. Therm. Fluid Sci. 2015, 60, 45–53. [Google Scholar] [CrossRef]
- Ramaswamy, C.; Joshi, Y.; Nakayama, W.; Johnson, W.B. Effects of Varying Geometrical Parameters on Boiling From Microfabricated Enhanced Structures. J. Heat Transf. 2003, 125, 103–109. [Google Scholar] [CrossRef]
- Pal, A.; Joshi, Y. Boiling at sub-atmospheric conditions with enhanced structures. In Proceedings of the Thermal and Thermomechanical Proceedings 10th Intersociety Conference on Phenomena in Electronics Systems, San Diego, CA, USA, 30 May–2 June 2006; pp. 620–629. [Google Scholar]
- Chao, D.F.; Zhang, N.; Yang, W.-J. Nucleate Pool Boiling on Copper-Graphite Composite Surfaces and Its Enhancement Mechanism. J. Thermophys. Heat Transf. 2004, 18, 236–242. [Google Scholar] [CrossRef]
- McNeil, D.; Burnside, B.; Rylatt, D.; Elsaye, E.; Baker, S. Shell-side boiling of water at sub-atmospheric pressures. Int. J. Heat Mass Transf. 2015, 85, 488–504. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, Z.; Li, Z.; Chen, Z.; Huang, S.; Zhang, J.; Guo, F. Numerical investigation on hydraulic and thermal performances of a mini-channel heat sink with twisted ribs. Int. J. Therm. Sci. 2022, 179, 107718. [Google Scholar] [CrossRef]
- Ding, B.; Zhang, Z.-H.; Gong, L.; Xu, M.-H.; Huang, Z.-Q. A novel thermal management scheme for 3D-IC chips with multi-cores and high power density. Appl. Therm. Eng. 2020, 168, 114832. [Google Scholar] [CrossRef]
- Ajalingam, R.; Chakraborty, S. Effect of shape and arrangement of micro-structures in a microchannel heat sink on the thermo-hydraulic performance. Appl. Therm. Eng. 2021, 190, 116755. [Google Scholar]
- Brackbill, J.; Kothe, D.; Zemach, C. A continuum method for modeling surface tension. J. Comput. Phys. 1992, 100, 335–354. [Google Scholar] [CrossRef]
- Mohsin, M.; Kaushal, D.R. 3D CFD validation of invert trap efficiency for sewer solid management using VOF model. Water Sci. Eng. 2016, 9, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Rohsenow, W.M. A method of correlating heat transfer data for surface boiling of liquids. J. Heat Transfer. 1952, 74, 969–973. [Google Scholar] [CrossRef]
- Schrage, R.W. A Thermal Study of Interface Mass Transfer; Columbia University Press: New York, NY, USA, 1953; pp. 160–256. [Google Scholar]
- Knudsen, M. The Kinetic Theory of Gases, Some Modern Aspects. In Methuen’s Monographs on Physical Subjects; Methuen & Company Limited: London, UK, 1934. [Google Scholar]
- Liu, X.; Yu, J. Numerical study on performances of mini-channel heat sinks with non-uniform inlets. Appl. Therm. Eng. 2016, 93, 856–864. [Google Scholar] [CrossRef]
- Yang, S.M.; Tao, W.Q. Heat Transfer; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Khoshvaght-Aliabadi, M.; Hormozi, F.; Zamzamian, A. Role of channel shape on performance of plate-fin heat exchangers: Experimental assessment. Int. J. Therm. Sci. 2014, 79, 183–193. [Google Scholar] [CrossRef]
- Kline, S.J.; McClintock, F.A. Describing uncertainties in single-sample experiments. Mech. Eng. 1953, 75, 3–8. [Google Scholar]
- Moffat, R.J. Describing the uncertainties in experimental results. Exp. Therm. Fluid Sci. 1988, 1, 3–17. [Google Scholar] [CrossRef]




| Parameters | Variable | Dimension/mm |
|---|---|---|
| Height of water evaporator | hw | 14 |
| Length of water evaporator | lc | 22 |
| Length of simulated water region | Lwater | 20 |
| Width of simulated water region | wwater | 20 |
| Height of simulated water region | hwater | 20 |
| Type | Description | Structural Parameters/mm | |
|---|---|---|---|
| Height (h) | Discontinuity Length (δl) | ||
| A1 | OSF structure | 2.5 | - |
| A2 | OSF discontinuous structure | 2.5 | 0.6 |
| A3 | Short OSF discontinuous structure | 1.2 | 0.6 |
| A4 | WF discontinuous structure | 1.2 | 0.6 |
| A5 | Short WF discontinuous structure | 2.5 | 0.6 |
| Type | Description | Structural Parameters/mm | |||
|---|---|---|---|---|---|
| Interval Width (w) | Thickness (σ) | Height (h) | Block Size | ||
| B1 | Rectangular straight wave | 2.5 | 0.2 | 6.5 | - |
| B2 | Rectangular block through wave | 2.5 | 0.2 | 6.5 | 0.1 × 4 |
| Type | Internal Channels | External Fins |
|---|---|---|
| Hex0 | A1 | B1 |
| Hex1 | A1 | B2 |
| Hex2 | A2 | B2 |
| Hex3 | A3 | B2 |
| Hex4 | A4 | B2 |
| Hex5 | A5 | B2 |
| Fluid | Density (kg/m3) | Specific Heat (J/kg·K) | Thermal Conductivity (W/m·K) | Viscosity (Pa·s) |
|---|---|---|---|---|
| Water | 997.6 | 4182 | 0.62 | 8.9 × 10−4 |
| Antifreeze | 1056 | 3317 | 0.3937 | 6.2 × 10−4 |
| Experiment | Simulation | Error | |
|---|---|---|---|
| Tout (°C) | 48 | 50.56 | 5.06% |
| K (W·m−2·K−1) | 1500 | 1562 | 3.9% |
| hf (W·m−2·K−1) | 2876 | 3020.1 | 5.01% |
| Qf (kW) | 29 | 30.1 | 3.78% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, L.; Ma, D.; Zhang, Y.; Yang, X. Experimental and Numerical Investigation on Heat Transfer Performance of Water Evaporators with Different Channels and Fin Structures in a Sub-Atmosphere Environment. Aerospace 2022, 9, 697. https://doi.org/10.3390/aerospace9110697
Pang L, Ma D, Zhang Y, Yang X. Experimental and Numerical Investigation on Heat Transfer Performance of Water Evaporators with Different Channels and Fin Structures in a Sub-Atmosphere Environment. Aerospace. 2022; 9(11):697. https://doi.org/10.3390/aerospace9110697
Chicago/Turabian StylePang, Liping, Desheng Ma, Yadan Zhang, and Xiaodong Yang. 2022. "Experimental and Numerical Investigation on Heat Transfer Performance of Water Evaporators with Different Channels and Fin Structures in a Sub-Atmosphere Environment" Aerospace 9, no. 11: 697. https://doi.org/10.3390/aerospace9110697




