Abstract
Research article abstracts, the second most-read part of research papers after titles, generally follow disciplinary conventions, which are often manifested in their language use. This study analyzed lexical bundles or multi-word sequences in move texts of a one-million-word corpus of English-language medical research article abstracts, with particular attention to vocabulary levels. The most frequent lexical bundles, such as “the primary end point was”, often occurred once per text and predominantly took part in realizing a move. The coverage of the first thousand New General Service List was 63.6% for the entire corpus but was around 80% for bundles in Move 3, describing principal results, and those in Move 4, evaluating the results. Many of the sequences were research-oriented bundles, used to express research contexts. The bundles were made up of relatively accessible word items, but the sequences occurred to realize highly specific research contexts. The findings suggest that becoming familiar with the bundle may need increasing awareness of disciplinary conventions such as guideline adherences and statistical procedures. This study may offer insights on the need for learners to familiarize themselves with these bundles.
1. Introduction
In an era where information floods every aspect of academic and professional life, understanding the conventions of academic writing genres, including textual and vocabulary features, is essential (Swales 1990; Bhatia 2019; Coxhead 2020). Over the past 50 years, the academic landscape has undergone unprecedented changes, marked by a substantial increase in the number of academic papers (Hyland and Jiang 2019). This rapid growth leads to a need for learners, especially medical students, to become proficient in reading and writing research article abstracts because of the specialized nature of their disciplinary texts (Coxhead 2016; Dang 2020; Simpson 2022; Tang and Liu 2019).
Functioning as an academic, especially in English, entails engaging in a sophisticated activity framework of communication practices, particularly when constructing and disseminating scientific claims (Belcher 2016). In this system, language plays a pivotal role in forging consensus and persuading the target discourse community, a group of individuals with shared goals (Swales 1990). Academic discourse is navigated through genre texts, which are types of spoken or written texts used for specific communicative purposes (Hyon 2018, p. 3).
Written communication can be distinguished by differences in audience, purposes, content, form, style, and context (Robinson et al. 2008). Academic written language is known to be differ from academic spoken language, particularly from a vocabulary perspective (Dang et al. 2017; Coxhead and Dang 2019). Students, particularly those learning English as an additional language (EAL) need to learn the genres and conventions commonly used by community members (Brooks et al. 2023; Samraj 2016; Flowerdew 2000). This mastery is the focus of English for academic purposes (EAP) research. EAP research has two main pillars: analyzing genre-specific texts to identify communicative functions and structures (Samraj 2016), and vocabulary studies (Coxhead 2016). Both aim to aid learners’ academic development (Hyland 2016).
EAP research often involves constructing corpora to facilitate these analyses (Handford 2010). One of the central pillars of this research is genre analysis (Swales 1990). The other main pillar of EAP research, vocabulary studies, also employs corpus linguistics techniques (Nation 2005). As Storch et al. (2016) indicated, approaches to EAP are influenced by various factors, including differences in higher education systems across countries. Therefore, research-based instruction integrating multiple approaches is recommended (Hyland 2016; Storch et al. 2016). In this context, linking vocabulary quantification with rhetorical moves has been attempted by several studies (Cortes 2013; Mizumoto et al. 2017; Qi and Pan 2020; Casal and Kessler 2020). These studies illustrate the importance of combining move analysis and vocabulary studies to shed light on the features of disciplinary texts. However, to our knowledge, studies examining the vocabulary levels of word items in lexical bundles within the moves of abstracts have been scarce. Therefore, this study aimed to examine lexical bundles across moves in disciplinary research article abstracts with a focus on the vocabulary levels.
2. Literature Review
2.1. Move Analysis
As a key element of genre analysis framework, move analysis is “a text analytical method developed by Swales in 1981” (Moreno and Swales 2018, p. 40). Moves, as defined by Swales (2004, pp. 228–29), are “discoursal or rhetorical units performing coherent communicative functions in texts”, exhibiting variability in length and other features. The “revised Create a Research Space (CARS) model” (Swales 1990, p. 140), preceded by “a 4-Move Schema” (Swales 1981, p. 15), provides an analytical approach to understanding the structure of research article introductions. Swales (2004) refined the CARS model to include distinct move structures: “Move 1 Establishing a territory”, “Move 2 Establishing a niche”, and “Move 3 Presenting the present work” (pp. 230–32). These moves, denoting their communicative purposes, can be segmented into “Steps” (Swales 2004, p. 230) that realize “functional components” (Tankó 2017, p. 43). The move analysis approach has been applied extensively to examine research articles as a whole (Kanoksilapatham 2005; Maswana et al. 2015; Mizumoto et al. 2017; Nwogu 1997; Stoller and Robinson 2013) and abstracts (Hyland 2000; Kanoksilapatham 2009; Pho 2008; Salager-Meyer 1991, 1992; Tankó 2017) in various disciplines, including medicine.
2.2. Vocabulary Studies
Teaching common words in specific contexts helps learners effectively improve their vocabulary (Nation 2001). In support of learners, various word lists have been developed from collections of specialized texts (Browne 2013; Coxhead 2000; Coxhead and Hirsh 2007; Fraser 2007; Tang and Liu 2019; Wang et al. 2008). An early example is Thorndike’s word book for instructors (Thorndike 1921), which was later updated by Thorndike and Lorge (1944). The General Service List (GSL; West 1953) was subsequently compiled after extensive international discussions before and after World War II (Gilner 2011). Developed from a five-million-word corpus, the GSL is tailored for ESL/EFL learners and is organized into two groups of word families (Coxhead 2000). The GSL has frequently been used as a basis for developing other word lists, including the Academic Word List (AWL) of 570 word families prepared by Coxhead (2000).
Many studies utilize the GSL and AWL to develop specialized word lists. Coxhead and Hirsh (2007) created “the pilot science corpus” (p. 70) from textbooks and discipline-specific reading materials to compile a word list covering items not included in the GSL or AWL. Fraser (2007) quantified the vocabulary required by pharmacology students and compiled the Pharmacology Word List (PWL) from international pharmacology journal articles. Wang et al. (2008) investigated key medical vocabulary to support curriculum design and learning objectives and created a Medical Academic Word List (MAWL). To support medical students, Quero and Coxhead (2018) also compiled word type lists based on their medical corpora, which included texts from medical textbooks. These studies are just a few examples of the many that have used the GSL and AWL to examine the features of their corpora.
While the original GSL has been a foundational tool in language learning over 50 years, it has faced some criticism in recent time (Green and Lambert 2018). The approach of utilizing word families in the GSL and AWL has been the subject of some debate among researchers (Gardner and Davies 2014). In response to these discussions, the New General Service List (NGSL), developed by Browne and his colleagues (Browne 2013), was designed as a modern update of the original GSL. Consisting of 2801 high-frequency words with a clearer definition of a “word” and a higher coverage of general English (Dang and Webb 2016), the NGSL is viewed by some researchers as more suitable for EAL learners (Mizumoto et al. 2021). It has been suggested that EAL undergraduates may find the NGSL to offer a slightly easier learning curve, potentially making it more appropriate for learners compared to the GSL (Culligan 2019). Subsequently, the New Academic Word List (NAWL), consisting of 963 words, was developed to align with the NGSL along with other specific purpose word lists targeting mid-frequency range vocabulary. It is reported that the NGSL and the NAWL combined offer an average of 92% coverage of academic texts and lectures (Browne 2021, p. 4). These updates reflect ongoing efforts to improve vocabulary learning tools for English language learners.
2.3. Lexical Bundles
In vocabulary studies that support learning academic texts, research on multiword units, often referred to as ”lexical bundles”, is also significant (Nation 2022, p. 454). Corpus-based studies have identified these lexical bundles (Samraj 2016). The term lexical bundles is defined as “sequences of word forms” (Biber et al. 1999, p. 990) that occur frequently across texts, characterizing a genre or discipline. This concept has gained prominence in corpus linguistics research (Biber et al. 1999; Cortes 2004, 2013; Hyland 2008a). Lexical bundles have been studied extensively for helping learners develop a repertoire that is sensitive to disciplinary norms (Hyland 2012, p. 17).
A study by Cortes (2013) associated the identification of lexical bundles and rhetorical analysis of moves and steps in research article introductions. This approach has contributed to revealing essential relationships between forms and functions (Gray et al. 2020, p. 139), showing the values of prefabricated word sequences (Biber et al. 2004, p. 376) within the linguistic contexts that constitute “communicative events” (Swales 1990, p. 9).
Following Cortes (2013), several scholars have observed move-specific bundles (Mizumoto et al. 2017; Omidian et al. 2018; Qi and Pan 2020). Mizumoto et al. (2017) observed 25 moves across 1000 research articles as a whole in applied linguistics, identifying frequently occurring lexical bundles in each move. Omidian et al. (2018) identified lexical bundles in each move in research article abstracts from six disciplines such as mechanical engineering, physics, and applied linguistics. Their study has shown a marked difference in the frequency of bundles across disciplines, suggesting that researchers from different fields prioritize different aspects when presenting their work in academic abstracts (Omidian et al. 2018, p. 12). Qi and Pan (2020) performed move analysis according to Hanidar’s (2016) four-move structure and extracted lexical bundles, suggesting the role of lexical bundles in achieving the communicative goals of rhetorical sections.
These bundles, also known as “multi-word sequences” (Biber et al. 2004, p. 373; Mizumoto 2015, p. 30) or “recurrent word combination” (Chen and Baker 2010, p. 31), and synonymous with “n-grams” (Mizumoto 2015, p. 31; Stubbs and Barth 2003, p. 61), play a crucial role in the structure of discourse. These bundles often encapsulate shorter sequences, such as three-word bundles, within their four-word structures (Cortes 2004, p. 401; Hyland 2008a, p. 6). Although Stubbs and Barth (2003) question the status of n-grams as linguistic units and refer to them as “chains of word-forms” (p. 62), they acknowledge that these recurring sequences typify specific text genres. Traditionally, studies have focused on the prevalence of four-grams in academic writing, with phrases like “as a result of” and “in the context of” serving as organizers of specific texts (Cortes 2013, p. 34).
Hyland (2008a, p. 6) has shown that frequency counts “drop dramatically” when quantitating five-grams compared to four-grams, stating that “many four and five word strings” share three-grams. Cortes (2024, pp. 120–21) discusses “potential overlaps” such as the five-gram “as a result of the” extending beyond shorter sequences like “as a result”. Furthermore, Hyland and Jiang (2019, p. 110) have demonstrated that longer strings, such as the five-gram “at the beginning of the” which extend beyond the four-gram “the beginning of the” may offer deeper insights into the pedagogical applications of lexical bundles. Golparvar and Barabadi (2020, p. 6) analyzed “phrase frames” (p-frames; Cortes 2024, p. 105) in the discussion section of research articles and suggested that five-word p-frames could often be “more specific to a particular genre” compared to four-word p-frames. The most frequently occurring five-word p-frame was “are * likely to withdraw”, with fillers such as “less” and “more”, while the leading four-word p-frame was “the * of technology”, with fillers such as “use” and “adoption”. Liu and Chen (2022) have shown that five-word and six-word p-frames in university lectures reveal knowledge-disseminating and content-oriented features. Casal and Kessler (2020), who studied frequent p-frames in grant applications, demonstrated a strong association between the writers’ use of five-word p-frames and their rhetorical intentions. Using a bundle-driven approach, Li et al. (2020) found that five-word bundles contributed to identifying moves of PhD abstracts. These findings indicate that extended sequences such as five-grams may be useful for genre-specific language use and potentially provide linguistic patterns beneficial for learners.
2.4. Medical Research Abstracts
Abstracts are the most-read texts in a research paper, second only to titles, and serve as a vital standalone tool of communication (Hyland 2000). Scientists increasingly rely on abstracts as “short, concise, complete, and accurate sources of information” (Salager-Meyer et al. 2014, p. 222). Research paper abstracts act as “advance indicators of the content and structure of the following text” (Swales 1990, p. 179) and encapsulate the key findings of the research (Huckin and Olsen 1983, p. 359).
Structured abstracts have become the norm in medical literature, largely because of long-standing endorsements by journal editors, such as those from the International Committee of Medical Journal Editors (ICMJE) since 1993 (Salager-Meyer et al. 2014, p. 223). Salager-Meyer (1991) noted that among 77 medical research article abstracts, half were deemed “poorly structured” in adherence to “the Introduction, the Methods, the Results, and the Discussion (IMRAD) pattern” (p. 528). While Anderson and Maclean (1997) examined “unstructured abstracts” (p. 2) of medical article abstracts and identified a five-move structure, including an optional background, along with the purpose, method, results, and conclusion, Hanidar (2016), analyzing abstracts from various disciplines including medicine, advocated for a four-move structure, comprising “Move 1: Creating a research space, ” “Move 2: Describing research procedure”, “Move 3: Summarizing principal results”, and “Move 4: Evaluating results”. This four-move approach aligns with our corpus texts structured according to the journal’s guidelines. The studies of structured abstracts, notably by James Hartley, highlights the benefits of structured texts over traditional formats (Hartley 1993, p. 90). Hartley (1999, 2003) argued that structured abstracts, already used in medicine, could be “appropriate for applied ergonomics” (1999, p. 535) and could be “introduced into psychology journals” (2003, p. 366). His extensive work on structured abstracts was comprehensively reviewed by Zhang and Liu (2011), who have underscored “the advantages of structured abstracts over traditional ones” (p. 575).
In medical research publications, articles are often categorized by study design and must meet the requirements of specific reporting standards (Millar et al. 2019; Stosic 2022). High-quality reporting is facilitated by over 616 standardized reporting guidelines from the EQUATOR Network (2024), up from around 400 different sets (Millar et al. 2019, p. 150). Failure to adopt the guidelines may cause a manuscript to be regarded as inferior in quality by the ICMJE (Millar et al. 2019, p. 141). The ICMJE (2024) aims to enhance the quality and transparency of medical reporting. Their recommendations are broadly “accepted by biomedical journals” (Luo and Hyland 2019, p. 39) and play a crucial role (Millar et al. 2012, p. 393) in shaping the research writing standards. These initiatives appear to accelerate the standardization of research writing (Swales 2017, p. 249).
While there are no specific guidelines on language use (Stosic 2022), medical abstract conventions have been studied from the perspective of linguistic features. Salager-Meyer (1992) analyzed 84 medical abstracts and revealed the choice of verb tense and modality associated with rhetorical functions. Abdollahpour and Gholami (2018) gathered 1800 medical article abstracts from various journals. They identified four-word lexical bundles and classified them into general and technical groups by two qualified raters (Abdollahpour and Gholami 2018). Nam et al. (2016), who aimed to reformat unstructured abstracts into the IMRAD format, have found that linguistic features such as a five-word sequence “aim of this study was” would improve the effectiveness of abstract sentence classification. These studies have contributed to understanding the textual features of medical research article abstracts.
2.5. Application of Move Analysis and Vocabulary Research
Most recent studies have integrated move analysis and vocabulary research to envisage academic writing instruction (Casal and Kessler 2020; Li et al. 2020; Qi and Pan 2020). As a practical application, Mizumoto et al. (2017) developed an academic writing support tool called the “Academic Word Suggestion Machine (AWSuM)”. AWSuM integrates move analysis with lexical bundle analysis. John Morley’s (2023) “The Academic Phrasebank” serves as a general resource for academic writers. It offers examples of phraseological components organized according to the main sections of a research paper or dissertation. This integrated approach is particularly beneficial for EAL learners. For instance, first-year medical students are shown to have difficulties in reading medical research abstracts (Shimizu 2019). It was pointed out that the boundary of methods and results sections is hard to identify when the learners face sentences like “Of the 19,114 persons who were enrolled in the trial, 9525 were assigned to receive aspirin and 9589 to receive placebo” (Shimizu 2019, p. 85). These findings echo Tardy and Jwa’s (2016) observations about the challenges of learning academic writing within a discipline, suggesting the importance of integrating genre and vocabulary research for future instructional practices.
3. The Current Study
The purpose of this study was to investigate the vocabulary levels of medical research article abstracts, especially lexical bundles across moves in disciplinary research article abstracts. This study identified lexical bundles across moves of research article abstracts and used the New General Service List (NGSL; Browne 2013) for examining the vocabulary levels of word items in the move texts and lexical bundles across moves. Our study poses the following research questions:
- Research Question 1: Which lexical bundles occur most frequently in specific moves within medical research abstracts?
- Research Question 2: What are the language features of lexical bundles across moves in medical research abstracts, such as the coverage of the NGSL, forms, and functions?
4. Data and Methods
4.1. Corpus
The data used in this study contain abstracts from the original research articles published in the years 2002–2020 in The New England Journal of Medicine (Table 1). These abstracts were selected for their “representativity, reputation, and accessibility”, according to Nwogu’s (1997, p. 121) criteria. The journal’s articles are also valued for providing regional students with essential insights into evaluating medical literature (Ogawa 2014) and understanding the practical use of language in medical writing (Jego 2012). Additionally, the journal provides official translations in the local language (Nankodo 2024).1

Table 1.
Corpus of research article abstracts.
Each article was segmented into individual sentences. Drawing on the studies by Cortes (2013, p. 36), who identified lexical bundles in research article introductions and mapped them to specific rhetorical moves, and Mizumoto et al. (2017, p. 902), who concentrated on extracting “move-specific lexical bundles”, we segmented our corpus by sections (moves). For move identification, we adopted the methodology of Qi and Pan (2020), dividing our corpus texts “based on their own headings” (p. 112)—Background, Methods, Results, and Conclusions—to create subcorpora “Move 1: Creating a research space”, “Move 2: Describing research procedure”, “Move 3: Summarizing principal results”, and “Move 4: Evaluating results” according to Hanidar (2016, p. 14). This four-move approach aligns with our corpus texts structured according to the journal’s guidelines (The New England Journal of Medicine 2024).
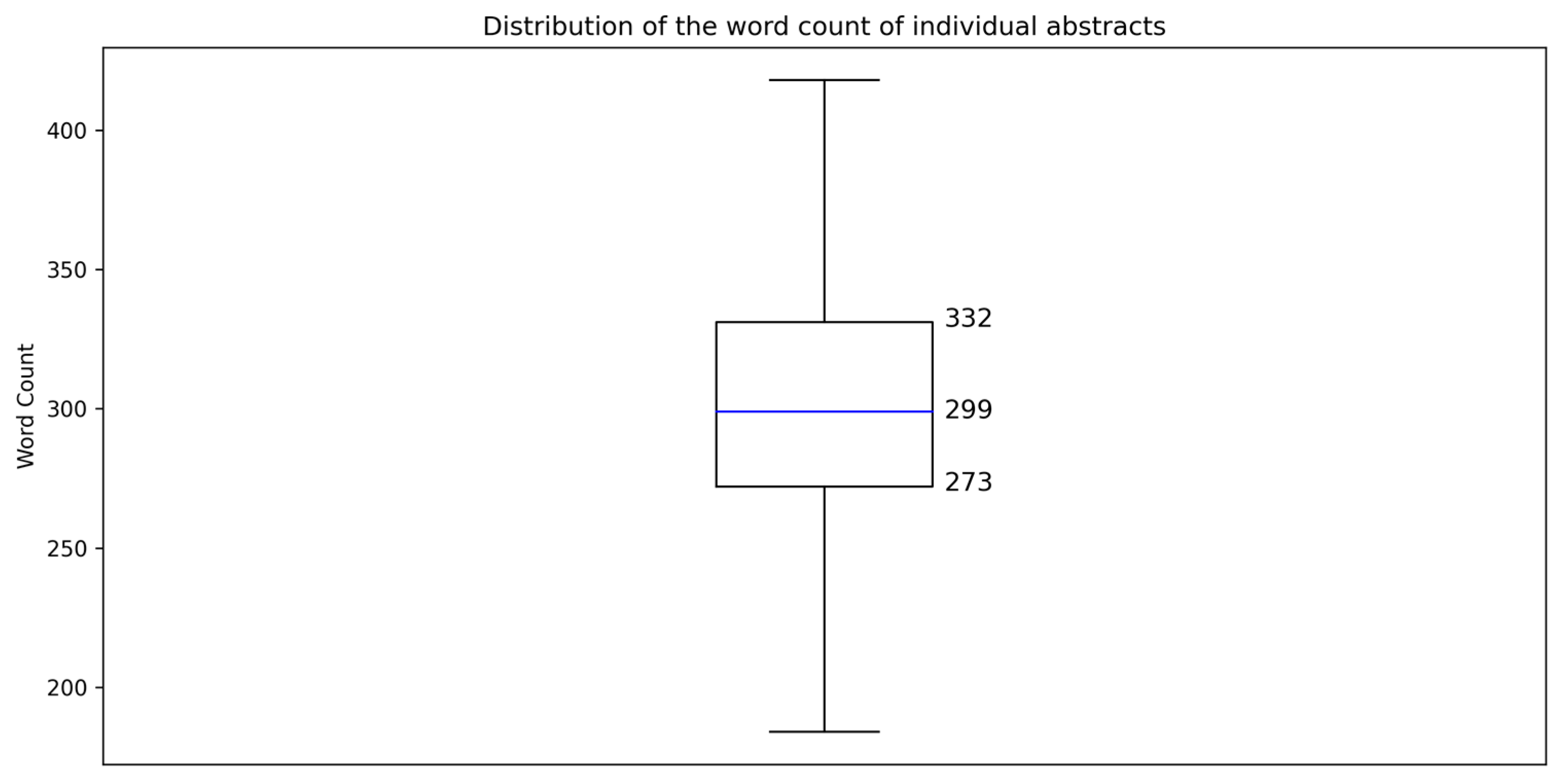
The abstract texts had 1,148,583 words, as determined using AntConc (Version 4.2.4; Anthony 2023).2 The number of words in each text was quantitated using CasualConc (Version 3.0.8; Imao 2024) and was visualized using Google Colaboratory’s Python environment (Version 3.10.12). The average word count of the abstracts was 303 words, with a standard deviation of 44 words. The distribution of abstract word counts showed that 50% of the texts fell between 273 and 332 words, with a median value of 299 words (Figure 1). These findings indicate a relatively consistent length across the corpus texts.
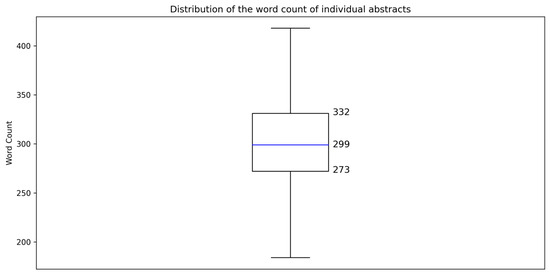
Figure 1.
Distribution of the word count of individual abstracts.
The most frequent words in the medical research abstract corpus included many function words, with the and of ranked the first and second (Table 2). The top 100 words accounted for over 51% of the texts (Table 3). These findings suggest that a limited set of words were quite frequently used for presenting research information. Furthermore, frequent instances of these function words and the scarcity of specialized terms suggest that studies on diverse topics were reported using similar basic vocabulary.

Table 2.
List of the most frequent words in the abstract corpus.

Table 3.
Cumulative coverage of the top 100 words in the abstract corpus.
For the analysis with the NGSL (Version 1.01, Browne 2013) and NAWL (Version 1.01, Browne et al. 2013), we used a corpus tool, CasualConc (Imao 2024). The NGSL comprises a total of 2801 words. Separate lists categorizing the top 1000 words by frequency (first NGSL), the next 1000 words (second NGSL), and the remaining 801 words (third NGSL) were available from the AntWordProfiler website (Anthony 2024) as a resource for vocabulary profiling. In addition, a supplementary list of 174 basic words, which were not included in the aforementioned three lists, and the NAWL, consisting of 963 words, were also downloaded from the same site. By combining these five lists cumulatively, the following four stopword lists were created and imported into CasualConc for coverage analysis.
- First NGSL + Supplement
- First NGSL + Second NGSL + Supplement
- First NGSL + Second NGSL + Third NGSL + Supplement
- First NGSL + Second NGSL + Third NGSL + NAWL + Supplement
Using the stopword function of CasualConc, the word items in the lists were applied to automatically remove the target items in the preparatory step (Sarica and Luo 2021). To determine the covered word count, the word count after removal was subtracted from the total word count of the texts using Microsoft Excel (Version 2406). This procedure was repeated using the aforementioned stopword lists one by one, and the results were visualized using Python 3.10.12 in the Google Colaboratory environment (Figure 2).
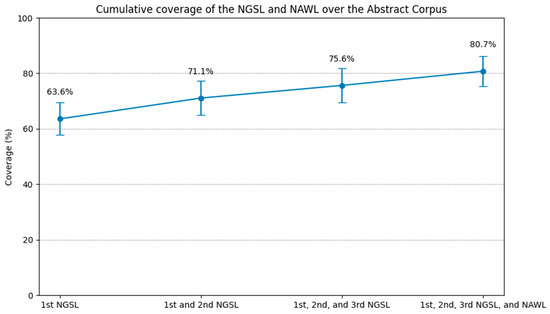
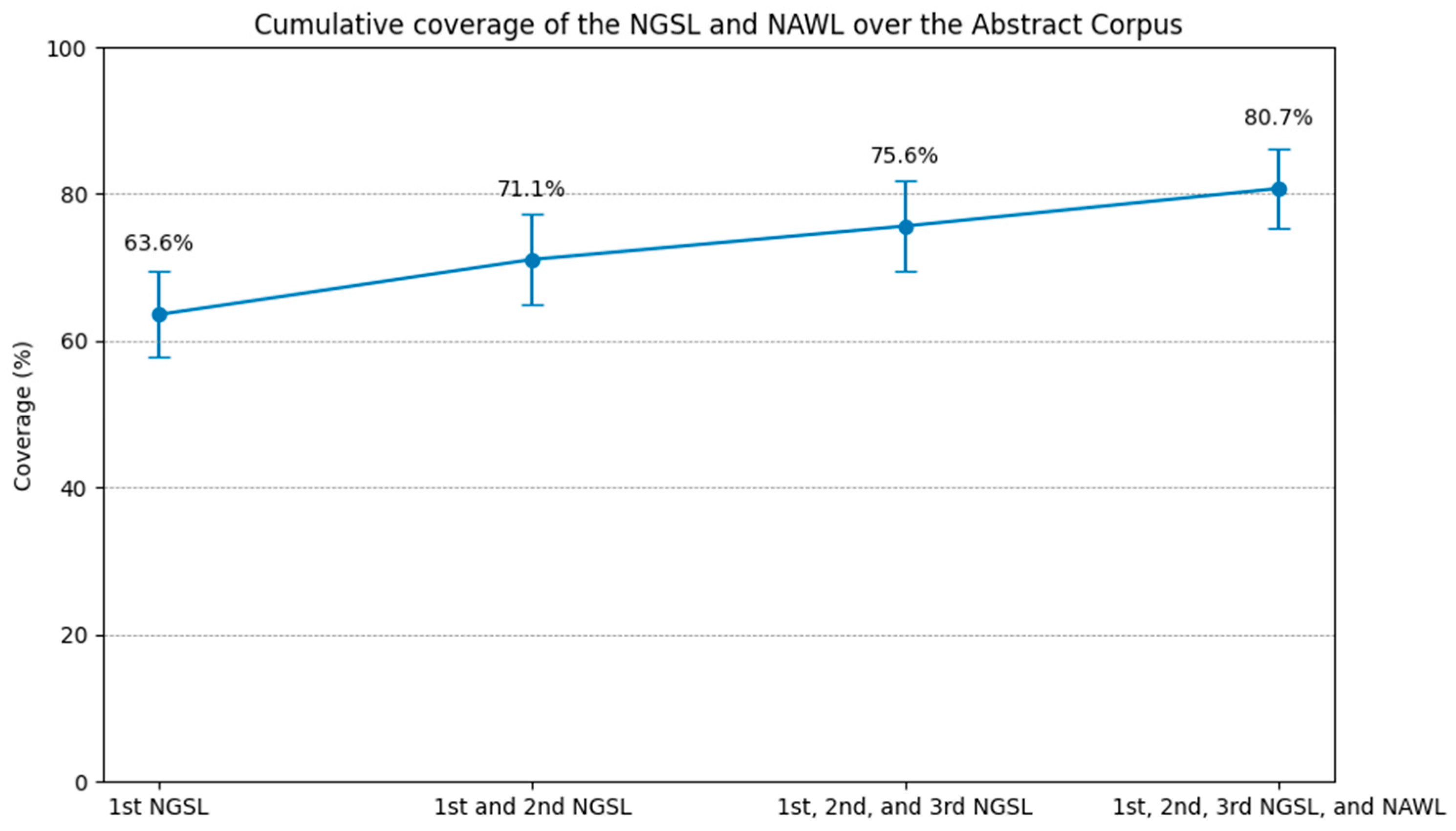
Figure 2.
Cumulative coverage of the NGSL and NAWL over the abstract corpus.
The first 1000 words plus the supplementary 172 words covered 63.6% of the entire corpus, 71.1% with the first 2000 words, and 75.6% with the addition of the remaining 801 words. The NAWL yielded an additional 5.1% coverage, reaching a total coverage of 80.7%.
The comparison with previous studies showed that while the NGSL and NAWL provided greater coverage in other texts, they did not cover as much of our medical research article abstract corpus. The 2801 high-frequency words from the NGSL provided between 95% and 97% overall text coverage of English reading passages of Japan’s national university entrance examination from 2015 to 2019 (MacDonald 2019, p. 22). The NGSL and NAWL combined to provide an average coverage of 84.8% of a 7.8-million-word civil engineering research article corpus (Gilmore and Millar 2018). In contrast, the coverage of our medical research abstract corpus was lower, indicating its highly specialized vocabulary level.
4.2. Data Processing
Hyland and Jiang (2019, p. 111), following Cortes (2015, p. 205), argued that while bundle frequencies are often standardized per 10,000 words, this normalization may result in higher instances of bundles in smaller corpora. This potentially raises the frequency of word combinations that are not usually common enough to surpass the threshold. Consequently, phrases that are infrequently used might still be classified as lexical bundles after normalization (Hyland and Jiang 2019, p. 111). To mitigate this issue, we adopted multi-step approach to bundle identification, drawing on methodologies mainly from Cortes (2004), Hyland (2008a), Hyland and Jiang (2019), and Lake and Cortes (2020). Our initial step was to ensure that each abstract in our corpus had broadly similar word count in English and quantitated the abstracts (Figure 1).
Recent studies have shown that five-word bundles are likely to realize rhetorical intentions (Casal and Kessler 2020) and represent a particular genre (Golparvar and Barabadi 2020). Nam et al. (2016) studied several linguistic features for classifying unstructured abstract sentences into the IMRAD format. They found that n-grams produced the best results. However, increasing the value of “n” in n-grams did not necessarily improve classification performance. Better results were obtained with sequences such as five-grams and six-grams (Nam et al. 2016). Therefore, the present study focused on identifying five-word bundles. Initially, following the criteria for five-word p-frames set by Casal and Kessler (2020), five-word bundles appearing in at least five different texts with a raw frequency of five were identified. This was achieved with the N-Gram function of AntConc (Version 4.2.4, Anthony 2023). This process revealed 433 bundles in Move 1, 1901 in Move 2, 3349 in Move 3, and 840 in Move 4. Given the size of the move corpora (Table 1), we followed the idea of Bestgen (2020) to avoid the potential bias of smaller corpora yielding more bundles by using higher frequency thresholds. For further analysis, a threshold of appearing in at least five different texts with a raw frequency of 10 was applied.
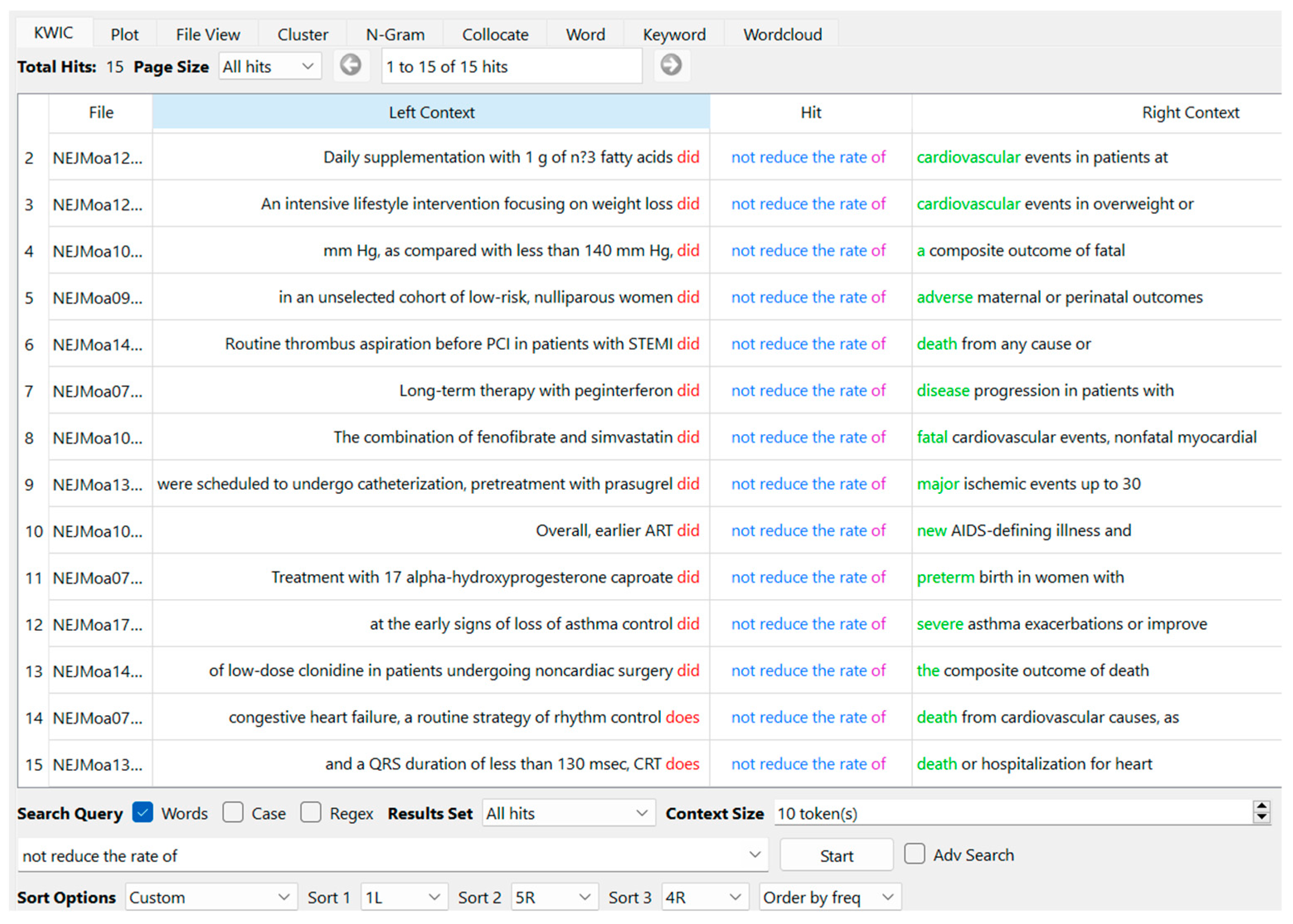
We followed the approach of Hyland and Jiang (2019, p. 109), which involved manually removing bundles containing text-dependent noun phrases, such as “the United States”. However, we retained phrases such as “the primary end point”. This is because primary end points serve as key measures of study outcomes, as noted by Qi and Pan (2020, p. 115), and because of the substantial instructional value of items conforming to disciplinary requirements (Salager-Meyer et al. 2014; Luo and Hyland 2019; Millar et al. 2019). This strategy aimed at extracting bundles while preserving “the observation of language in use” (Sinclair 1991, p. 39) as much as possible. In this process, we followed Durrant’s (2017, p. 170) study for the exclusion of “punctuation and numerals”. We utilized AntConc’s feature for automatically omitting punctuation and digits (Viana and O’Boyle 2022, p. 119). Then, we manually excluded “overlapping word sequences” (Chen and Baker 2010, p. 33) with reference to the procedure outlined by Qi and Pan (2020, p. 113). This was done by placing the extracted bundles on spreadsheets (Microsoft Excel) and examining AntConc’s concordance lines. For example, the bundle “did not reduce the rate” showed 13 times in Move 4; all instances were from the six-word string “did not reduce the rate of”. The five-word string “not reduce the rate of” occurred 15 times, including two instances of a six-word sequence, “does not reduce the rate of” (Figure 3). Therefore, the bundle “not reduce the rate of” was listed.
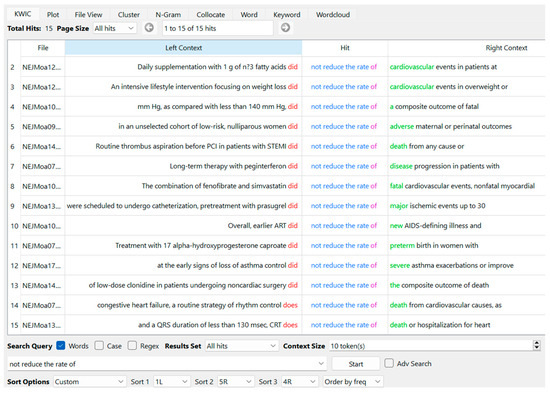
Figure 3.
Example screen of AntConc showing “not reduce the rate of” as the node word with 13 instances of “did” and 2 instances of “does” at L1 or one word to the left (order by frequency of L1).
The structures of the bundles were examined with reference to Biber et al. (1999, pp. 1014–24). We also referred to the procedure by Hyland (2008a, p. 10) and examples by Qi and Pan (2020, pp. 125–28). Biber et al. (1999) presented taxonomies for lexical bundles in academic prose, providing example bundles embedded in sentences. Hyland (2008a), citing Biber et al. (1999), also gave example bundles in sentences. By comparing our identified bundles to those examples, we classified our bundles accordingly. Bundles that did not fall into the categories based on the examples, such as “than in the group that”, were labeled as others.
The functions of the bundles were analyzed based on Hyland’s (2008a) classification. Hyland (2008a, p. 13) explained that research-oriented bundles “help writers to structure their activities and experiences of the real world”. Accordingly, our observation included both less specific items such as “with a use of the” and highly specific combinations such as “hazard ratio confidence interval ci” in this category. Text-oriented bundles are “concerned with the organisation of the text and its meaning as a message or argument”, and participant-oriented bundles are “focused on the writer or reader of the text” (Hyland 2008a, pp. 13–14). Based on these definitions, bundles such as “associated with an increase in” were differentiated from research-oriented sequences such as “associated with an increased risk” in classification.
5. Results
5.1. Lexical Bundles in Move Texts
5.1.1. Frequencies
We found a total of 1286 five-word lexical bundles in medical research abstracts: 71 in Move 1, 305 in Move 2, 848 in Move 3, and 62 in Move 4. The most frequently occurring bundles are shown in Table 4, Table 5, Table 6 and Table 7, and all 1286 bundles are shown in Table A1 in Appendix A. The most frequent five-word bundle “the efficacy and safety of” appeared 98 times in Move 1 with a range of 96; “the primary end point was” in Move 2 occurred 708 times in 705 texts. The bundle “confidence interval ci to p” occurred 681 times in 681 texts in Move 3. The original sequence was “confidence interval [CI], n# to n#; P<n#” as shown in an example sentence below, after the exclusion of punctuation and digits (Durrant 2017, p. 170; Viana and O’Boyle 2022, p. 119). In Move 4, the most frequently occurring bundle “associated with an increased risk” appeared 59 times in 56 texts. These instances indicate that many high-frequency sequences were used only once per abstract. The raw frequency and range values suggest a consistent use of bundles across the corpus texts.

Table 4.
Most frequent five-word lexical bundles in Move 1.

Table 5.
Most frequent five-word lexical bundles in Move 2.

Table 6.
Most frequent five-word lexical bundles in Move 3.

Table 7.
Most frequent five-word lexical bundles in Move 4.
- 1.
- In total, 457 patients (22.8%) in the surgery group and 539 patients (26.4%) in the control group died (hazard ratio, 0.77; 95% confidence interval [CI], 0.68 to 0.87; p < 0.001).
While the top bundle in Move 1 did not exceed a raw frequency of 100 (Table 4), the bundle “the primary end point was” in Move 2 was notably prevalent and was found 708 times in 705 texts (Table 5). The prevalence of this bundle indicates common practices in the disciplinary research where the sequence is used to define main variable or parameter to be measured. The combination reflects a highly technical aspect, setting the stage for the methodological framework although the individual lexical items—“the”, “primary”, “end”, “point”, and “was”—have been found to be accessible from a vocabulary perspective (Asano and Fujieda 2024). For instance, the following example illustrates the essential role of such lexical bundles in presenting the main parameters of a study in a succinct manner:
- 2.
- The primary end point was histologic improvement in the 10-mg group as compared with the placebo group.
The bundle “confidence interval ci to p” also occurred as high as 681 times in 681 texts in Move 3, as represented by the fragment “95% confidence interval [CI], 0.68 to 0.87; p < 0.001” in the abovementioned example sentence. This indicates a noticeably consistent appearance in statistical contexts. The abbreviation “CI” indicates multiple occurrences of the term “confidence interval” within the text. The consistent appearance of the five-word bundle “confidence interval ci to p” across various texts suggests its routine use in statistical analyses within medical research. This bundle helps in presenting critical statistical information in a uniform manner, which facilitates clear and comparable interpretations of study results. For instance, the text uses this bundle to report the statistical measures of effect and significance, such as the confidence interval and a p-value. The text illustrates the bundle’s role in showing quantitative aspects of the study findings. This uniform application of statistical terminology ensures that the findings are communicated in a precise and standardized format that can be easily understood and evaluated by others in the scientific community. In Move 4, high-frequency bundles contained word items related to discussing changes or statistical results, such as “associated with an increased risk” and “there was no significant difference”, as described later in Section 5.1.3. Structure and Functions of Lexical Bundles.
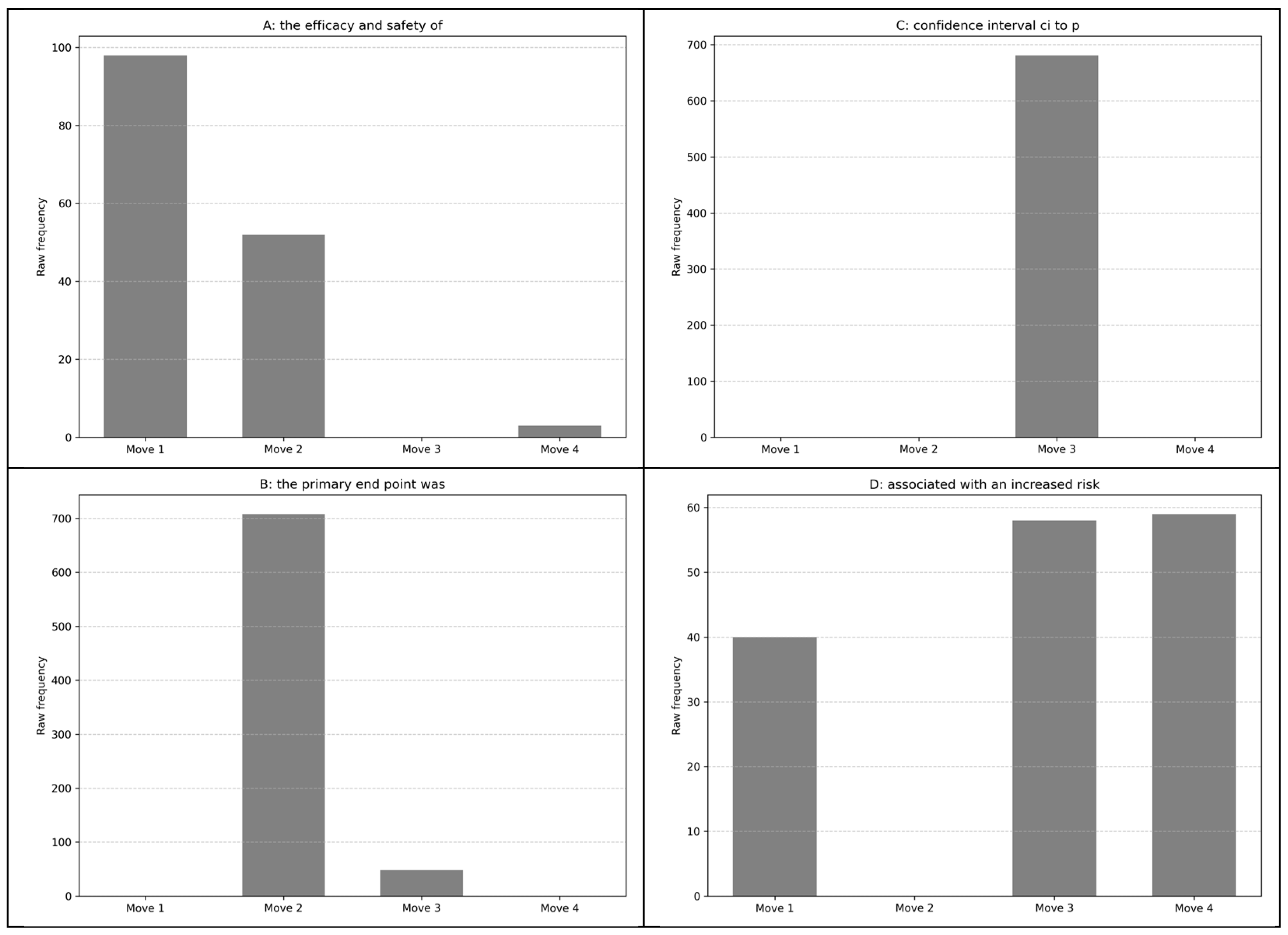
Figure 4 shows the frequency of the leading bundles in each move. This indicates that the individual bundles have unique roles in the texts, as shown by Nam et al. (2016). For example, Panel A in Figure 4 shows that “the efficacy and safety of” occurred most often in Move 1. Example concordance lines indicated that the bundle took the technical part of the sentence for realizing study objectives.
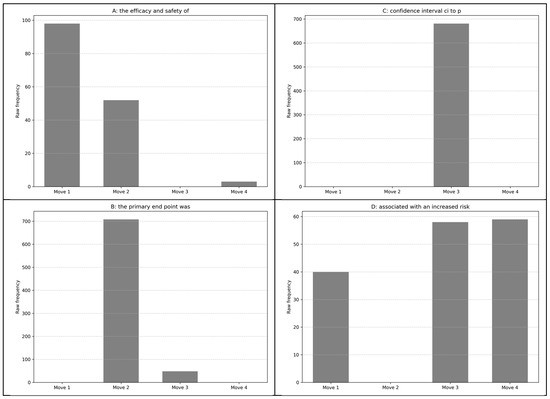
Figure 4.
Frequency of move-specific most frequent five-word bundles. (A): the efficacy and safety of; (B): the primary end point was; (C): confidence interval ci to p; (D): associated with an increased risk.
- 3.
- We assessed the efficacy and safety of a paclitaxel-coated balloon in this setting.
Panel B in Figure 4 indicates that the instances of “the primary end point was” were dominant in Move 2. The bundle “confidence interval ci to p” only occurred in Move 3 (Panel C), indicating its essential function for presenting statistical findings. The sequence “associated with an increased risk” was rather exceptional as it was common in Moves 3 and 4 and also occurred in Move 1. However, the instances in these moves were below 60; therefore, the bundle may have been used only when the bundle fitted appropriately in realizing the authors’ intentions. The findings suggest that these combinations may have pedagogical value. Becoming aware of where these bundles typically appear and their function within the abstracts could greatly enhance learners’ familiarity with disciplinary research article abstracts.
5.1.2. The NSGL Coverage of the Bundles
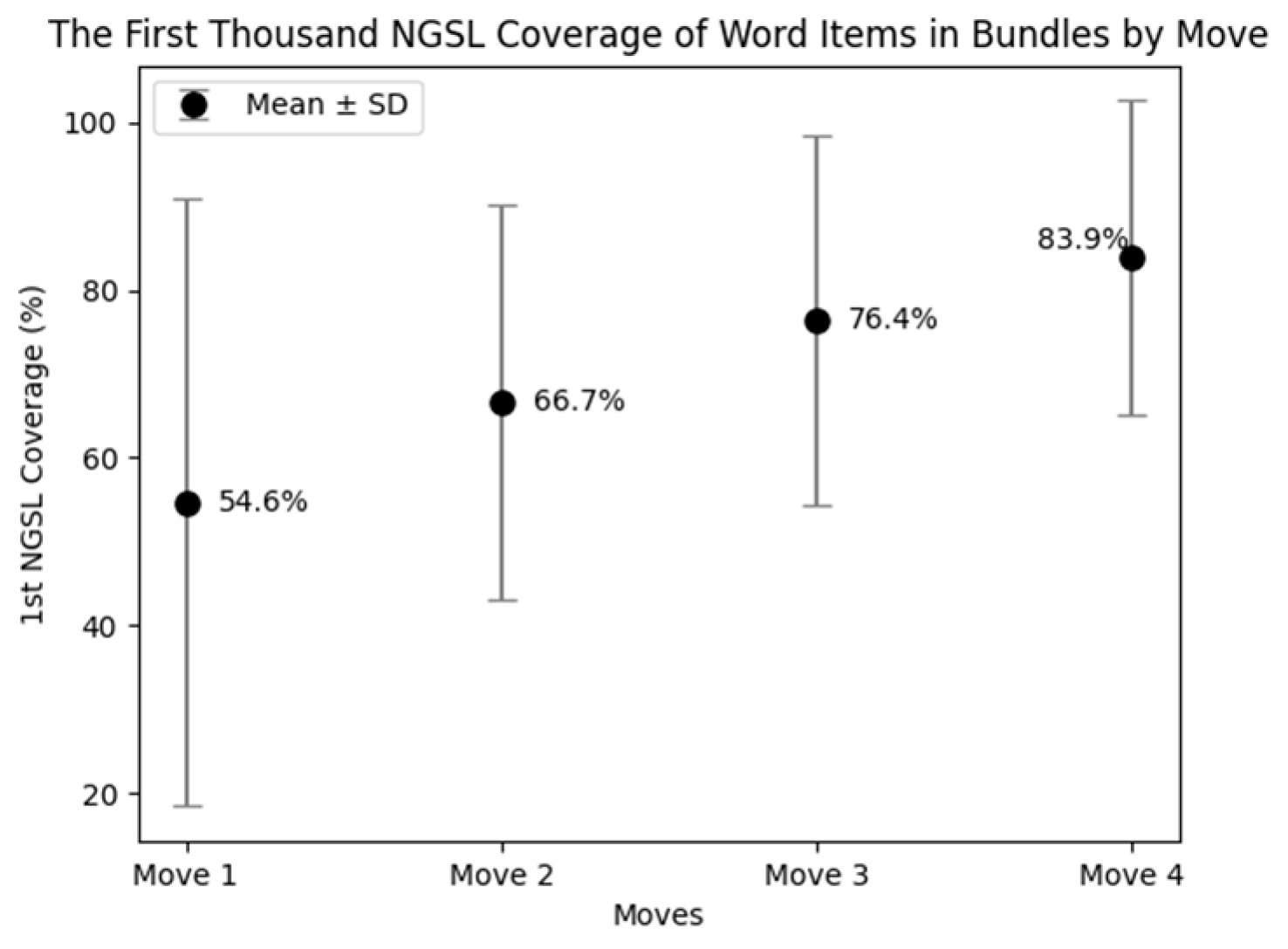
The coverage of the first thousand NGSL varied across the moves (Figure 5). The word list covered up to 83.9% of the word items in bundles occurred in Move 4, followed by 76.4% in Move 3, 66.7% in Move 2, and 54.6% in Move 1. These variations are explicitly depicted in Figure 6. This figure shows the percentages of word items in each five-word bundle covered by the word list. For instance, a five-word bundle was classified as 100% when all five individual word items were covered by the word list. It was marked as 80% when four out of the five word items were covered. When none of the word items were covered by the word list, the bundle was marked as 0%.
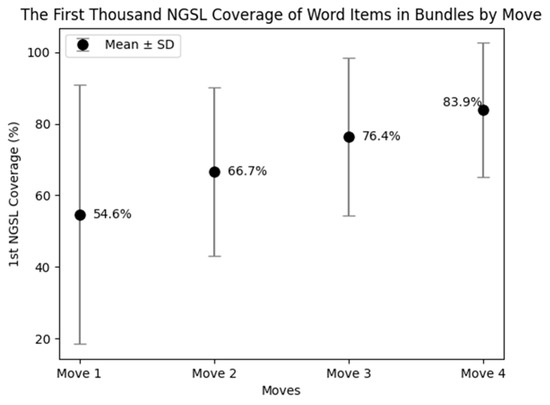
Figure 5.
The first thousand NGSL coverage of word items in lexical bundles by move.
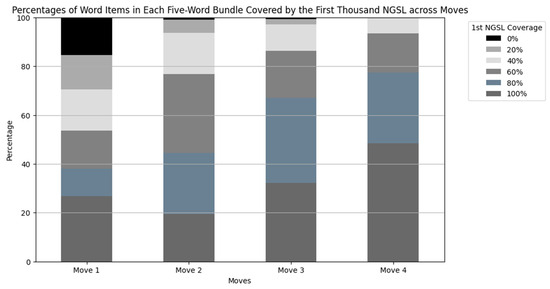
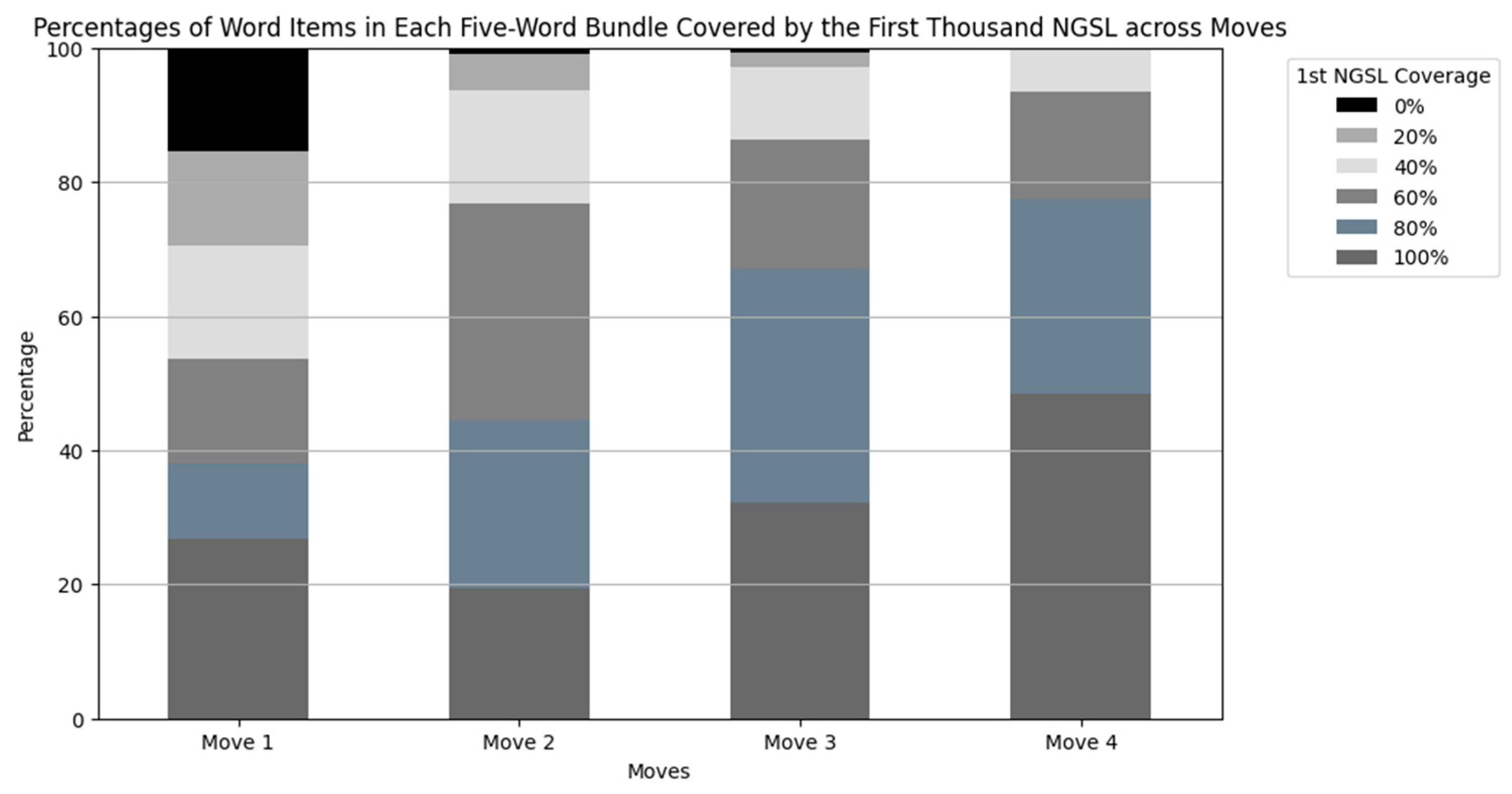
Figure 6.
Percentages of word items in each five-word bundle covered by the first thousand NGSL across moves. From the bottom, bars in dark gray represent 100%, those in blue gray represent 80%, those in dim gray represent 60%, those in silver represent 40%, those in light gray represent 20%, and those in black represent 0%.
In Move 4, about a half of the bundles consisted of words in the first NGSL only, such as “with an increased risk of”, “did not result in a”, and “was not associated with a”. The coverage of the word list was 40% or greater in all the bundles in Move 4. These bundles were often used for discussing the changes or statistical results.
On the other hand, 15.5% of the bundles in Move 1 contained no word items covered by the word list, such as “hepatitis c virus hcv infection” (Table 8). Additionally, 14.1% of the sequences had only one word item covered by the word list, such as the fourth most frequently occurring bundle “low density lipoprotein ldl cholesterol” (Table 4). These combinations were used to introduce the research area as described in the following Section 5.1.3.

Table 8.
Concordance lines with the node word of “hepatitis C virus (HCV) infection” in Move 1.
Move 3 also had many bundles comprised of words covered by the word list, but they were different from the bundles in Move 4 in that most of the bundles in Move 3 were used to accurately report results with numerals, such as “percent confidence interval to p”, “of the patients in the”, and “than in the placebo group” (Table 6). Although each word item was rather accessible, the bundles appeared to convey information specific to the research (Table 9).

Table 9.
Concordance lines with the node word of “of the patients in the” in Move 3.
Similar tendencies were seen in Move 2, where many frequent bundles, such as “the primary end point was” and “we randomly assigned patients with”, were embedded in the context of research as shown in the next section. There were 31 instances of the first-person pronoun, and they were generally used to build research-oriented bundles. Here again, while the individual word items seemed accessible, the combined use of the words tended to reflect the disciplinary conventions.
5.1.3. Structure and Functions of Lexical Bundles
The texts showed that main structures of bundles were noun phrases, similar to the findings in academic written discourse such as those by Biber et al. (1999), Hyland (2008a), and Golparvar and Barabadi (2020). Noun phrases most often occurred in Move 1 (Table 10). Many were used for presenting disciplinary content words like “low-density lipoprotein (LDL) cholesterol” and “chronic obstructive pulmonary disease (COPD)” for setting the scene:

Table 10.
Principal structures of bundles across moves (%).
- 4.
- Non–small-cell lung cancer with sensitive mutations of the epidermal growth factor receptor (EGFR) is highly responsive to EGFR tyrosine kinase inhibitors such as gefitinib, but little is known about how its efficacy and safety profile compares with that of standard chemotherapy.
On the other hand, of-phrase fragments such as “a significantly lower rate of” appeared frequently in expressing the conclusions (Move 4). Most sequences were part of descriptions used to indicate comparisons:
- 5.
- In children with chronic hepatitis B, 52 weeks of treatment with lamivudine was associated with a significantly higher rate of virologic response than was placebo.
The results (Move 3) made by far the most use of bundles beginning with a prepositional phrase. Such items included “of the patients in the”, “in the medical therapy group”, and “as compared with the placebo”. These were most likely used to describe classifications and comparisons:
- 6.
- At 3 years, the criterion for the primary end point was met by 5% of the patients in the medical-therapy group, as compared with 38% of those in the gastric-bypass group (p < 0.001) and 24% of those in the sleeve-gastrectomy group (p = 0.01).
Verb phrases were most used for describing methods (Move 2). These included passive bundles preceded by “patients”, such as “patients were randomly assigned to”, and constructions with the presence of the first-person pronoun, such as “we evaluated the efficacy of”. The following is an example with the first-person pronoun:
- 7.
- We conducted a double-blind, randomized, placebo-controlled trial of intravenous remdesivir in adults who were hospitalized with COVID-19 and had evidence of lower respiratory tract infection.
There were very few usages of the anticipatory it structure among five-word bundles. The only sequence we found was “it is not known whether” in Move 1:
- 8.
- It is not known whether infants conceived with use of intracytoplasmic sperm injection or in vitro fertilization have a higher risk of birth defects than infants conceived naturally.
Many sequences were research-oriented bundles (Table 11). These bundles were used to introduce the research area in Move 1, such as “coronary artery bypass grafting cabg”. The specific word combinations were used and defined to engage readers and direct their focus to the research areas early in the abstracts.

Table 11.
Distribution of bundle functions by move (%).
- 9.
- Some studies suggest that combination antiretroviral therapy in pregnant women with human immunodeficiency virus type 1 (HIV-1) infection increases the risk of premature birth and other adverse outcomes of pregnancy.
Almost all bundles were shown to describe study-related matters in Move 2. They were used to describe “location—indicating time/place” (Hyland 2008a, p. 13) such as “at the time of the”, procedure such as “we randomly assigned to a”, and quantification such as “a scale from to with”. These bundles functioned to contextualize the research processes.
- 10.
- Each videotape was rated in various domains of technical skill on a scale of 1 to 5 (with higher scores indicating more advanced skill) by at least 10 peer surgeons who were unaware of the identity of the operating surgeon.
Although the results section (Move 3) was also predominantly composed of research-oriented bundles, it showed a few participant-oriented bundles like “were more likely to be”. Such bundles were accompanied by specific numbers, or values, and results of statistical analyses. The sequences helped to support the summarized data to describe major findings.
- 11.
- According to univariate analysis, patients with S. marcescens bacteremia stayed in the surgical intensive care unit longer than controls (13.5 vs. 4.0 days, p < 0.001), were more likely to have received fentanyl in the surgical intensive care unit (odds ratio, 31; p < 0.001), and were more likely to have been exposed to two particular respiratory therapists (odds ratios, 13.1 and 5.1; p < 0.001 for both comparisons).
Move 4 showed outstanding differences in that it had various text-oriented bundles. They were related to changes seen in the studies in relation to certain interventions, such as “associated with an increase in” or indicating statistical results, such as “no significant difference in the”. These bundles helped briefly discuss and conclude the study findings in abstract texts.
- 12.
- Knowledge of the fetal oxygen saturation is not associated with a reduction in the rate of cesarean delivery or with improvement in the condition of the newborn.
The instances of “participant-oriented bundles” (Hyland 2008a, p. 14) were fewer than expected, indicating a preference for diverse word combinations to construct participant-oriented discourse. Specifically, the bundle “may reduce the risk of” and “may increase the risk of”, which incorporate the modal “may”, were extracted only in Moves 1 and 4, with more frequent occurrences in Move 1. The bundles such as “more likely to have” and “more likely to be” were found in Moves 3 and 4. These bundles were consistently paired with quantifiers like “more” or “less”, likely reflecting the authors’ intention to contextualize and interpret their results within comparative frameworks.
Despite the simplicity of the individual lexical items such as prepositions, pronouns, and verbs like “know”, their combination into bundles plays significant roles in structuring academically contextualized sequences. This synergy illustrates how domain-specific language is formulated from simple components to convey complex ideas, which is crucial for precise communication in scientific contexts.
6. Discussion
Our main purpose in this study was to examine the lexical bundles across moves in the research article abstracts with a focus on the vocabulary levels. We aimed to address the two research questions. Our findings on the most frequently occurring five-word bundles in each move revealed many sequences similar to the 185 bundles extracted by Qi and Pan (2020) but showed a substantial difference in numbers. We identified 1286 five-word lexical bundles in total; about two-thirds of them occurred in Move 3, one-quarter in Move 2, and the remaining bundles were in either Move 1 or Move 4.
Our findings on the structures and functions of bundles support studies by Biber et al. (1999) and Hyland (2008a), which showed that the main structures of bundles are noun phrases, with the majority of sequences being research-oriented bundles. We have extended these studies by examining the coverage of the first thousand NGSL for bundles in each move. We found fluctuations in the coverage of the first thousand NGSL among bundles in different moves.
Although the word list covered around 80% of the word items in the bundles in Move 3 and Move 4, differences were seen in the forms and functions of the bundles in these moves. In Move 3, noun-phrase bundles accounted for about 50%, and many sequences were used to summarize research findings, describing essential data and presenting statistical information. In Move 4, however, about one-third of the bundles were verb phrases, and text-oriented bundles comprised 40%. The bundles in Move 2 were made up of word items in which the coverage of the word list was slightly lower than in the Move 3 bundles and were predominantly research-oriented. The first-person pronoun used in the Move 2 bundles was mainly part of research-oriented sequences. These pronouns were used to describe the researchers’ adherence to disciplinary requirements (Hyland 2008a). Although many five-word lexical bundles were built with basic word items, the combined sequences often realized the contexts of research. Becoming familiar with these bundles may require learners to raise their awareness of the contextual knowledge of formal and rhetorical features of the genre texts (Tardy 2009). These findings may have implications for teaching specific lexical bundles in educational settings especially for novice learners such as undergraduate medical students.
Our study has several limitations. One is that we identified lexical bundles primarily based on frequency information. Nation (2001) argued that items with higher frequency are used more often and thus have educational value. Biber et al. (2004) suggested that examination of frequently occurring bundles can reveal the real-world use of multi-word sequences. However, there are counterarguments. Flowerdew (2012), citing Widdowson (1991) and Cook (1998), pointed out “the danger of equating frequency with pedagogic relevance” (p. 191). This is pertinent because Simpson-Vlach and Ellis (2010) argued that “sequences such as ‘on the other hand’ and ‘at the same time’ are more psycholinguistically salient than sequences such as ‘to do with the’, or ‘I think it was’” (p. 490). They created an academic formula list by taking into consideration the psycholinguistic salience in addition to frequency count.
A notable finding from Graetz (1982) is that there is a high correlation between the disciplines in which abstracts appear in journals and lecturers’ reliance on abstracts, along with their belief that students should be taught how to read abstracts (p. 26). This perspective is supported by Nation (2022), who emphasized that the characteristics and usage of language items should substantially influence teaching and learning strategies (p. 435). However, our study primarily focused on analyzing the features of lexical bundles in abstract texts, without exploring into classroom applications.
In one of our prior studies, we used a seven-year segment of our corpus to develop the Medical English Education Support System (MEESUS), which was implemented in language courses at a university (Asano et al. 2022a). The complexity of contextual meanings of lexical items in disciplinary discourse has been pointed out, such as the term “clinical” in “clinical trials” and “a clinical decision” (Coxhead 2016, p. 179). To tackle this difficulty, around 100 first-year “genre students” (Hyland 2008a, p. 20), with an average TOEFL ITP score of 475.04, engaged with the MEESUS in our previous study (Asano et al. 2022a). They used its concordance tool to explore language items and reported their insights on terms like “mean” for an average and “case” for a subject, gaining awareness of their their usage in the academic contexts.
In another study, fourth-year medical students, averaging a TOEFL ITP score of 455.9, used disciplinary “guidelines” (Millar et al. 2019, p. 150) to examine and summarize research abstracts (Asano et al. 2022b). Despite different thoughts about “learner-directed corpus projects” (Ballance and Coxhead 2022, p. 412), such activities have been shown to enhance linguistic “awareness” and “tolerance”, preparing learners for real-world applications (Cook 2010, pp. 117–18). Simpson-Vlach and Ellis (2010) suggested that “organization of constructions according to academic needs and purposes is essential” in transforming language resources into effective tools for pedagogy (p. 510).
In the future, it will be necessary to expand upon these prior works by conducting classroom activities. Such activities may include observing lexical bundles and raising learners’ awareness of the form and function of the sequences in context.
Many studies have shown the linguistic features of lexical items in disciplinary texts (Biber et al. 1999; Cortes 2004, 2013; Hyland 2008a, 2008b; Mizumoto et al. 2017; Omidian et al. 2018; Qi and Pan 2020; Li et al. 2020). These studies have aimed to address the challenges faced by “novice and seasoned scientists” (Kanoksilapatham 2005, p. 288). This includes the notion that becoming proficient in a language requires an awareness of the preferred word sequences used by experts (Hyland 2008b, p. 44). Our study found similar results regarding five-word lexical bundles in the moves of research article abstracts (Biber et al. 1999; Hyland 2008a). Our examination revealed that although the bundles identified in our texts consisted of relatively accessible individual vocabulary items, their combined sequences reflected the context of research and adherence to the required academic conventions. Several difficulties in learning multiword items have been pointed out (Boers 2020). Becoming familiar with these patterns could assist students in “learning productive chunks of language” (Reppen and Olson 2020, p. 177). In helping learners succeed in their learning contexts (Tribble 2017), the findings in this study suggest that raising awareness of specific lexical bundles can be beneficial for aiding students in joining their disciplinary communities.
Author Contributions
Conceptualization, M.A. and M.F.; text processing and corpus compilation K.H.; analyses, M.A., K.H. and M.F.; writing—original draft preparation, M.A., K.H. and M.F.; funding acquisition, M.A. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Grants-in-Aid for Scientific Research awarded by the Japan Society for the Promotion of Science (JSPS) grant numbers 23K02800 and 18K02966. The APC was funded by the Author Voucher discount code (0736e63e42811a20).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data from our corpus are currently not accessible to the public. However, data used in this study can be provided upon request to the corresponding author.
Acknowledgments
We would like to express our gratitude to the two anonymous reviewers for their insightful comments. We also thank the academic editors of Languages for their generous assistance throughout the review process. The authors are especially grateful to Junichiro Taki, a fourth-year medical student at Osaka Medical and Pharmaceutical University, for his invaluable assistance in data processing and corpus compilation. Any remaining errors are our responsibility.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
All lexical bundles identified are listed by move in the order of frequency (Table A1). The abbreviations are as follows: Freq stands for frequency, 1st N for the coverage of the first NGSL, NP for noun phrase, VP for verb phrase, PP or prepositional phrase, Ant it for anticipatory it structure, Res for research-oriented, Text for text-oriented, and Participant for participant-oriented bundles.

Table A1.
All lexical bundles identified by move, frequency, first NGSL coverage, structure, and function.
Table A1.
All lexical bundles identified by move, frequency, first NGSL coverage, structure, and function.
| Move | Bundles | Freq | Range | 1st N | Structure | Function |
|---|---|---|---|---|---|---|
| 1 | the efficacy and safety of | 98 | 96 | 60 | NP | Res |
| 1 | non small cell lung cancer | 47 | 47 | 40 | NP | Res |
| 1 | the safety and efficacy of | 45 | 45 | 60 | NP | Res |
| 1 | low density lipoprotein ldl cholesterol | 42 | 42 | 20 | NP | Res |
| 1 | with an increased risk of | 42 | 41 | 100 | PP | Res |
| 1 | human immunodeficiency virus type hiv | 40 | 40 | 100 | NP | Res |
| 1 | associated with an increased risk | 40 | 39 | 40 | VP | Res |
| 1 | in patients with type diabetes | 37 | 32 | 80 | PP | Res |
| 1 | chronic obstructive pulmonary disease copd | 31 | 31 | 20 | NP | Res |
| 1 | coronary artery bypass grafting cabg | 31 | 31 | 0 | NP | Res |
| 1 | it is not known whether | 31 | 31 | 100 | Ant it | Text |
| 1 | small cell lung cancer nsclc | 30 | 30 | 40 | NP | Res |
| 1 | human immunodeficiency virus hiv infection | 28 | 28 | 20 | NP | Res |
| 1 | we tested the hypothesis that | 27 | 27 | 80 | VP | Res |
| 1 | little is known about the | 26 | 26 | 100 | VP | Participant |
| 1 | out of hospital cardiac arrest | 23 | 21 | 60 | NP | Res |
| 1 | are at high risk for | 22 | 22 | 100 | VP | Res |
| 1 | human epidermal growth factor receptor | 22 | 22 | 60 | NP | Res |
| 1 | we conducted a study to | 22 | 22 | 80 | VP | Res |
| 1 | years of age or older | 21 | 20 | 100 | NP | Res |
| 1 | we sought to determine whether | 20 | 20 | 100 | VP | Res |
| 1 | hepatitis c virus hcv infection | 19 | 19 | 0 | NP | Res |
| 1 | of human immunodeficiency virus hiv | 19 | 19 | 40 | PP | Res |
| 1 | influenza a h n virus | 18 | 18 | 20 | NP | Res |
| 1 | high density lipoprotein hdl cholesterol | 17 | 17 | 20 | NP | Res |
| 1 | is a major cause of | 17 | 17 | 100 | VP | Participant |
| 1 | the human immunodeficiency virus hiv | 17 | 17 | 40 | NP | Res |
| 1 | acute respiratory distress syndrome ards | 16 | 16 | 0 | NP | Res |
| 1 | hepatitis c virus hcv genotype | 16 | 16 | 0 | NP | Res |
| 1 | in patients with atrial fibrillation | 16 | 15 | 60 | PP | Res |
| 1 | to reduce the risk of | 16 | 16 | 100 | PP | Res |
| 1 | we conducted a randomized trial | 16 | 16 | 40 | VP | Res |
| 1 | are at increased risk for | 15 | 15 | 100 | VP | Res |
| 1 | has been shown to reduce | 15 | 15 | 100 | VP | Text |
| 1 | proprotein convertase subtilisin kexin type | 15 | 15 | 20 | NP | Res |
| 1 | transcatheter aortic valve replacement tavr | 15 | 15 | 0 | NP | Res |
| 1 | we evaluated the effect of | 15 | 15 | 80 | VP | Res |
| 1 | with hepatitis c virus hcv | 15 | 15 | 20 | PP | Res |
| 1 | epidermal growth factor receptor egfr | 14 | 14 | 40 | NP | Res |
| 1 | has been shown to be | 14 | 13 | 100 | VP | Text |
| 1 | may reduce the risk of | 14 | 14 | 100 | VP | Participant |
| 1 | we evaluated the efficacy of | 14 | 14 | 60 | VP | Res |
| 1 | we evaluated the safety and | 14 | 14 | 60 | VP | Res |
| 1 | with human immunodeficiency virus hiv | 14 | 14 | 40 | PP | Res |
| 1 | diffuse large b cell lymphoma | 13 | 9 | 40 | NP | Res |
| 1 | st segment elevation myocardial infarction | 13 | 13 | 0 | NP | Res |
| 1 | the treatment of patients with | 13 | 13 | 100 | NP | Res |
| 1 | allogeneic hematopoietic stem cell transplantation | 12 | 12 | 20 | NP | Res |
| 1 | angiotensin converting enzyme ace inhibitors | 12 | 12 | 0 | NP | Res |
| 1 | in patients with heart failure | 12 | 12 | 80 | PP | Res |
| 1 | primary percutaneous coronary intervention pci | 12 | 12 | 0 | NP | Res |
| 1 | the most common cause of | 12 | 12 | 100 | NP | Participant |
| 1 | we assessed the efficacy and | 12 | 12 | 60 | VP | Res |
| 1 | cardiovascular events in patients with | 11 | 10 | 80 | NP | Res |
| 1 | continuous positive airway pressure cpap | 11 | 11 | 40 | NP | Res |
| 1 | cystic fibrosis transmembrane conductance regulator | 11 | 11 | 0 | NP | Res |
| 1 | has not been well studied | 11 | 11 | 100 | VP | Participant |
| 1 | methicillin resistant staphylococcus aureus mrsa | 11 | 11 | 0 | NP | Res |
| 1 | patients with chronic kidney disease | 11 | 9 | 60 | NP | Res |
| 1 | patients with relapsed or refractory | 11 | 10 | 60 | NP | Res |
| 1 | patients with type diabetes mellitus | 11 | 11 | 60 | NP | Res |
| 1 | severe acute respiratory syndrome coronavirus | 11 | 11 | 0 | NP | Res |
| 1 | the long term effects of | 11 | 11 | 100 | NP | Res |
| 1 | the risk of cardiovascular events | 11 | 11 | 80 | NP | Res |
| 1 | we examined the effect of | 11 | 11 | 80 | VP | Res |
| 1 | with acute myeloid leukemia aml | 11 | 11 | 20 | PP | Res |
| 1 | data are lacking on the | 10 | 10 | 100 | VP | Participant |
| 1 | graft versus host disease gvhd | 10 | 10 | 20 | NP | Res |
| 1 | mutations in the gene encoding | 10 | 10 | 40 | NP | Res |
| 1 | of death from any cause | 10 | 10 | 100 | PP | Res |
| 1 | patients with severe aortic stenosis | 10 | 10 | 40 | NP | Res |
| 2 | the primary end point was | 708 | 705 | 80 | VP | Res |
| 2 | at a dose of mg | 325 | 252 | 60 | PP | Res |
| 2 | we randomly assigned patients with | 294 | 294 | 60 | VP | Res |
| 2 | were randomly assigned to receive | 285 | 278 | 60 | VP | Res |
| 2 | primary end point was the | 278 | 277 | 80 | VP | Res |
| 2 | per kilogram of body weight | 211 | 211 | 80 | PP | Res |
| 2 | the primary outcome was the | 190 | 190 | 60 | VP | Res |
| 2 | in a ratio to receive | 183 | 178 | 80 | PP | Res |
| 2 | randomly assigned in a ratio | 151 | 147 | 40 | VP | Res |
| 2 | patients were randomly assigned to | 149 | 148 | 60 | VP | Res |
| 2 | randomized double blind placebo controlled | 134 | 134 | 20 | NP | Res |
| 2 | assigned in a ratio to | 127 | 123 | 60 | VP | Res |
| 2 | to with higher scores indicating | 125 | 105 | 100 | PP | Res |
| 2 | years of age or older | 125 | 121 | 100 | NP | Res |
| 2 | were randomly assigned in a | 124 | 120 | 60 | VP | Res |
| 2 | double blind placebo controlled trial | 120 | 120 | 20 | NP | Res |
| 2 | was a composite of death | 93 | 88 | 80 | VP | Res |
| 2 | the primary outcome was a | 90 | 90 | 60 | VP | Res |
| 2 | outcome was a composite of | 79 | 77 | 60 | VP | Res |
| 2 | we randomly assigned patients to | 79 | 79 | 60 | VP | Res |
| 2 | per square meter of body | 75 | 75 | 60 | PP | Res |
| 2 | we conducted a randomized double | 72 | 72 | 40 | VP | Res |
| 2 | the primary efficacy end point | 71 | 70 | 60 | NP | Res |
| 2 | we randomly assigned patients who | 70 | 70 | 60 | VP | Res |
| 2 | secondary end points included the | 53 | 53 | 80 | VP | Res |
| 2 | with the use of a | 53 | 52 | 100 | PP | Res |
| 2 | with the use of the | 53 | 50 | 100 | PP | Res |
| 2 | the efficacy and safety of | 52 | 51 | 60 | NP | Res |
| 2 | scores range from to with | 51 | 42 | 100 | NP | Res |
| 2 | of death from any cause | 50 | 47 | 100 | PP | Res |
| 2 | reverse transcriptase polymerase chain reaction | 50 | 50 | 0 | NP | Res |
| 2 | with higher scores indicating more | 45 | 43 | 100 | PP | Res |
| 2 | a total of patients with | 44 | 44 | 100 | NP | Res |
| 2 | between the ages of and | 44 | 43 | 100 | PP | Res |
| 2 | to years of age with | 44 | 43 | 100 | PP | Res |
| 2 | the primary end points were | 43 | 43 | 80 | VP | Res |
| 2 | we conducted a double blind | 43 | 43 | 40 | VP | Res |
| 2 | in this randomized double blind | 41 | 41 | 40 | PP | Res |
| 2 | mg per deciliter mmol per | 40 | 29 | 40 | NP | Res |
| 2 | the primary outcome measure was | 39 | 39 | 60 | VP | Res |
| 2 | to years of age who | 39 | 39 | 100 | PP | Res |
| 2 | we conducted a multicenter randomized | 38 | 38 | 40 | VP | Res |
| 2 | double blind placebo controlled phase | 37 | 37 | 20 | NP | Res |
| 2 | of death from cardiovascular causes | 37 | 33 | 80 | PP | Res |
| 2 | the primary efficacy outcome was | 37 | 37 | 40 | VP | Res |
| 2 | a double blind placebo controlled | 34 | 33 | 40 | NP | Res |
| 2 | change from baseline in the | 34 | 31 | 80 | NP | Res |
| 2 | the primary outcome was death | 34 | 34 | 60 | VP | Res |
| 2 | was death from any cause | 34 | 34 | 100 | VP | Res |
| 2 | were to years of age | 34 | 33 | 100 | VP | Res |
| 2 | were randomly assigned to undergo | 33 | 33 | 40 | VP | Res |
| 2 | ml per minute per m | 31 | 23 | 80 | NP | Res |
| 2 | this double blind placebo controlled | 31 | 31 | 40 | NP | Res |
| 2 | at a dose of µg | 30 | 24 | 60 | PP | Res |
| 2 | forced expiratory volume in second | 30 | 30 | 60 | NP | Res |
| 2 | in a randomized double blind | 30 | 30 | 40 | PP | Res |
| 2 | on the basis of the | 29 | 29 | 100 | PP | Text |
| 2 | outcome was the rate of | 29 | 27 | 80 | VP | Res |
| 2 | were years of age or | 29 | 29 | 100 | VP | Res |
| 2 | assessed with the use of | 28 | 27 | 80 | VP | Res |
| 2 | end point was the percentage | 28 | 28 | 80 | VP | Res |
| 2 | the presence or absence of | 28 | 28 | 60 | NP | Text |
| 2 | we randomly assigned patients in | 28 | 28 | 60 | VP | Res |
| 2 | assigned to receive mg of | 27 | 26 | 60 | VP | Res |
| 2 | or death from cardiovascular causes | 26 | 24 | 80 | NP | Res |
| 2 | the primary safety end point | 26 | 24 | 60 | NP | Res |
| 2 | trial we assigned patients with | 26 | 26 | 60 | VP | Res |
| 2 | double blind randomized placebo controlled | 25 | 25 | 20 | NP | Res |
| 2 | mg per day or placebo | 25 | 24 | 60 | NP | Res |
| 2 | nonfatal myocardial infarction nonfatal stroke | 25 | 24 | 0 | NP | Res |
| 2 | double blind trial we randomly | 24 | 24 | 20 | NP | Res |
| 2 | placebo controlled trial we randomly | 24 | 24 | 40 | NP | Res |
| 2 | on the modified rankin scale | 23 | 20 | 40 | PP | Res |
| 2 | to receive either mg of | 23 | 23 | 80 | VP | Res |
| 2 | a total of patients were | 22 | 22 | 100 | NP | Res |
| 2 | end point was death from | 22 | 22 | 100 | VP | Res |
| 2 | placebo controlled phase trial we | 22 | 22 | 40 | NP | Res |
| 2 | the change from baseline to | 22 | 21 | 80 | NP | Res |
| 2 | the two primary end points | 22 | 22 | 80 | NP | Res |
| 2 | we conducted a randomized trial | 22 | 22 | 40 | VP | Res |
| 2 | weight in kilograms divided by | 22 | 22 | 60 | NP | Res |
| 2 | divided by the square of | 21 | 21 | 60 | VP | Res |
| 2 | patients with moderate to severe | 21 | 21 | 60 | NP | Res |
| 2 | performed with the use of | 21 | 21 | 100 | VP | Res |
| 2 | randomized double blind trial we | 21 | 21 | 20 | NP | Res |
| 2 | secondary end points included overall | 21 | 21 | 60 | NP | Res |
| 2 | the patients were randomly assigned | 21 | 21 | 60 | VP | Res |
| 2 | was a sustained virologic response | 21 | 21 | 60 | VP | Res |
| 2 | weeks after the end of | 21 | 21 | 100 | NP | Res |
| 2 | assigned patients with type diabetes | 20 | 20 | 60 | VP | Res |
| 2 | at a dose of or | 20 | 17 | 80 | PP | Res |
| 2 | composite of death myocardial infarction | 20 | 19 | 40 | NP | Res |
| 2 | end point was a sustained | 20 | 20 | 80 | VP | Res |
| 2 | health related quality of life | 20 | 20 | 100 | NP | Res |
| 2 | in the score on the | 20 | 18 | 100 | PP | Res |
| 2 | nonfatal myocardial infarction or nonfatal | 20 | 20 | 20 | NP | Res |
| 2 | out of hospital cardiac arrest | 20 | 18 | 60 | NP | Res |
| 2 | we randomly assigned adults with | 20 | 20 | 60 | VP | Res |
| 2 | children to months of age | 19 | 19 | 100 | NP | Res |
| 2 | end points were overall survival | 19 | 19 | 60 | VP | Res |
| 2 | free survival and overall survival | 19 | 19 | 40 | NP | Res |
| 2 | in the intention to treat | 19 | 18 | 80 | PP | Res |
| 2 | on a scale of to | 19 | 17 | 80 | PP | Res |
| 2 | or placebo in addition to | 19 | 19 | 80 | NP | Res |
| 2 | outcome was the composite of | 19 | 18 | 60 | VP | Res |
| 2 | to mg per deciliter to | 19 | 16 | 60 | PP | Res |
| 2 | a time to event analysis | 18 | 15 | 100 | NP | Res |
| 2 | at a dose of to | 18 | 16 | 80 | PP | Res |
| 2 | double blind phase trial we | 18 | 18 | 20 | NP | Res |
| 2 | ejection fraction of or less | 18 | 18 | 60 | NP | Res |
| 2 | randomly assigned to one of | 18 | 18 | 60 | VP | Res |
| 2 | the primary composite end point | 18 | 18 | 60 | NP | Res |
| 2 | was the composite of death | 18 | 17 | 80 | VP | Res |
| 2 | after the end of treatment | 17 | 17 | 100 | PP | Res |
| 2 | at the end of the | 17 | 16 | 100 | PP | Res |
| 2 | end point was the composite | 17 | 15 | 80 | VP | Res |
| 2 | hours after the onset of | 17 | 17 | 80 | NP | Res |
| 2 | in this phase trial we | 17 | 17 | 60 | PP | Res |
| 2 | investigator assessed progression free survival | 17 | 17 | 20 | NP | Res |
| 2 | mg per kilogram every weeks | 17 | 10 | 60 | NP | Res |
| 2 | non small cell lung cancer | 17 | 16 | 40 | NP | Res |
| 2 | patients were assigned to receive | 17 | 15 | 80 | VP | Res |
| 2 | progression free survival and overall | 17 | 17 | 40 | NP | Res |
| 2 | we conducted a retrospective cohort | 17 | 17 | 40 | VP | Res |
| 2 | were randomly assigned to a | 17 | 17 | 60 | VP | Res |
| 2 | a left ventricular ejection fraction | 16 | 16 | 20 | NP | Res |
| 2 | a scale from to with | 16 | 15 | 80 | NP | Res |
| 2 | key secondary end point was | 16 | 16 | 80 | VP | Res |
| 2 | mg twice daily or placebo | 16 | 15 | 40 | NP | Res |
| 2 | or death from any cause | 16 | 16 | 100 | NP | Res |
| 2 | randomization was stratified according to | 16 | 16 | 60 | VP | Res |
| 2 | randomized placebo controlled double blind | 16 | 16 | 20 | NP | Res |
| 2 | secondary end points were the | 16 | 16 | 80 | VP | Res |
| 2 | the between group difference in | 16 | 14 | 100 | NP | Text |
| 2 | the coprimary end points were | 16 | 16 | 80 | VP | Res |
| 2 | the median follow up was | 16 | 16 | 80 | VP | Res |
| 2 | the primary end point of | 16 | 16 | 80 | NP | Res |
| 2 | the safety and efficacy of | 16 | 16 | 60 | NP | Res |
| 2 | the time to the first | 16 | 14 | 100 | NP | Res |
| 2 | to with lower scores indicating | 16 | 14 | 100 | PP | Res |
| 2 | we conducted an open label | 16 | 16 | 60 | VP | Res |
| 2 | we enrolled patients who had | 16 | 16 | 80 | VP | Res |
| 2 | who had not previously received | 16 | 16 | 80 | VP | Res |
| 2 | a composite of death myocardial | 15 | 15 | 60 | NP | Res |
| 2 | according to the intention to | 15 | 15 | 80 | PP | Res |
| 2 | analyzed with the use of | 15 | 13 | 80 | VP | Res |
| 2 | during the period from through | 15 | 14 | 100 | PP | Res |
| 2 | end points were progression free | 15 | 15 | 80 | VP | Res |
| 2 | every weeks for up to | 15 | 14 | 100 | NP | Res |
| 2 | in this multicenter double blind | 15 | 15 | 40 | PP | Res |
| 2 | of to mg per deciliter | 15 | 13 | 60 | PP | Res |
| 2 | open label phase trial we | 15 | 15 | 40 | NP | Res |
| 2 | the composite of death from | 15 | 15 | 80 | NP | Res |
| 2 | the percentage of patients with | 15 | 13 | 80 | NP | Res |
| 2 | the primary safety outcome was | 15 | 15 | 40 | VP | Res |
| 2 | a composite of cardiovascular death | 14 | 12 | 60 | NP | Res |
| 2 | after the end of therapy | 14 | 14 | 80 | PP | Res |
| 2 | as compared with placebo in | 14 | 14 | 80 | PP | Res |
| 2 | at the time of the | 14 | 13 | 100 | PP | Res |
| 2 | death myocardial infarction or stroke | 14 | 14 | 40 | NP | Res |
| 2 | free survival as assessed by | 14 | 14 | 60 | NP | Res |
| 2 | in a ratio to undergo | 14 | 14 | 60 | PP | Res |
| 2 | mg per kilogram per day | 14 | 11 | 60 | NP | Res |
| 2 | or hospitalization for heart failure | 14 | 14 | 60 | NP | Res |
| 2 | patients who had a response | 14 | 13 | 100 | NP | Res |
| 2 | to years of age and | 14 | 14 | 100 | PP | Res |
| 2 | was the percentage of patients | 14 | 14 | 80 | VP | Res |
| 2 | we conducted a randomized controlled | 14 | 14 | 60 | VP | Res |
| 2 | we randomly assigned women with | 14 | 14 | 60 | VP | Res |
| 2 | a composite of death or | 13 | 13 | 80 | PP | Res |
| 2 | and randomly assigned them to | 13 | 13 | 60 | VP | Res |
| 2 | bmi the weight in kilograms | 13 | 13 | 60 | NP | Res |
| 2 | boundary of the confidence interval | 13 | 13 | 40 | NP | Res |
| 2 | end point was disease free | 13 | 13 | 100 | VP | Res |
| 2 | end point was the first | 13 | 9 | 100 | VP | Res |
| 2 | in a double blind fashion | 13 | 13 | 40 | PP | Res |
| 2 | in this double blind phase | 13 | 13 | 40 | PP | Res |
| 2 | key secondary end points were | 13 | 13 | 80 | VP | Res |
| 2 | of body surface area and | 13 | 13 | 80 | PP | Res |
| 2 | patients with hcv genotype infection | 13 | 10 | 40 | NP | Res |
| 2 | patients with relapsed or refractory | 13 | 13 | 60 | NP | Res |
| 2 | placebo for weeks the primary | 13 | 12 | 60 | NP | Res |
| 2 | point was investigator assessed progression | 13 | 13 | 40 | VP | Res |
| 2 | primary end point was disease | 13 | 13 | 80 | VP | Res |
| 2 | primary end point was survival | 13 | 13 | 60 | VP | Res |
| 2 | psoriasis area and severity index | 13 | 13 | 40 | NP | Res |
| 2 | randomized trial we assigned patients | 13 | 13 | 40 | NP | Res |
| 2 | sustained virologic response at weeks | 13 | 13 | 60 | NP | Res |
| 2 | time to event analysis was | 13 | 12 | 100 | NP | Res |
| 2 | to years of age to | 13 | 12 | 100 | PP | Res |
| 2 | trial to evaluate the efficacy | 13 | 13 | 40 | NP | Res |
| 2 | we performed a randomized double | 13 | 13 | 60 | VP | Res |
| 2 | were followed for up to | 13 | 13 | 100 | VP | Res |
| 2 | who did not have a | 13 | 13 | 100 | VP | Res |
| 2 | years of age or younger | 13 | 13 | 100 | NP | Res |
| 2 | a dose of either mg | 12 | 11 | 60 | NP | Res |
| 2 | aspirin at a dose of | 12 | 11 | 60 | NP | Res |
| 2 | at a daily dose of | 12 | 11 | 80 | PP | Res |
| 2 | at a dose of iu | 12 | 10 | 60 | PP | Res |
| 2 | between and years of age | 12 | 12 | 100 | PP | Res |
| 2 | body mass index bmi the | 12 | 12 | 40 | NP | Res |
| 2 | determined with the use of | 12 | 12 | 100 | VP | Res |
| 2 | end point was the annualized | 12 | 12 | 80 | VP | Res |
| 2 | estimated with the use of | 12 | 12 | 100 | VP | Res |
| 2 | evaluated with the use of | 12 | 12 | 80 | VP | Res |
| 2 | for a median of years | 12 | 12 | 80 | PP | Res |
| 2 | in this double blind trial | 12 | 12 | 40 | PP | Res |
| 2 | measured with the use of | 12 | 12 | 100 | VP | Res |
| 2 | mg once daily or placebo | 12 | 12 | 60 | NP | Res |
| 2 | of patients who had a | 12 | 12 | 100 | PP | Res |
| 2 | on the basis of a | 12 | 12 | 100 | PP | Text |
| 2 | real time polymerase chain reaction | 12 | 12 | 40 | NP | Res |
| 2 | secondary end point was the | 12 | 12 | 80 | VP | Res |
| 2 | st segment elevation myocardial infarction | 12 | 12 | 0 | NP | Res |
| 2 | than years of age who | 12 | 12 | 100 | PP | Res |
| 2 | the percentage of patients who | 12 | 11 | 80 | PP | Res |
| 2 | the primary composite outcome was | 12 | 12 | 40 | VP | Res |
| 2 | the primary objective was to | 12 | 12 | 60 | VP | Res |
| 2 | the primary outcome was day | 12 | 12 | 60 | VP | Res |
| 2 | the primary outcome was survival | 12 | 12 | 40 | VP | Res |
| 2 | the primary outcomes were the | 12 | 12 | 60 | VP | Res |
| 2 | the secondary end points were | 12 | 12 | 80 | VP | Res |
| 2 | to years of age in | 12 | 11 | 100 | PP | Res |
| 2 | trial in which patients with | 12 | 12 | 80 | NP | Res |
| 2 | was the time to the | 12 | 11 | 100 | VP | Res |
| 2 | we evaluated the effect of | 12 | 12 | 80 | VP | Res |
| 2 | we evaluated the efficacy of | 12 | 12 | 60 | VP | Res |
| 2 | we performed a multicenter randomized | 12 | 12 | 60 | VP | Res |
| 2 | were at high risk for | 12 | 12 | 100 | VP | Res |
| 2 | were randomly assigned to the | 12 | 12 | 60 | VP | Res |
| 2 | with a by factorial design | 12 | 12 | 80 | PP | Res |
| 2 | a by factorial design we | 11 | 11 | 80 | NP | Res |
| 2 | a noninferiority margin of percentage | 11 | 11 | 40 | NP | Res |
| 2 | an estimated glomerular filtration rate | 11 | 11 | 60 | NP | Res |
| 2 | an intention to treat basis | 11 | 11 | 80 | NP | Res |
| 2 | assessed in a time to | 11 | 8 | 80 | VP | Res |
| 2 | assigned to one of three | 11 | 11 | 80 | VP | Res |
| 2 | controlled trial involving patients with | 11 | 11 | 80 | PP | Res |
| 2 | death from coronary heart disease | 11 | 10 | 80 | PP | Res |
| 2 | disease patients were randomly assigned | 11 | 11 | 60 | VP | Res |
| 2 | dose of mg every weeks | 11 | 11 | 60 | NP | Res |
| 2 | dose of to mg per | 11 | 10 | 60 | NP | Res |
| 2 | factorial design we randomly assigned | 11 | 11 | 40 | NP | Res |
| 2 | from no symptoms to death | 11 | 11 | 80 | PP | Res |
| 2 | in the intensive care unit | 11 | 11 | 80 | PP | Res |
| 2 | in this double blind randomized | 11 | 11 | 40 | PP | Res |
| 2 | of less than mm hg | 11 | 7 | 60 | PP | Res |
| 2 | of the confidence interval for | 11 | 11 | 60 | PP | Res |
| 2 | open label randomized controlled trial | 11 | 11 | 40 | NP | Res |
| 2 | patients at high risk for | 11 | 11 | 100 | NP | Res |
| 2 | patients were stratified according to | 11 | 11 | 80 | VP | Res |
| 2 | patients who had had a | 11 | 11 | 100 | NP | Res |
| 2 | patients with type diabetes who | 11 | 11 | 80 | NP | Res |
| 2 | peginterferon alfa a and ribavirin | 11 | 7 | 40 | NP | Res |
| 2 | per square meter on day | 11 | 8 | 60 | NP | Res |
| 2 | randomly assigned them to receive | 11 | 11 | 60 | VP | Res |
| 2 | safety outcome was major bleeding | 11 | 11 | 40 | VP | Res |
| 2 | single nucleotide polymorphisms snps in | 11 | 11 | 40 | NP | Res |
| 2 | the principal safety outcome was | 11 | 11 | 40 | VP | Res |
| 2 | the rate of death from | 11 | 10 | 100 | NP | Res |
| 2 | to evaluate the efficacy and | 11 | 11 | 60 | VP | Res |
| 2 | to one of three groups | 11 | 11 | 100 | PP | Res |
| 2 | was a secondary end point | 11 | 11 | 80 | VP | Res |
| 2 | was death from cardiovascular causes | 11 | 11 | 80 | VP | Res |
| 2 | we randomly assigned patients years | 11 | 11 | 60 | VP | Res |
| 2 | who had a response to | 11 | 11 | 100 | VP | Res |
| 2 | who received a diagnosis of | 11 | 10 | 80 | VP | Res |
| 2 | within hours after symptom onset | 11 | 11 | 60 | PP | Res |
| 2 | a glycated hemoglobin level of | 10 | 8 | 60 | NP | Res |
| 2 | a multicenter double blind randomized | 10 | 10 | 20 | NP | Res |
| 2 | a multicenter randomized open label | 10 | 10 | 40 | NP | Res |
| 2 | and the primary end point | 10 | 10 | 80 | NP | Res |
| 2 | area under the curve auc | 10 | 10 | 60 | NP | Res |
| 2 | bone mineral density at the | 10 | 8 | 40 | NP | Res |
| 2 | cardiovascular death myocardial infarction or | 10 | 10 | 40 | NP | Res |
| 2 | cisplatin mg per square meter | 10 | 10 | 20 | NP | Res |
| 2 | clinical trial we randomly assigned | 10 | 10 | 20 | NP | Res |
| 2 | controlled study we randomly assigned | 10 | 10 | 60 | NP | Res |
| 2 | coprimary end points were the | 10 | 10 | 80 | VP | Res |
| 2 | data and safety monitoring board | 10 | 10 | 60 | NP | Res |
| 2 | death from any cause myocardial | 10 | 10 | 80 | NP | Res |
| 2 | estimated glomerular filtration rate gfr | 10 | 10 | 40 | NP | Res |
| 2 | evaluation criteria in solid tumors | 10 | 10 | 20 | NP | Res |
| 2 | had been randomly assigned to | 10 | 9 | 60 | VP | Res |
| 2 | less than copies per milliliter | 10 | 10 | 60 | Oth | Res |
| 2 | less than per cubic millimeter | 10 | 9 | 60 | Oth | Res |
| 2 | median follow up was years | 10 | 10 | 80 | VP | Res |
| 2 | noninferiority trial we randomly assigned | 10 | 10 | 20 | NP | Res |
| 2 | of at least mg per | 10 | 8 | 80 | PP | Res |
| 2 | older than years of age | 10 | 10 | 100 | Oth | Res |
| 2 | patients in the placebo group | 10 | 10 | 80 | NP | Res |
| 2 | proportional hazards models were used | 10 | 10 | 60 | VP | Res |
| 2 | ranges from to with higher | 10 | 10 | 100 | VP | Res |
| 2 | tested for an association between | 10 | 9 | 80 | VP | Res |
| 2 | the primary outcome measures were | 10 | 10 | 60 | VP | Res |
| 2 | to evaluate the efficacy of | 10 | 10 | 60 | VP | Res |
| 2 | to receive subcutaneous injections of | 10 | 10 | 60 | VP | Res |
| 2 | trial we enrolled patients with | 10 | 10 | 60 | NP | Res |
| 2 | we conducted a randomized open | 10 | 10 | 60 | VP | Res |
| 2 | we conducted a randomized placebo | 10 | 10 | 40 | VP | Res |
| 2 | we randomly assigned participants with | 10 | 10 | 40 | VP | Res |
| 2 | weeks days to weeks days | 10 | 9 | 100 | NP | Res |
| 2 | were assigned in a ratio | 10 | 10 | 60 | VP | Res |
| 2 | were randomly assigned to either | 10 | 10 | 60 | VP | Res |
| 2 | were randomly assigned to treatment | 10 | 10 | 60 | VP | Res |
| 3 | confidence interval ci to p | 681 | 681 | 20 | NP | Res |
| 3 | hazard ratio ci to p | 421 | 272 | 20 | NP | Res |
| 3 | percent confidence interval to p | 310 | 155 | 40 | NP | Res |
| 3 | of the patients in the | 291 | 192 | 100 | PP | Res |
| 3 | hazard ratio confidence interval ci | 230 | 230 | 0 | NP | Res |
| 3 | in of patients in the | 225 | 149 | 100 | PP | Res |
| 3 | than in the placebo group | 221 | 173 | 80 | Oth | Res |
| 3 | similar in the two groups | 208 | 193 | 100 | Oth | Text |
| 3 | in the placebo group p | 185 | 138 | 60 | PP | Res |
| 3 | a total of patients were | 180 | 180 | 100 | VP | Res |
| 3 | and in the placebo group | 180 | 138 | 80 | PP | Res |
| 3 | did not differ significantly between | 176 | 163 | 60 | VP | Text |
| 3 | a median follow up of | 168 | 165 | 80 | NP | Res |
| 3 | in of the patients in | 168 | 114 | 100 | PP | Res |
| 3 | occurred in patients in the | 160 | 110 | 100 | VP | Res |
| 3 | there was no significant difference | 152 | 142 | 100 | VP | Text |
| 3 | the placebo group hazard ratio | 149 | 107 | 40 | NP | Res |
| 3 | was confidence interval ci to | 148 | 148 | 40 | VP | Res |
| 3 | occurred in of the patients | 145 | 119 | 100 | VP | Res |
| 3 | were similar in the two | 145 | 143 | 100 | VP | Text |
| 3 | in the placebo group hazard | 143 | 103 | 60 | PP | Res |
| 3 | patients in the placebo group | 142 | 105 | 80 | NP | Res |
| 3 | as compared with in the | 139 | 118 | 100 | PP | Text |
| 3 | group hazard ratio ci to | 138 | 109 | 40 | NP | Res |
| 3 | there were no significant differences | 134 | 128 | 100 | VP | Text |
| 3 | percent percent confidence interval to | 131 | 58 | 60 | NP | Res |
| 3 | percent confidence interval to percent | 123 | 62 | 60 | NP | Res |
| 3 | mg per deciliter mmol per | 121 | 60 | 40 | NP | Res |
| 3 | occurred in of patients in | 117 | 102 | 100 | VP | Res |
| 3 | hazard ratio percent confidence interval | 115 | 63 | 20 | NP | Res |
| 3 | relative risk percent confidence interval | 115 | 55 | 40 | NP | Res |
| 3 | a total of patients underwent | 109 | 109 | 80 | VP | Res |
| 3 | confidence interval ci to and | 107 | 107 | 40 | NP | Res |
| 3 | difference percentage points ci to | 104 | 70 | 60 | NP | Res |
| 3 | group hazard ratio confidence interval | 97 | 97 | 20 | NP | Res |
| 3 | in the intention to treat | 96 | 84 | 80 | PP | Res |
| 3 | odds ratio ci to p | 96 | 61 | 20 | NP | Res |
| 3 | percentage points confidence interval ci | 96 | 96 | 20 | NP | Res |
| 3 | was similar in the two | 95 | 89 | 100 | VP | Text |
| 3 | percent confidence interval to and | 94 | 71 | 60 | NP | Res |
| 3 | confidence interval ci to in | 93 | 93 | 40 | NP | Res |
| 3 | difference percentage points confidence interval | 90 | 90 | 40 | NP | Res |
| 3 | ci to and ci to | 89 | 67 | 60 | NP | Res |
| 3 | relative risk ci to p | 88 | 51 | 40 | NP | Res |
| 3 | was percent confidence interval to | 85 | 66 | 60 | VP | Res |
| 3 | interval ci to p and | 84 | 84 | 40 | NP | Res |
| 3 | median progression free survival was | 83 | 78 | 40 | VP | Res |
| 3 | group as compared with the | 79 | 74 | 100 | NP | Text |
| 3 | vs hazard ratio ci to | 79 | 44 | 20 | PP | Res |
| 3 | than in the control group | 78 | 60 | 100 | Oth | Res |
| 3 | group and in in the | 76 | 63 | 100 | NP | Text |
| 3 | group as compared with of | 74 | 65 | 100 | NP | Text |
| 3 | group confidence interval ci to | 74 | 74 | 40 | NP | Res |
| 3 | group and patients in the | 71 | 53 | 100 | NP | Res |
| 3 | ratio ci to p and | 71 | 59 | 40 | NP | Res |
| 3 | the most common adverse events | 71 | 71 | 80 | NP | Res |
| 3 | ratio confidence interval to p | 69 | 69 | 20 | NP | Res |
| 3 | those in the placebo group | 69 | 54 | 80 | NP | Res |
| 3 | and of patients in the | 68 | 60 | 100 | PP | Res |
| 3 | risk confidence interval ci to | 68 | 68 | 40 | NP | Res |
| 3 | in the control group p | 66 | 48 | 80 | PP | Res |
| 3 | of death from any cause | 66 | 61 | 100 | PP | Res |
| 3 | years of age or older | 66 | 54 | 100 | NP | Res |
| 3 | a total of of the3 | 65 | 63 | 100 | NP | Text |
| 3 | after a median follow up | 65 | 64 | 80 | PP | Res |
| 3 | in the placebo group and | 64 | 50 | 80 | PP | Res |
| 3 | the median progression free survival | 64 | 60 | 40 | NP | Res |
| 3 | there were no significant between | 64 | 63 | 100 | VP | Text |
| 3 | were randomly assigned to receive | 63 | 57 | 60 | VP | Res |
| 3 | group and percent in the | 62 | 49 | 100 | NP | Res |
| 3 | in the group that received | 62 | 34 | 100 | PP | Res |
| 3 | hazard ratio for disease progression | 61 | 61 | 40 | NP | Res |
| 3 | of the primary end point | 61 | 56 | 80 | PP | Res |
| 3 | patients were randomly assigned to | 61 | 60 | 60 | VP | Res |
| 3 | patients in the control group | 60 | 40 | 100 | NP | Res |
| 3 | respectively hazard ratio ci to | 60 | 46 | 20 | Oth | Res |
| 3 | the intention to treat analysis | 60 | 58 | 80 | NP | Res |
| 3 | and ci to in the | 59 | 51 | 80 | NP | Res |
| 3 | associated with an increased risk | 58 | 54 | 100 | VP | Res |
| 3 | death confidence interval ci to | 58 | 58 | 40 | NP | Res |
| 3 | in the medical therapy group | 58 | 26 | 60 | PP | Res |
| 3 | adverse events of grade or | 57 | 54 | 60 | NP | Res |
| 3 | as compared with of those | 57 | 47 | 100 | PP | Text |
| 3 | ci to p for noninferiority | 57 | 51 | 40 | NP | Res |
| 3 | group and the placebo group | 56 | 45 | 80 | NP | Res |
| 3 | group hazard ratio for death | 56 | 53 | 60 | NP | Res |
| 3 | to and ci to respectively | 56 | 47 | 60 | PP | Res |
| 3 | at a median follow up | 55 | 53 | 80 | PP | Res |
| 3 | of patients in the placebo | 55 | 50 | 80 | PP | Res |
| 3 | percentage points ci to p | 54 | 41 | 40 | NP | Res |
| 3 | adverse events were similar in | 53 | 53 | 80 | VP | Res |
| 3 | between group difference in the | 53 | 50 | 100 | NP | Res |
| 3 | for disease progression or death | 53 | 52 | 80 | PP | Res |
| 3 | of the patients who received | 53 | 36 | 100 | PP | Res |
| 3 | the median overall survival was | 53 | 48 | 40 | VP | Res |
| 3 | hazard ratio confidence interval to | 52 | 52 | 20 | NP | Res |
| 3 | survival was months in the | 52 | 47 | 80 | VP | Res |
| 3 | in the placebo group had | 51 | 45 | 80 | VP | Res |
| 3 | ratio for disease progression or | 51 | 51 | 60 | NP | Res |
| 3 | significant between group difference in | 51 | 49 | 100 | NP | Text |
| 3 | the primary outcome occurred in | 51 | 51 | 60 | VP | Res |
| 3 | group and in of patients | 50 | 41 | 100 | NP | Res |
| 3 | of those in the placebo | 50 | 37 | 80 | PP | Res |
| 3 | percentage points confidence interval to | 50 | 50 | 40 | NP | Res |
| 3 | as compared with of patients | 49 | 43 | 100 | PP | Res |
| 3 | by the log rank test | 48 | 36 | 60 | PP | Res |
| 3 | the primary end point was | 48 | 44 | 80 | VP | Res |
| 3 | in the combination therapy group | 47 | 22 | 60 | PP | Res |
| 3 | in the standard therapy group | 47 | 20 | 80 | PP | Res |
| 3 | there was no significant between | 47 | 46 | 100 | VP | Text |
| 3 | a total of patients in | 46 | 45 | 100 | NP | Res |
| 3 | as compared with percent of | 46 | 40 | 100 | PP | Res |
| 3 | group and months in the | 46 | 42 | 100 | NP | Res |
| 3 | serious adverse events occurred in | 46 | 44 | 80 | VP | Res |
| 3 | in of those in the | 45 | 37 | 100 | PP | Text |
| 3 | ml per minute per m | 45 | 19 | 80 | NP | Res |
| 3 | was significantly higher in the | 45 | 44 | 80 | VP | Text |
| 3 | adjusted odds ratio ci to | 44 | 26 | 20 | NP | Res |
| 3 | and in of the patients | 44 | 38 | 100 | PP | Res |
| 3 | of in the placebo group | 44 | 33 | 80 | PP | Res |
| 3 | were more common in the | 44 | 42 | 100 | VP | Text |
| 3 | p for the comparison of | 43 | 21 | 60 | NP | Res |
| 3 | primary end point occurred in | 43 | 39 | 80 | VP | Res |
| 3 | the modified intention to treat | 43 | 35 | 60 | NP | Res |
| 3 | a total of of patients4 | 42 | 40 | 100 | NP | Res |
| 3 | adverse events occurred in of | 42 | 39 | 80 | VP | Res |
| 3 | ci to p and the | 42 | 37 | 60 | NP | Res |
| 3 | in the intervention group and | 42 | 24 | 80 | PP | Res |
| 3 | per person years in the | 42 | 21 | 100 | PP | Res |
| 3 | was significantly lower in the | 42 | 41 | 80 | VP | Text |
| 3 | as compared with of the | 41 | 39 | 100 | PP | Text |
| 3 | at a dose of mg | 41 | 26 | 60 | PP | Res |
| 3 | during a median follow up | 41 | 41 | 80 | PP | Res |
| 3 | group and of in the | 41 | 35 | 100 | NP | Res |
| 3 | the proportion of patients with | 41 | 37 | 80 | NP | Res |
| 3 | of the patients who underwent | 40 | 39 | 80 | PP | Res |
| 3 | the median follow up was | 40 | 38 | 80 | VP | Res |
| 3 | confidence interval ci to with | 39 | 39 | 40 | NP | Res |
| 3 | as compared with the placebo | 38 | 36 | 80 | PP | Res |
| 3 | confidence interval ci to for | 38 | 38 | 40 | NP | Res |
| 3 | in of the patients who | 38 | 25 | 100 | PP | Res |
| 3 | increase in the risk of | 38 | 28 | 100 | NP | Res |
| 3 | the percentage of patients with | 38 | 33 | 80 | NP | Res |
| 3 | and percent confidence interval to | 37 | 30 | 60 | NP | Res |
| 3 | group relative risk ci to | 37 | 29 | 60 | NP | Res |
| 3 | group relative risk confidence interval | 37 | 37 | 40 | NP | Res |
| 3 | in the placebo group relative | 37 | 30 | 60 | PP | Res |
| 3 | the rate of death from | 37 | 33 | 100 | NP | Res |
| 3 | were confidence interval ci to | 37 | 37 | 40 | VP | Res |
| 3 | as compared with the control | 36 | 32 | 100 | PP | Res |
| 3 | ci to in the placebo | 36 | 29 | 60 | NP | Res |
| 3 | of death from cardiovascular causes | 36 | 35 | 80 | PP | Res |
| 3 | or death ci to p | 36 | 34 | 60 | NP | Res |
| 3 | reduction in the risk of | 36 | 25 | 80 | NP | Res |
| 3 | vs relative risk ci to | 36 | 16 | 40 | PP | Res |
| 3 | adjusted hazard ratio ci to | 35 | 22 | 20 | NP | Res |
| 3 | person years of follow up | 35 | 29 | 100 | NP | Res |
| 3 | the rate of the primary | 35 | 27 | 80 | NP | Res |
| 3 | group hazard ratio in the | 34 | 33 | 60 | NP | Res |
| 3 | in the group assigned to | 34 | 17 | 80 | PP | Res |
| 3 | odds ratio confidence interval to | 34 | 34 | 20 | NP | Res |
| 3 | the placebo group in the | 34 | 30 | 80 | NP | Res |
| 3 | hazard ratio ci to and | 33 | 29 | 40 | NP | Res |
| 3 | in of participants in the | 33 | 18 | 80 | PP | Res |
| 3 | percent in the placebo group | 33 | 30 | 80 | NP | Res |
| 3 | progression or death confidence interval | 33 | 33 | 40 | NP | Res |
| 3 | underwent randomization were assigned to | 33 | 33 | 40 | VP | Res |
| 3 | in the risk of death | 32 | 28 | 100 | PP | Res |
| 3 | in the two study groups | 32 | 31 | 100 | PP | Res |
| 3 | median follow up period of | 32 | 32 | 80 | NP | Res |
| 3 | of the participants in the | 32 | 22 | 80 | PP | Res |
| 3 | the incidence of adverse events | 32 | 30 | 60 | NP | Res |
| 3 | were more likely to have | 32 | 29 | 100 | VP | Participant |
| 3 | in percent of the patients | 31 | 28 | 100 | PP | Res |
| 3 | group confidence interval to p | 30 | 30 | 40 | NP | Res |
| 3 | p for the comparison with | 30 | 22 | 60 | NP | Res |
| 3 | the hazard ratio for death | 30 | 23 | 60 | NP | Res |
| 3 | the presence or absence of | 30 | 28 | 60 | NP | Text |
| 3 | adjusted odds ratio percent confidence | 29 | 20 | 20 | NP | Res |
| 3 | adverse events was similar in | 29 | 29 | 80 | VP | Res |
| 3 | group and in of the | 29 | 25 | 100 | NP | Res |
| 3 | group than in the standard | 29 | 20 | 100 | NP | Res |
| 3 | in and of the patients | 29 | 28 | 100 | PP | Res |
| 3 | in the mg group and | 29 | 20 | 80 | PP | Res |
| 3 | months vs. months hazard ratio | 29 | 23 | 40 | NP | Res |
| 3 | patients in the rivaroxaban group | 29 | 15 | 80 | NP | Res |
| 3 | percent and percent respectively p | 29 | 25 | 60 | NP | Res |
| 3 | percent of patients in the | 29 | 15 | 100 | NP | Res |
| 3 | percent of those in the | 29 | 22 | 100 | NP | Res |
| 3 | reduction in the rate of | 29 | 25 | 80 | NP | Res |
| 3 | relative risk confidence interval to | 29 | 29 | 40 | NP | Res |
| 3 | the percentage of patients who | 29 | 24 | 80 | NP | Res |
| 3 | younger than years of age | 29 | 26 | 100 | Oth | Res |
| 3 | adverse events were reported in | 28 | 28 | 80 | VP | Res |
| 3 | patients who could be evaluated | 28 | 24 | 80 | NP | Res |
| 3 | the median age of the | 28 | 28 | 80 | NP | Res |
| 3 | a mean follow up of | 27 | 27 | 100 | NP | Res |
| 3 | among the patients in the | 27 | 22 | 100 | PP | Res |
| 3 | and of the patients in | 27 | 26 | 100 | PP | Res |
| 3 | differences in the rates of | 27 | 26 | 100 | NP | Res |
| 3 | incidence rate ratio ci to | 27 | 12 | 40 | NP | Res |
| 3 | patients were assigned to the | 27 | 25 | 80 | VP | Res |
| 3 | percent of the patients in | 27 | 19 | 100 | NP | Res |
| 3 | percent vs. percent p and | 27 | 26 | 60 | NP | Res |
| 3 | between the ages of and | 26 | 17 | 100 | PP | Res |
| 3 | group p for both comparisons | 26 | 23 | 60 | NP | Res |
| 3 | in the standard care group | 26 | 12 | 100 | PP | Res |
| 3 | months confidence interval ci to | 26 | 26 | 40 | NP | Res |
| 3 | odds ratio ci to and | 26 | 19 | 40 | NP | Res |
| 3 | percent percent and percent respectively | 26 | 15 | 80 | NP | Res |
| 3 | rate ratio ci to p | 26 | 15 | 40 | NP | Res |
| 3 | the median age was years | 26 | 26 | 80 | VP | Res |
| 3 | a primary end point event | 25 | 24 | 80 | NP | Res |
| 3 | adjusted odds ratio confidence interval | 25 | 25 | 0 | NP | Res |
| 3 | an intention to treat analysis | 25 | 25 | 80 | NP | Res |
| 3 | as compared with patients in | 25 | 25 | 100 | PP | Res |
| 3 | change from baseline in the | 25 | 22 | 80 | NP | Text |
| 3 | for death from any cause | 25 | 24 | 100 | PP | Res |
| 3 | in the control group vs | 25 | 20 | 80 | PP | Res |
| 3 | in the intensive therapy group | 25 | 12 | 60 | PP | Res |
| 3 | mmol per liter in the | 25 | 11 | 60 | NP | Res |
| 3 | months ci to in the | 25 | 17 | 80 | NP | Res |
| 3 | of adverse events were similar | 25 | 25 | 80 | VP | Res |
| 3 | outcome occurred in of patients | 25 | 25 | 80 | VP | Res |
| 3 | progression free survival was significantly | 25 | 24 | 40 | VP | Res |
| 3 | received at least one dose | 25 | 23 | 80 | VP | Res |
| 3 | reported in of the patients | 25 | 25 | 100 | VP | Res |
| 3 | the data and safety monitoring | 25 | 25 | 60 | NP | Res |
| 3 | the risk of death from | 25 | 19 | 100 | NP | Res |
| 3 | a difference of percentage points | 24 | 17 | 80 | NP | Res |
| 3 | a total of patients with | 24 | 24 | 100 | NP | Res |
| 3 | and were included in the | 24 | 24 | 100 | VP | Text |
| 3 | death percent confidence interval to | 24 | 18 | 60 | NP | Res |
| 3 | group and the control group | 24 | 23 | 100 | NP | Res |
| 3 | group percent confidence interval to | 24 | 15 | 60 | NP | Res |
| 3 | in of patients who received | 24 | 17 | 100 | PP | Res |
| 3 | in the rivaroxaban group and | 24 | 12 | 80 | PP | Res |
| 3 | in the vaccine group and | 24 | 15 | 80 | PP | Res |
| 3 | no significant differences in the | 24 | 22 | 100 | NP | Text |
| 3 | of the patients treated with | 24 | 19 | 100 | PP | Res |
| 3 | and of the patients respectively | 23 | 20 | 80 | NP | Res |
| 3 | as compared with those who | 23 | 17 | 100 | PP | Text |
| 3 | associated with an increase in | 23 | 23 | 100 | VP | Text |
| 3 | during a mean follow up | 23 | 23 | 100 | PP | Res |
| 3 | end point occurred in patients | 23 | 19 | 100 | VP | Res |
| 3 | events occurred in of the | 23 | 23 | 100 | VP | Res |
| 3 | follow up of years the | 23 | 23 | 100 | NP | Res |
| 3 | followed for a median of | 23 | 23 | 80 | VP | Res |
| 3 | in the high dose group | 23 | 12 | 80 | PP | Res |
| 3 | of percentage points ci to | 23 | 21 | 60 | PP | Res |
| 3 | of the patients assigned to | 23 | 16 | 80 | PP | Res |
| 3 | outcome occurred in patients in | 23 | 22 | 80 | VP | Res |
| 3 | the weight in kilograms divided | 23 | 23 | 60 | NP | Res |
| 3 | therapy group than in the | 23 | 17 | 80 | NP | Res |
| 3 | there were deaths in the | 23 | 23 | 100 | VP | Res |
| 3 | vs odds ratio ci to | 23 | 18 | 20 | PP | Res |
| 3 | with a median follow up | 23 | 23 | 80 | PP | Res |
| 3 | with of patients in the | 23 | 22 | 100 | PP | Res |
| 3 | a dose of mg per | 22 | 14 | 60 | NP | Res |
| 3 | as compared with percent in | 22 | 21 | 100 | PP | Res |
| 3 | as compared with placebo was | 22 | 19 | 80 | VP | Res |
| 3 | between the groups in the | 22 | 21 | 100 | PP | Res |
| 3 | confidence interval to p for | 22 | 17 | 40 | NP | Res |
| 3 | difference confidence interval ci to | 22 | 22 | 40 | NP | Res |
| 3 | difference in the rate of | 22 | 22 | 100 | NP | Res |
| 3 | events occurred in of patients | 22 | 20 | 100 | NP | Res |
| 3 | follow up period of years | 22 | 22 | 100 | NP | Res |
| 3 | group hazard ratio for disease | 22 | 22 | 60 | NP | Res |
| 3 | had a sustained virologic response | 22 | 15 | 60 | VP | Res |
| 3 | in the per protocol analysis | 22 | 20 | 80 | PP | Res |
| 3 | in the usual care group | 22 | 13 | 80 | PP | Res |
| 3 | months in the placebo group | 22 | 20 | 80 | NP | Res |
| 3 | patients were included in the | 22 | 22 | 100 | VP | Res |
| 3 | percentage of patients with a | 22 | 19 | 80 | NP | Res |
| 3 | placebo hazard ratio ci to | 22 | 20 | 20 | NP | Res |
| 3 | rates of serious adverse events | 22 | 22 | 80 | NP | Res |
| 3 | ratio for death in the | 22 | 22 | 80 | NP | Res |
| 3 | the ages of and years | 22 | 16 | 100 | NP | Res |
| 3 | the objective response rate was | 22 | 22 | 80 | VP | Res |
| 3 | the upper limit of the | 22 | 20 | 80 | NP | Res |
| 3 | there was no evidence of | 22 | 22 | 100 | VP | Text |
| 3 | vs in the placebo group | 22 | 20 | 60 | PP | Res |
| 3 | was ci to in the | 22 | 19 | 80 | VP | Res |
| 3 | was significantly longer in the | 22 | 22 | 80 | VP | Text |
| 3 | with respect to the primary | 22 | 22 | 80 | PP | Res |
| 3 | a total of patients received | 21 | 21 | 100 | NP | Res |
| 3 | at years was in the | 21 | 15 | 100 | PP | Res |
| 3 | end point occurred in of | 21 | 21 | 100 | VP | Res |
| 3 | free survival was significantly longer | 21 | 20 | 60 | VP | Res |
| 3 | hazard ratio for death p | 21 | 20 | 40 | NP | Res |
| 3 | in the per protocol population | 21 | 16 | 80 | PP | Res |
| 3 | in the placebo group of | 21 | 20 | 80 | PP | Res |
| 3 | median age of the patients | 21 | 21 | 80 | NP | Res |
| 3 | occurred in and of the | 21 | 20 | 100 | VP | Res |
| 3 | of the patients who were | 21 | 19 | 100 | PP | Res |
| 3 | on the basis of the | 21 | 20 | 100 | PP | Text |
| 3 | participants in the placebo group | 21 | 16 | 60 | NP | Res |
| 3 | patients in the intervention group | 21 | 11 | 80 | NP | Res |
| 3 | patients were assigned to receive | 21 | 19 | 80 | VP | Res |
| 3 | rate of sustained virologic response | 21 | 16 | 60 | NP | Res |
| 3 | serious adverse events were reported | 21 | 21 | 80 | VP | Res |
| 3 | significant difference between the two | 21 | 21 | 100 | NP | Text |
| 3 | survival was months ci to | 21 | 18 | 60 | VP | Res |
| 3 | the group given mg of | 21 | 10 | 80 | NP | Res |
| 3 | the overall response rate was | 21 | 20 | 80 | VP | Res |
| 3 | there were no serious adverse | 21 | 21 | 80 | VP | Res |
| 3 | was associated with a significantly | 21 | 20 | 80 | VP | Text |
| 3 | were included in the analysis | 21 | 21 | 100 | VP | Res |
| 3 | were percent confidence interval to | 21 | 17 | 60 | VP | Res |
| 3 | were reported in of the | 21 | 21 | 100 | VP | Res |
| 3 | a percent reduction in the | 20 | 14 | 80 | NP | Res |
| 3 | adjusted hazard ratio confidence interval | 20 | 20 | 0 | NP | Res |
| 3 | as compared with months in | 20 | 17 | 100 | PP | Res |
| 3 | at the end of the | 20 | 18 | 100 | PP | Res |
| 3 | ci to p and a | 20 | 19 | 60 | NP | Res |
| 3 | control group hazard ratio for | 20 | 18 | 60 | NP | Res |
| 3 | difference between the two groups | 20 | 20 | 100 | NP | Text |
| 3 | for the primary end point | 20 | 19 | 80 | PP | Res |
| 3 | group odds ratio ci to | 20 | 15 | 40 | NP | Res |
| 3 | group odds ratio confidence interval | 20 | 20 | 20 | NP | Res |
| 3 | in of infants in the | 20 | 8 | 80 | PP | Res |
| 3 | in of patients assigned to | 20 | 15 | 80 | PP | Res |
| 3 | in the intensive care unit | 20 | 20 | 80 | PP | Res |
| 3 | interquartile range to in the | 20 | 10 | 80 | NP | Res |
| 3 | less than copies per milliliter | 20 | 15 | 60 | Oth | Res |
| 3 | occurred in of patients receiving | 20 | 19 | 100 | VP | Res |
| 3 | the mean age of the | 20 | 20 | 100 | NP | Res |
| 3 | the median duration of response | 20 | 20 | 60 | NP | Res |
| 3 | therapy was associated with a | 20 | 18 | 80 | VP | Res |
| 3 | to years of age and | 20 | 18 | 100 | PP | Res |
| 3 | were followed for a median | 20 | 20 | 80 | VP | Res |
| 3 | were more frequent in the | 20 | 19 | 80 | VP | Text |
| 3 | were randomly assigned to the | 20 | 20 | 60 | VP | Res |
| 3 | were to years of age | 20 | 13 | 100 | VP | Res |
| 3 | years of follow up the | 20 | 18 | 100 | NP | Res |
| 3 | a total of participants were | 19 | 19 | 80 | NP | Res |
| 3 | confidence interval to in the | 19 | 12 | 60 | NP | Res |
| 3 | confidence interval to percent and | 19 | 16 | 60 | NP | Res |
| 3 | did not differ significantly among | 19 | 18 | 60 | VP | Text |
| 3 | follow up period of months | 19 | 19 | 100 | NP | Res |
| 3 | forced expiratory volume in second | 19 | 19 | 60 | NP | Res |
| 3 | group as compared with percent | 19 | 18 | 100 | NP | Res |
| 3 | group percent vs. percent p | 19 | 16 | 60 | NP | Res |
| 3 | hazard ratio for death from | 19 | 16 | 60 | NP | Res |
| 3 | in of the patients and | 19 | 18 | 100 | PP | Res |
| 3 | modified intention to treat population | 19 | 15 | 60 | NP | Res |
| 3 | of adverse events was similar | 19 | 19 | 80 | VP | Res |
| 3 | of percentage points confidence interval | 19 | 19 | 40 | PP | Res |
| 3 | of years of follow up | 19 | 18 | 100 | PP | Res |
| 3 | p for the comparison between | 19 | 13 | 60 | NP | Res |
| 3 | patients who underwent randomization were | 19 | 19 | 60 | VP | Res |
| 3 | per deciliter μmol per liter | 19 | 10 | 40 | PP | Res |
| 3 | primary outcome occurred in patients | 19 | 19 | 60 | VP | Res |
| 3 | rate of progression free survival | 19 | 16 | 60 | NP | Res |
| 3 | relative risk ci to and | 19 | 17 | 60 | NP | Res |
| 3 | survival was months confidence interval | 19 | 19 | 40 | VP | Res |
| 3 | vs hazard ratio confidence interval | 19 | 19 | 0 | PP | Res |
| 3 | who were randomly assigned to | 19 | 15 | 60 | VP | Res |
| 3 | a median of years of | 18 | 17 | 80 | NP | Res |
| 3 | absolute risk difference percentage points | 18 | 15 | 60 | NP | Res |
| 3 | and percent of those in | 18 | 17 | 100 | NP | Res |
| 3 | and the placebo group in | 18 | 16 | 80 | NP | Res |
| 3 | associated with a reduction in | 18 | 17 | 80 | VP | Text |
| 3 | ci to p by the | 18 | 12 | 60 | NP | Res |
| 3 | confidence interval for the difference | 18 | 15 | 60 | NP | Res |
| 3 | confidence interval to p by | 18 | 16 | 40 | NP | Res |
| 3 | duration of follow up was | 18 | 18 | 80 | NP | Res |
| 3 | end point event occurred in | 18 | 17 | 100 | VP | Res |
| 3 | in the control group had | 18 | 17 | 100 | PP | Res |
| 3 | in the group given mg | 18 | 8 | 80 | PP | Res |
| 3 | in the sirolimus stent group | 18 | 8 | 60 | PP | Res |
| 3 | intervention group and in the | 18 | 15 | 80 | NP | Res |
| 3 | level was mg per deciliter | 18 | 15 | 60 | VP | Res |
| 3 | months hazard ratio for death | 18 | 18 | 60 | NP | Res |
| 3 | occurred more frequently in the | 18 | 17 | 80 | VP | Res |
| 3 | of ci to in the | 18 | 11 | 80 | PP | Res |
| 3 | on the modified rankin scale | 18 | 15 | 40 | PP | Res |
| 3 | p and the rate of | 18 | 16 | 80 | NP | Res |
| 3 | p for both comparisons with | 18 | 16 | 60 | NP | Res |
| 3 | percent confidence interval to as | 18 | 15 | 60 | NP | Res |
| 3 | rates of adverse events were | 18 | 18 | 80 | NP | Res |
| 3 | respectively relative risk ci to | 18 | 15 | 40 | NP | Res |
| 3 | significant differences between the two | 18 | 18 | 100 | NP | Text |
| 3 | the patients in the placebo | 18 | 16 | 80 | NP | Res |
| 3 | therapy group and in the | 18 | 13 | 80 | NP | Res |
| 3 | to with higher scores indicating | 18 | 18 | 100 | PP | Text |
| 3 | was associated with a higher | 18 | 18 | 100 | VP | Text |
| 3 | was associated with a reduction | 18 | 18 | 80 | VP | Text |
| 3 | was associated with a significant | 18 | 18 | 100 | VP | Text |
| 3 | with a reduction in the | 18 | 17 | 80 | PP | Text |
| 3 | and death from any cause | 17 | 14 | 100 | NP | Res |
| 3 | did not differ between the | 17 | 17 | 80 | VP | Text |
| 3 | group and in the mg | 17 | 12 | 80 | NP | Res |
| 3 | group and in the standard | 17 | 10 | 100 | NP | Res |
| 3 | group hazard ratio for progression | 17 | 17 | 40 | NP | Res |
| 3 | hazard ratio for death with | 17 | 15 | 60 | NP | Res |
| 3 | in of the patients with | 17 | 16 | 100 | PP | Res |
| 3 | in the placebo group percent | 17 | 16 | 80 | PP | Res |
| 3 | intervention group than in the | 17 | 14 | 80 | NP | Res |
| 3 | mean follow up of years | 17 | 17 | 100 | NP | Res |
| 3 | of patients who underwent randomization | 17 | 17 | 60 | PP | Res |
| 3 | of those who received placebo | 17 | 15 | 80 | PP | Res |
| 3 | patients were enrolled in the | 17 | 15 | 80 | VP | Res |
| 3 | per kilogram of body weight | 17 | 17 | 80 | PP | Res |
| 3 | per person years ci to | 17 | 8 | 80 | PP | Res |
| 3 | reached the primary end point | 17 | 16 | 80 | VP | Res |
| 3 | the adjusted hazard ratio for | 17 | 13 | 40 | NP | Res |
| 3 | the time to the first | 17 | 13 | 100 | NP | Res |
| 3 | to the primary end point | 17 | 16 | 80 | PP | Res |
| 3 | was associated with a lower | 17 | 17 | 100 | VP | Text |
| 3 | were percent percent and percent | 17 | 11 | 100 | VP | Res |
| 3 | were significantly higher in the | 17 | 17 | 80 | VP | Text |
| 3 | adverse events were more common | 16 | 15 | 80 | VP | Res |
| 3 | and to the control group | 16 | 16 | 100 | PP | Res |
| 3 | as compared with ci to | 16 | 14 | 80 | PP | Res |
| 3 | as compared with patients with | 16 | 15 | 100 | PP | Res |
| 3 | associated with the risk of | 16 | 14 | 100 | VP | Res |
| 3 | characteristics were similar in the | 16 | 16 | 80 | VP | Text |
| 3 | confidence interval ci to or | 16 | 16 | 40 | NP | Res |
| 3 | did not differ significantly from | 16 | 16 | 60 | VP | Text |
| 3 | differences between the groups in | 16 | 16 | 100 | NP | Text |
| 3 | grade or adverse events were | 16 | 16 | 60 | VP | Res |
| 3 | group as compared with months | 16 | 14 | 100 | NP | Res |
| 3 | health related quality of life | 16 | 16 | 100 | NP | Res |
| 3 | in an intention to treat | 16 | 16 | 80 | PP | Res |
| 3 | in the aspirin group and | 16 | 9 | 80 | PP | Res |
| 3 | in the intervention group than | 16 | 13 | 80 | PP | Res |
| 3 | in the rate of death | 16 | 14 | 100 | PP | Res |
| 3 | in the two groups respectively | 16 | 15 | 80 | PP | Res |
| 3 | in the zoledronic acid group | 16 | 5 | 60 | PP | Res |
| 3 | incidence of adverse events was | 16 | 16 | 60 | NP | Res |
| 3 | mean age of the patients | 16 | 16 | 100 | NP | Res |
| 3 | median duration of response was | 16 | 16 | 60 | NP | Res |
| 3 | occurred in patients assigned to | 16 | 13 | 80 | VP | Res |
| 3 | of grade or higher were | 16 | 16 | 80 | VP | Res |
| 3 | or death from any cause | 16 | 14 | 100 | NP | Res |
| 3 | patients in the rituximab group | 16 | 7 | 80 | NP | Res |
| 3 | per person years hazard ratio | 16 | 6 | 60 | PP | Res |
| 3 | percent confidence interval to respectively | 16 | 14 | 40 | NP | Res |
| 3 | percent in the control group | 16 | 12 | 100 | NP | Res |
| 3 | percent vs. percent relative risk | 16 | 12 | 60 | NP | Res |
| 3 | percentage points ci to and | 16 | 13 | 60 | NP | Res |
| 3 | significant difference between the groups | 16 | 12 | 100 | NP | Res |
| 3 | the group receiving mg of | 16 | 9 | 80 | NP | Res |
| 3 | the hazard ratio for the | 16 | 14 | 60 | NP | Res |
| 3 | the incidence of the primary | 16 | 15 | 60 | NP | Res |
| 3 | the median time to the | 16 | 14 | 80 | NP | Res |
| 3 | the rate of adverse events | 16 | 16 | 80 | NP | Res |
| 3 | those years of age or | 16 | 14 | 100 | NP | Res |
| 3 | to in the control group | 16 | 15 | 100 | PP | Res |
| 3 | to percent confidence interval to | 16 | 10 | 60 | PP | Res |
| 3 | was not associated with a | 16 | 16 | 100 | VP | Text |
| 3 | was significantly greater in the | 16 | 15 | 80 | VP | Text |
| 3 | were percent and percent respectively | 16 | 13 | 80 | VP | Res |
| 3 | were years of age or | 16 | 16 | 100 | VP | Res |
| 3 | years hazard ratio ci to | 16 | 9 | 40 | NP | Res |
| 3 | a significant reduction in the | 15 | 13 | 80 | NP | Res |
| 3 | a total of patients had | 15 | 15 | 100 | VP | Res |
| 3 | a total of women were | 15 | 15 | 100 | VP | Res |
| 3 | among the patients who received | 15 | 13 | 100 | NP | Res |
| 3 | among those who received placebo | 15 | 14 | 80 | NP | Res |
| 3 | and months respectively hazard ratio | 15 | 11 | 40 | NP | Res |
| 3 | as compared with those with | 15 | 15 | 100 | PP | Res |
| 3 | blood pressure was mm hg | 15 | 13 | 40 | VP | Res |
| 3 | body mass index the weight | 15 | 15 | 60 | NP | Res |
| 3 | bone mineral density at the | 15 | 10 | 40 | NP | Res |
| 3 | ci to p for superiority | 15 | 14 | 40 | NP | Res |
| 3 | death confidence interval to p | 15 | 15 | 40 | NP | Res |
| 3 | for a median of years | 15 | 15 | 80 | PP | Res |
| 3 | free survival was months with | 15 | 14 | 80 | VP | Res |
| 3 | from any cause hazard ratio | 15 | 13 | 60 | PP | Res |
| 3 | group p for the comparison | 15 | 12 | 60 | NP | Res |
| 3 | group vs. in the placebo | 15 | 14 | 60 | NP | Res |
| 3 | in and of patients respectively | 15 | 14 | 80 | PP | Res |
| 3 | in the control group and | 15 | 13 | 100 | PP | Res |
| 3 | in the pci group and | 15 | 10 | 80 | PP | Res |
| 3 | in the placebo group difference | 15 | 14 | 80 | PP | Res |
| 3 | in the placebo group for | 15 | 12 | 80 | PP | Res |
| 3 | in the placebo group were | 15 | 15 | 80 | VP | Res |
| 3 | in the surgery group and | 15 | 8 | 80 | PP | Res |
| 3 | in the two groups in | 15 | 14 | 100 | PP | Res |
| 3 | incidence of serious adverse events | 15 | 15 | 60 | NP | Res |
| 3 | less than mg per deciliter | 15 | 7 | 60 | Oth | Res |
| 3 | median follow up was years | 15 | 15 | 80 | VP | Res |
| 3 | mg group and in the | 15 | 12 | 80 | NP | Res |
| 3 | months in the control group | 15 | 15 | 100 | NP | Res |
| 3 | occurred in of participants in | 15 | 11 | 80 | VP | Res |
| 3 | occurred in of patients who | 15 | 15 | 100 | VP | Res |
| 3 | occurred in of the participants | 15 | 12 | 80 | VP | Res |
| 3 | of progression free survival was | 15 | 13 | 60 | VP | Res |
| 3 | of the women in the | 15 | 10 | 100 | PP | Res |
| 3 | over a median follow up | 15 | 15 | 80 | PP | Res |
| 3 | patients who had a response | 15 | 14 | 100 | NP | Res |
| 3 | per minute per m of | 15 | 13 | 100 | PP | Res |
| 3 | percent as compared with percent | 15 | 15 | 100 | NP | Res |
| 3 | randomization were assigned to the | 15 | 15 | 60 | VP | Res |
| 3 | respectively hazard ratio confidence interval | 15 | 15 | 0 | NP | Res |
| 3 | serious adverse events were similar | 15 | 15 | 80 | VP | Res |
| 3 | than among those who received | 15 | 15 | 100 | Oth | Res |
| 3 | the placebo group and the | 15 | 14 | 80 | NP | Res |
| 3 | the primary efficacy end point | 15 | 15 | 60 | NP | Res |
| 3 | therapy group as compared with | 15 | 14 | 80 | NP | Res |
| 3 | vaccine group and in the | 15 | 11 | 80 | NP | Res |
| 3 | was associated with an increase | 15 | 15 | 100 | VP | Text |
| 3 | was confidence interval to p | 15 | 15 | 40 | VP | Res |
| 3 | was months and months respectively | 15 | 10 | 80 | VP | Res |
| 3 | was observed in of patients | 15 | 14 | 80 | VP | Res |
| 3 | with an increase in the | 15 | 15 | 100 | PP | Res |
| 3 | with the use of the | 15 | 14 | 100 | PP | Res |
| 3 | years of age or younger | 15 | 13 | 100 | NP | Res |
| 3 | a total of participants underwent | 14 | 14 | 60 | VP | Res |
| 3 | adverse events occurred in patients | 14 | 13 | 80 | VP | Res |
| 3 | an increased risk of death | 14 | 13 | 100 | NP | Res |
| 3 | and in the mg group | 14 | 11 | 80 | PP | Res |
| 3 | between the two treatment groups | 14 | 14 | 100 | PP | Res |
| 3 | by confidence interval ci to | 14 | 14 | 40 | PP | Res |
| 3 | cause hazard ratio ci to | 14 | 11 | 40 | NP | Res |
| 3 | ci to for death from | 14 | 7 | 80 | NP | Res |
| 3 | ci to in the group | 14 | 6 | 80 | NP | Res |
| 3 | ci to p and in | 14 | 14 | 60 | NP | Res |
| 3 | ci to p for the | 14 | 9 | 60 | NP | Res |
| 3 | ci to p hazard ratio | 14 | 11 | 20 | NP | Res |
| 3 | death from any cause or | 14 | 13 | 100 | NP | Res |
| 3 | death from any cause was | 14 | 14 | 100 | VP | Res |
| 3 | difference in the incidence of | 14 | 14 | 80 | NP | Res |
| 3 | group p for all comparisons | 14 | 11 | 60 | NP | Res |
| 3 | groups did not differ significantly | 14 | 14 | 60 | VP | Text |
| 3 | in the change from baseline | 14 | 14 | 80 | PP | Res |
| 3 | in the control group in | 14 | 13 | 100 | PP | Res |
| 3 | in the endovascular repair group | 14 | 5 | 60 | PP | Res |
| 3 | in the group receiving mg | 14 | 6 | 80 | PP | Res |
| 3 | in the low dose group | 14 | 6 | 80 | PP | Res |
| 3 | in the placebo group as | 14 | 14 | 80 | PP | Res |
| 3 | in the subgroup of patients | 14 | 11 | 80 | PP | Res |
| 3 | in the treatment group and | 14 | 5 | 100 | PP | Res |
| 3 | in the two treatment groups | 14 | 14 | 100 | PP | Res |
| 3 | length of stay in the | 14 | 12 | 80 | NP | Res |
| 3 | major bleeding occurred in patients | 14 | 11 | 80 | VP | Res |
| 3 | no serious adverse events were | 14 | 14 | 80 | VP | Res |
| 3 | of patients vs. of patients | 14 | 11 | 80 | PP | Res |
| 3 | patients confidence interval ci to | 14 | 14 | 40 | NP | Res |
| 3 | patients years of age or | 14 | 10 | 100 | NP | Res |
| 3 | percent confidence interval for the | 14 | 11 | 60 | NP | Res |
| 3 | primary outcome event occurred in | 14 | 13 | 60 | VP | Res |
| 3 | rates of sustained virologic response | 14 | 13 | 60 | NP | Res |
| 3 | ratio ci to p but | 14 | 14 | 40 | NP | Res |
| 3 | ratio ci to p or | 14 | 14 | 40 | NP | Res |
| 3 | response rate was ci to | 14 | 12 | 80 | VP | Res |
| 3 | the change from baseline in | 14 | 13 | 80 | NP | Res |
| 3 | the control group in the | 14 | 13 | 100 | NP | Res |
| 3 | the most common adverse event | 14 | 14 | 80 | NP | Res |
| 3 | the proportion of patients who | 14 | 14 | 80 | NP | Res |
| 3 | the rates of adverse events | 14 | 14 | 80 | NP | Res |
| 3 | the trial was stopped early | 14 | 14 | 80 | VP | Res |
| 3 | there were also no significant | 14 | 14 | 100 | VP | Text |
| 3 | times the upper limit of | 14 | 13 | 80 | NP | Res |
| 3 | treatment related adverse events of | 14 | 13 | 80 | NP | Res |
| 3 | was achieved in of patients | 14 | 10 | 100 | VP | Res |
| 3 | was of patients in the | 14 | 12 | 100 | VP | Res |
| 3 | a median of days after | 13 | 11 | 80 | NP | Res |
| 3 | and months ci to in | 13 | 11 | 80 | NP | Res |
| 3 | and of women in the | 13 | 9 | 100 | NP | Res |
| 3 | as compared with those in | 13 | 12 | 100 | PP | Res |
| 3 | at the time of the | 13 | 13 | 100 | PP | Res |
| 3 | cases per person years in | 13 | 7 | 100 | NP | Res |
| 3 | ci to p as was | 13 | 13 | 60 | VP | Res |
| 3 | ci to p for trend | 13 | 7 | 40 | NP | Res |
| 3 | common grade or adverse events | 13 | 13 | 60 | NP | Res |
| 3 | confidence interval to p as | 13 | 13 | 40 | NP | Res |
| 3 | days confidence interval ci to | 13 | 13 | 40 | NP | Res |
| 3 | death from coronary heart disease | 13 | 9 | 80 | NP | Res |
| 3 | difference between the groups in | 13 | 11 | 100 | NP | Res |
| 3 | difference in risk percentage points | 13 | 8 | 80 | NP | Res |
| 3 | dose of mg per kilogram | 13 | 9 | 40 | NP | Res |
| 3 | duration of progression free survival | 13 | 12 | 40 | NP | Res |
| 3 | for a median of months | 13 | 13 | 80 | PP | Res |
| 3 | free survival was months and | 13 | 12 | 80 | VP | Res |
| 3 | group absolute difference percentage points | 13 | 10 | 60 | NP | Res |
| 3 | group and days in the | 13 | 13 | 100 | NP | Res |
| 3 | group in the mg group | 13 | 8 | 80 | NP | Res |
| 3 | group risk difference percentage points | 13 | 10 | 80 | NP | Res |
| 3 | hazard ratio for death or | 13 | 11 | 60 | NP | Res |
| 3 | hazard ratio was ci to | 13 | 12 | 40 | VP | Res |
| 3 | in of the patients receiving | 13 | 10 | 100 | PP | Res |
| 3 | in of those who received | 13 | 8 | 100 | PP | Res |
| 3 | in the combined therapy group | 13 | 5 | 60 | PP | Res |
| 3 | in the conventional therapy group | 13 | 8 | 60 | PP | Res |
| 3 | in the group receiving the | 13 | 8 | 100 | PP | Res |
| 3 | in the mg tofacitinib group | 13 | 5 | 60 | PP | Res |
| 3 | in the standard treatment group | 13 | 5 | 100 | PP | Res |
| 3 | observed in of the patients | 13 | 13 | 80 | VP | Res |
| 3 | of log copies per milliliter | 13 | 9 | 40 | PP | Res |
| 3 | of patients who had a | 13 | 12 | 100 | PP | Res |
| 3 | of serious adverse events was | 13 | 13 | 80 | VP | Res |
| 3 | of the composite end point | 13 | 10 | 80 | PP | Res |
| 3 | of the patients who had | 13 | 13 | 100 | PP | Res |
| 3 | of the primary outcome was | 13 | 13 | 60 | VP | Res |
| 3 | patients in the aspirin group | 13 | 8 | 80 | NP | Res |
| 3 | percentage of patients who were | 13 | 11 | 80 | NP | Res |
| 3 | placebo p for both comparisons | 13 | 11 | 40 | NP | Res |
| 3 | risk ratio ci to p | 13 | 12 | 40 | NP | Res |
| 3 | significantly more patients in the | 13 | 12 | 80 | NP | Res |
| 3 | survival hazard ratio for death | 13 | 12 | 40 | NP | Res |
| 3 | survival was months and months | 13 | 9 | 80 | VP | Res |
| 3 | that in the placebo group | 13 | 12 | 80 | NP | Res |
| 3 | the area under the curve | 13 | 11 | 80 | NP | Res |
| 3 | the rate of grade or | 13 | 10 | 80 | NP | Res |
| 3 | the two groups were similar | 13 | 13 | 100 | VP | Text |
| 3 | venous thromboembolism occurred in of | 13 | 11 | 60 | VP | Res |
| 3 | was also associated with a | 13 | 13 | 100 | VP | Text |
| 3 | was cells per cubic millimeter | 13 | 13 | 60 | VP | Res |
| 3 | was ci to and the | 13 | 11 | 80 | VP | Res |
| 3 | was more frequent in the | 13 | 12 | 80 | VP | Res |
| 3 | was observed in of the | 13 | 13 | 80 | VP | Res |
| 3 | were more likely to be | 13 | 13 | 100 | VP | Participant |
| 3 | with a reduced risk of | 13 | 10 | 100 | PP | Res |
| 3 | adjusted hazard ratio for death | 12 | 7 | 40 | NP | Res |
| 3 | among the patients who were | 12 | 11 | 100 | VP | Res |
| 3 | and p for the comparison | 12 | 8 | 60 | NP | Res |
| 3 | and percentage points ci to | 12 | 9 | 60 | NP | Res |
| 3 | and serious adverse events were | 12 | 12 | 80 | VP | Res |
| 3 | and the hazard ratio for | 12 | 12 | 60 | NP | Res |
| 3 | and were more likely to | 12 | 12 | 100 | VP | Participant |
| 3 | ci to and in the | 12 | 10 | 80 | NP | Res |
| 3 | ci to hazard ratio for | 12 | 11 | 40 | NP | Res |
| 3 | ci to p and death | 12 | 10 | 60 | NP | Res |
| 3 | ci to p for both | 12 | 12 | 60 | NP | Res |
| 3 | difference percent percent confidence interval | 12 | 9 | 60 | NP | Res |
| 3 | during the follow up period | 12 | 12 | 100 | PP | Res |
| 3 | end point of death from | 12 | 11 | 100 | NP | Res |
| 3 | end point was in the | 12 | 10 | 100 | VP | Res |
| 3 | from any cause ci to | 12 | 12 | 80 | PP | Res |
| 3 | group p and in the | 12 | 12 | 80 | NP | Res |
| 3 | had a higher rate of | 12 | 12 | 100 | VP | Text |
| 3 | had occurred in of patients | 12 | 10 | 100 | VP | Res |
| 3 | in the control group percent | 12 | 9 | 100 | PP | Res |
| 3 | in the ldl cholesterol level | 12 | 10 | 60 | PP | Res |
| 3 | in the placebo group died | 12 | 11 | 80 | PP | Res |
| 3 | in the placebo group with | 12 | 11 | 80 | PP | Res |
| 3 | in the proportion of patients | 12 | 11 | 80 | PP | Res |
| 3 | not associated with an increased | 12 | 12 | 100 | VP | Text |
| 3 | occurred in participants in the | 12 | 12 | 80 | VP | Res |
| 3 | of follow up was years | 12 | 12 | 100 | VP | Res |
| 3 | of in the control group | 12 | 11 | 100 | PP | Res |
| 3 | of the children in the | 12 | 8 | 100 | PP | Res |
| 3 | of the infants in the | 12 | 6 | 80 | PP | Res |
| 3 | of the patients in group | 12 | 5 | 100 | PP | Res |
| 3 | other adverse events were similar | 12 | 12 | 80 | VP | Res |
| 3 | outcome event occurred in of | 12 | 11 | 80 | VP | Res |
| 3 | patients in the surgery group | 12 | 9 | 80 | NP | Res |
| 3 | patients vs. patients hazard ratio | 12 | 9 | 40 | NP | Res |
| 3 | patients who did not have | 12 | 12 | 100 | NP | Res |
| 3 | percentage points ci to for | 12 | 6 | 60 | NP | Res |
| 3 | rate of overall survival was | 12 | 12 | 60 | NP | Res |
| 3 | response rate was in the | 12 | 12 | 100 | VP | Res |
| 3 | risk of death from any | 12 | 11 | 100 | NP | Res |
| 3 | serious adverse events in the | 12 | 12 | 80 | NP | Res |
| 3 | the median follow up period | 12 | 12 | 80 | NP | Res |
| 3 | the placebo group had a | 12 | 11 | 80 | VP | Res |
| 3 | the placebo group vs. p | 12 | 12 | 40 | NP | Res |
| 3 | the primary end point of | 12 | 12 | 80 | NP | Res |
| 3 | the risk of death was | 12 | 11 | 100 | VP | Res |
| 3 | there was a trend toward | 12 | 12 | 80 | VP | Text |
| 3 | to p as was the | 12 | 12 | 80 | PP | Res |
| 3 | vs hazard ratio for death | 12 | 11 | 40 | PP | Res |
| 3 | vs in the control group | 12 | 9 | 80 | PP | Res |
| 3 | was in the placebo group | 12 | 9 | 80 | VP | Res |
| 3 | year in the placebo group | 12 | 10 | 80 | NP | Res |
| 3 | a higher percentage of patients | 11 | 10 | 80 | NP | Res |
| 3 | a percent increase in the | 11 | 5 | 100 | NP | Res |
| 3 | a relative reduction of in | 11 | 11 | 60 | NP | Res |
| 3 | a significantly increased risk of | 11 | 10 | 80 | NP | Res |
| 3 | a total of children were | 11 | 11 | 100 | VP | Res |
| 3 | among patients who did not | 11 | 9 | 100 | PP | Text |
| 3 | among patients who had a | 11 | 9 | 100 | PP | Text |
| 3 | among patients years of age | 11 | 8 | 100 | PP | Res |
| 3 | and ci to for the | 11 | 9 | 80 | NP | Res |
| 3 | as compared with with placebo5 | 11 | 9 | 80 | PP | Res |
| 3 | at the end of treatment | 11 | 9 | 100 | PP | Res |
| 3 | between group difference percentage points | 11 | 10 | 80 | PP | Res |
| 3 | between the two study groups | 11 | 11 | 100 | PP | Res |
| 3 | death hazard ratio ci to | 11 | 11 | 40 | NP | Res |
| 3 | did not differ significantly in | 11 | 11 | 60 | VP | Text |
| 3 | event occurred in of patients | 11 | 10 | 100 | VP | Res |
| 3 | group and months ci to | 11 | 9 | 80 | NP | Res |
| 3 | group had died relative risk | 11 | 9 | 80 | VP | Res |
| 3 | groups in the rates of | 11 | 11 | 100 | NP | Res |
| 3 | had an increased risk of | 11 | 11 | 100 | VP | Res |
| 3 | had no significant effect on | 11 | 11 | 100 | VP | Text |
| 3 | hazard ratio for disease recurrence | 11 | 9 | 40 | NP | Res |
| 3 | in the active treatment group | 11 | 5 | 80 | PP | Res |
| 3 | in the apixaban group and | 11 | 6 | 80 | PP | Res |
| 3 | in the control group adjusted | 11 | 9 | 80 | PP | Res |
| 3 | in the hypothermia group and | 11 | 7 | 80 | PP | Res |
| 3 | in the paclitaxel stent group | 11 | 5 | 60 | PP | Res |
| 3 | in the placebo group incidence | 11 | 8 | 60 | PP | Res |
| 3 | in the t pr group | 11 | 5 | 80 | PP | Res |
| 3 | in the watchful waiting group | 11 | 6 | 80 | PP | Res |
| 3 | liter percent confidence interval to | 11 | 6 | 40 | NP | Res |
| 3 | mean change from baseline in | 11 | 10 | 80 | NP | Res |
| 3 | mg per deciliter in the | 11 | 5 | 60 | NP | Res |
| 3 | no significant differences among the | 11 | 11 | 100 | NP | Text |
| 3 | no significant differences were observed | 11 | 11 | 80 | VP | Text |
| 3 | occurred in and of patients | 11 | 9 | 100 | VP | Res |
| 3 | occurred in patients who received | 11 | 10 | 100 | VP | Res |
| 3 | occurred in percent of the | 11 | 11 | 100 | VP | Res |
| 3 | of grade or higher occurred | 11 | 11 | 80 | PP | Res |
| 3 | of less than mg per | 11 | 7 | 80 | PP | Res |
| 3 | patients had at least one | 11 | 11 | 100 | VP | Res |
| 3 | patients in the apixaban group | 11 | 6 | 80 | NP | Res |
| 3 | patients in the pci group | 11 | 6 | 80 | NP | Res |
| 3 | patients who did not receive | 11 | 9 | 100 | NP | Res |
| 3 | percent confidence interval to or | 11 | 10 | 60 | NP | Res |
| 3 | percent vs. percent p or | 11 | 11 | 60 | NP | Res |
| 3 | placebo hazard ratio confidence interval | 11 | 11 | 0 | NP | Res |
| 3 | progression free survival rate was | 11 | 10 | 60 | VP | Res |
| 3 | rate of serious adverse events | 11 | 11 | 80 | NP | Res |
| 3 | rate of the primary outcome | 11 | 11 | 60 | NP | Res |
| 3 | reported in of patients in | 11 | 11 | 100 | VP | Res |
| 3 | risk of death from cardiovascular | 11 | 11 | 80 | NP | Res |
| 3 | the end of the study | 11 | 11 | 100 | NP | Res |
| 3 | the most frequent adverse events | 11 | 11 | 60 | NP | Res |
| 3 | the patients randomly assigned to | 11 | 8 | 60 | NP | Res |
| 3 | the placebo group p and | 11 | 11 | 60 | NP | Res |
| 3 | the primary composite end point | 11 | 11 | 60 | NP | Res |
| 3 | the rate of freedom from | 11 | 9 | 80 | NP | Res |
| 3 | the rates of the primary | 11 | 11 | 80 | NP | Res |
| 3 | to hazard ratio ci to | 11 | 7 | 40 | PP | Res |
| 3 | was associated with a percent | 11 | 10 | 100 | VP | Text |
| 3 | was more common in the | 11 | 11 | 100 | VP | Text |
| 3 | were ci to ci to | 11 | 11 | 60 | VP | Res |
| 3 | were lost to follow up | 11 | 10 | 100 | VP | Res |
| 3 | were randomly assigned to a | 11 | 11 | 60 | VP | Res |
| 3 | who did not have a | 11 | 11 | 100 | VP | Res |
| 3 | who received at least one | 11 | 9 | 100 | VP | Res |
| 3 | who received placebo p for | 11 | 7 | 60 | VP | Res |
| 3 | women in the placebo group | 11 | 9 | 80 | NP | Res |
| 3 | years confidence interval ci to | 11 | 11 | 40 | NP | Res |
| 3 | a glycated hemoglobin level of | 10 | 7 | 60 | NP | Res |
| 3 | a significant increase in the | 10 | 9 | 100 | NP | Res |
| 3 | a significantly lower risk of | 10 | 9 | 80 | NP | Res |
| 3 | a total of cases of | 10 | 10 | 100 | NP | Res |
| 3 | a total of infants were | 10 | 10 | 80 | VP | Res |
| 3 | a total of participants in | 10 | 10 | 80 | NP | Res |
| 3 | an increase in the risk | 10 | 9 | 100 | NP | Res |
| 3 | and events per person years | 10 | 9 | 100 | NP | Res |
| 3 | and in the group receiving | 10 | 6 | 100 | PP | Res |
| 3 | and of participants in the | 10 | 7 | 80 | NP | Res |
| 3 | and percent in the group | 10 | 9 | 100 | NP | Res |
| 3 | and percent respectively in the | 10 | 6 | 80 | NP | Res |
| 3 | as compared with percent among | 10 | 10 | 100 | PP | Res |
| 3 | as compared with placebo p | 10 | 7 | 60 | PP | Res |
| 3 | at months the rate of | 10 | 10 | 100 | PP | Res |
| 3 | at to years of age | 10 | 7 | 100 | PP | Res |
| 3 | at years the rate of | 10 | 10 | 100 | PP | Res |
| 3 | bleeding occurred in of patients | 10 | 10 | 80 | VP | Res |
| 3 | by the end of the | 10 | 10 | 100 | PP | Res |
| 3 | ci to among patients with | 10 | 8 | 80 | NP | Res |
| 3 | ci to in the per | 10 | 7 | 80 | NP | Res |
| 3 | ci to p and for | 10 | 8 | 60 | NP | Res |
| 3 | confidence interval ci to not | 10 | 10 | 40 | NP | Res |
| 3 | control group than in the | 10 | 8 | 100 | NP | Res |
| 3 | data and safety monitoring committee | 10 | 10 | 40 | NP | Res |
| 3 | days of patients in the | 10 | 8 | 100 | NP | Res |
| 3 | death from any cause occurred | 10 | 10 | 100 | VP | Res |
| 3 | developed in of the patients | 10 | 10 | 100 | VP | Res |
| 3 | developed in patients in the | 10 | 10 | 100 | VP | Res |
| 3 | died as compared with of | 10 | 9 | 100 | VP | Res |
| 3 | differences in the rate of | 10 | 9 | 100 | NP | Res |
| 3 | for progression or death ci | 10 | 8 | 60 | PP | Res |
| 3 | for the comparison between the | 10 | 6 | 80 | PP | Text |
| 3 | free survival and overall survival | 10 | 9 | 40 | NP | Res |
| 3 | free survival hazard ratio for | 10 | 10 | 40 | NP | Res |
| 3 | free survival was in the | 10 | 9 | 80 | VP | Res |
| 3 | from any cause occurred in | 10 | 10 | 100 | PP | Res |
| 3 | group and in the medical | 10 | 10 | 80 | NP | Res |
| 3 | group and in the surgery | 10 | 9 | 80 | NP | Res |
| 3 | group in the intention to | 10 | 10 | 80 | NP | Res |
| 3 | group of patients vs. of | 10 | 10 | 80 | NP | Res |
| 3 | group rate ratio ci to | 10 | 10 | 60 | NP | Res |
| 3 | had a lower risk of | 10 | 10 | 100 | VP | Res |
| 3 | had received a median of | 10 | 10 | 80 | VP | Res |
| 3 | hazard ratios for death from | 10 | 8 | 60 | NP | Res |
| 3 | in the budesonide formoterol group | 10 | 5 | 60 | PP | Res |
| 3 | in the group that underwent | 10 | 6 | 80 | PP | Res |
| 3 | in the placebo group adjusted | 10 | 5 | 60 | PP | Res |
| 3 | in the rate of the | 10 | 10 | 100 | PP | Res |
| 3 | in the rates of death | 10 | 10 | 100 | PP | Res |
| 3 | in the surgery group p | 10 | 8 | 60 | PP | Res |
| 3 | in the two groups were | 10 | 10 | 100 | PP | Res |
| 3 | in the two groups with | 10 | 10 | 100 | PP | Res |
| 3 | increase in the incidence of | 10 | 8 | 80 | NP | Res |
| 3 | increased by a factor of | 10 | 9 | 100 | VP | Res |
| 3 | increased from in to in | 10 | 9 | 100 | VP | Text |
| 3 | independent data and safety monitoring | 10 | 10 | 40 | NP | Res |
| 3 | intention to treat analysis of | 10 | 10 | 80 | NP | Res |
| 3 | intervention group as compared with | 10 | 8 | 80 | NP | Res |
| 3 | kaplan meier estimates of the | 10 | 10 | 60 | NP | Res |
| 3 | level of mg per deciliter | 10 | 7 | 60 | NP | Res |
| 3 | major bleeding occurred in of | 10 | 10 | 80 | VP | Res |
| 3 | mg group as compared with | 10 | 6 | 80 | NP | Res |
| 3 | occurred in patients and patients | 10 | 9 | 100 | VP | Res |
| 3 | of the patients had a | 10 | 10 | 100 | PP | Res |
| 3 | of the patients in each | 10 | 10 | 100 | PP | Res |
| 3 | older than years of age | 10 | 10 | 100 | Oth | Res |
| 3 | outcomes did not differ significantly | 10 | 10 | 40 | VP | Res |
| 3 | patients in the enoxaparin group | 10 | 7 | 80 | NP | Res |
| 3 | patients in the respective groups | 10 | 10 | 80 | NP | Res |
| 3 | percent confidence interval to with | 10 | 6 | 60 | NP | Res |
| 3 | percent in the group given | 10 | 5 | 100 | NP | Res |
| 3 | progression free survival in the | 10 | 10 | 60 | NP | Res |
| 3 | progression or death in the | 10 | 10 | 80 | NP | Res |
| 3 | rate of disease free survival | 10 | 9 | 80 | NP | Res |
| 3 | reduction in the number of | 10 | 8 | 80 | NP | Res |
| 3 | risk of death hazard ratio | 10 | 10 | 60 | NP | Res |
| 3 | significant between group differences were | 10 | 10 | 100 | VP | Res |
| 3 | standard therapy group hazard ratio | 10 | 6 | 40 | NP | Res |
| 3 | surgery group and in the | 10 | 8 | 80 | NP | Res |
| 3 | survival was similar in the | 10 | 10 | 80 | VP | Res |
| 3 | sustained virologic response at weeks | 10 | 5 | 60 | NP | Res |
| 3 | than among those receiving placebo | 10 | 10 | 80 | Oth | Res |
| 3 | than in the group that | 10 | 10 | 100 | Oth | Res |
| 3 | than with placebo vs. p | 10 | 8 | 40 | Oth | Res |
| 3 | the between group difference in | 10 | 9 | 100 | NP | Res |
| 3 | the control group p and | 10 | 9 | 80 | NP | Res |
| 3 | the control group vs. p | 10 | 9 | 60 | NP | Res |
| 3 | the group that received the | 10 | 7 | 100 | NP | Res |
| 3 | the mg group and the | 10 | 5 | 80 | NP | Res |
| 3 | the most common grade or | 10 | 10 | 80 | NP | Res |
| 3 | the patients who had a | 10 | 10 | 100 | NP | Res |
| 3 | the patients who were enrolled | 10 | 10 | 80 | NP | Res |
| 3 | the placebo group and respectively | 10 | 9 | 60 | NP | Res |
| 3 | the rate of death was | 10 | 10 | 100 | VP | Res |
| 3 | the relative risk of death | 10 | 9 | 80 | NP | Res |
| 3 | the results were similar in | 10 | 10 | 100 | VP | Text |
| 3 | the subgroup of patients with | 10 | 10 | 80 | NP | Res |
| 3 | the two groups did not | 10 | 10 | 100 | VP | Text |
| 3 | those who received placebo p | 10 | 8 | 60 | NP | Res |
| 3 | treatment group than in the | 10 | 8 | 100 | NP | Res |
| 3 | vs p as was the | 10 | 10 | 60 | PP | Res |
| 3 | was days interquartile range to | 10 | 10 | 80 | VP | Res |
| 3 | was reported in of patients | 10 | 9 | 100 | VP | Res |
| 3 | was similar to that of | 10 | 10 | 100 | VP | Text |
| 3 | were enrolled in the study | 10 | 10 | 80 | VP | Res |
| 3 | were included in the analyses | 10 | 10 | 100 | VP | Res |
| 3 | were similar with respect to | 10 | 10 | 100 | VP | Text |
| 3 | women to years of age | 10 | 5 | 100 | NP | Res |
| 3 | women were randomly assigned to | 10 | 10 | 60 | VP | Res |
| 4 | associated with an increased risk | 59 | 56 | 100 | VP | Res |
| 4 | with an increased risk of | 58 | 55 | 100 | PP | Res |
| 4 | did not result in a | 38 | 38 | 100 | VP | Text |
| 4 | at a dose of mg | 31 | 27 | 60 | PP | Res |
| 4 | was associated with an increased | 30 | 30 | 100 | VP | Res |
| 4 | of death from any cause | 27 | 24 | 100 | PP | Res |
| 4 | there was no significant difference | 27 | 27 | 100 | VP | Text |
| 4 | than among those who received | 26 | 26 | 100 | Oth | Res |
| 4 | out of hospital cardiac arrest | 23 | 22 | 60 | NP | Res |
| 4 | was associated with a higher | 23 | 23 | 100 | VP | Res |
| 4 | did not differ significantly between | 22 | 22 | 60 | VP | Text |
| 4 | was not associated with a | 22 | 22 | 100 | VP | Text |
| 4 | did not significantly reduce the | 21 | 21 | 80 | VP | Text |
| 4 | a significantly lower rate of | 20 | 20 | 80 | NP | Text |
| 4 | years of age or older | 20 | 18 | 100 | NP | Res |
| 4 | is associated with an increased | 19 | 19 | 100 | VP | Text |
| 4 | reduction in the rate of | 19 | 19 | 80 | NP | Text |
| 4 | was associated with a lower | 19 | 18 | 100 | VP | Text |
| 4 | non small cell lung cancer | 18 | 18 | 40 | NP | Res |
| 4 | rates of sustained virologic response | 18 | 18 | 60 | NP | Res |
| 4 | in a lower incidence of | 17 | 17 | 80 | PP | Text |
| 4 | the rate of death from | 17 | 17 | 100 | NP | Res |
| 4 | was associated with a significantly | 17 | 17 | 80 | VP | Text |
| 4 | a significantly lower risk of | 16 | 16 | 80 | NP | Res |
| 4 | associated with a reduction in | 15 | 15 | 80 | VP | Text |
| 4 | lower among those who received | 15 | 15 | 100 | Oth | Res |
| 4 | not reduce the rate of | 15 | 15 | 100 | VP | Text |
| 4 | patients with moderate to severe | 15 | 15 | 60 | NP | Res |
| 4 | resulted in a significantly lower | 15 | 15 | 80 | VP | Text |
| 4 | and was associated with a | 14 | 14 | 100 | VP | Text |
| 4 | composite end point of death | 14 | 14 | 80 | NP | Res |
| 4 | free survival among patients with | 14 | 14 | 80 | NP | Res |
| 4 | in patients with type diabetes | 14 | 14 | 80 | PP | Res |
| 4 | in the rate of death | 14 | 13 | 100 | PP | Res |
| 4 | of death from cardiovascular causes | 14 | 14 | 80 | PP | Res |
| 4 | patients with relapsed or refractory | 14 | 14 | 60 | NP | Res |
| 4 | than those who received placebo | 14 | 14 | 80 | Oth | Res |
| 4 | an increased risk of death | 13 | 13 | 100 | NP | Res |
| 4 | free survival and overall survival | 13 | 13 | 40 | NP | Res |
| 4 | in patients with atrial fibrillation | 13 | 13 | 60 | PP | Res |
| 4 | not reduce the risk of | 13 | 13 | 100 | VP | Res |
| 4 | patients with hcv genotype infection | 13 | 12 | 40 | NP | Res |
| 4 | a significantly higher rate of | 12 | 12 | 80 | NP | Text |
| 4 | associated with an increase in | 12 | 12 | 100 | VP | Text |
| 4 | longer progression free survival than | 12 | 12 | 60 | NP | Res |
| 4 | with a lower rate of | 12 | 12 | 100 | PP | Text |
| 4 | with a lower risk of | 12 | 11 | 100 | PP | Res |
| 4 | among patients with type diabetes | 11 | 11 | 80 | NP | Res |
| 4 | had no significant effect on | 11 | 11 | 100 | VP | Res |
| 4 | in a lower rate of | 11 | 10 | 100 | PP | Text |
| 4 | in this trial involving patients | 11 | 11 | 80 | PP | Res |
| 4 | increase in the rate of | 11 | 10 | 100 | NP | Text |
| 4 | overall survival among patients with | 11 | 11 | 60 | NP | Res |
| 4 | than in the placebo group | 11 | 11 | 80 | Oth | Res |
| 4 | was associated with a significant | 11 | 11 | 100 | VP | Text |
| 4 | increase in the risk of | 10 | 8 | 100 | NP | Res |
| 4 | no significant difference in the | 10 | 10 | 100 | NP | Text |
| 4 | patients with acute ischemic stroke | 10 | 10 | 40 | NP | Res |
| 4 | patients with chronic kidney disease | 10 | 10 | 60 | NP | Res |
| 4 | the rate of the composite | 10 | 10 | 80 | NP | Res |
| 4 | the risk of death from | 10 | 9 | 100 | NP | Res |
| 4 | with a higher rate of | 10 | 9 | 100 | PP | Text |
Notes
| 1 | Although translations can sometimes be criticized for not perfectly reflecting “the content” of the original source texts or texts originally written in the target language (Rees 2022, p. 395), the Japanese translations of the journal’s abstracts, shown in the regional edition of the journal as well as on the website of the licensed publisher (Nankodo 2024), are noted for accurately reflecting the original texts. Noto (2015) specifically highlighted their effectiveness in conveying essential information to the domestic disciplinary readers, ensuring that critical medical insights remain accessible and reliable. |
| 2 | When measured in the Google Colaboratory’s Python environment, the English-language abstract texts comprised 1,201,780 words. However, using AntConc for quantification, the count was 1,209,851 words with punctuation included and 1,148,583 words without punctuation. For the purposes of this study, we adopt the more conservative word count of 1,148,583, excluding punctuation, to describe the size of our corpus. |
| 3 | The bundle “a total of of the” was extracted from fragments such as “a total of 444 of the 490 children” because punctuation and numerals were excluded when extracting bundles, following the methodology of a previous study (Durrant 2017, p. 170). |
| 4 | The bundle “a total of of patients” was extracted from fragments such as “a total of 128 of 212 patients”. |
| 5 | The bundle “as compared with with placebo” was extracted from fragments such as “as compared with 44% with placebo”. |
References
- Abdollahpour, Zeinab, and Javad Gholami. 2018. Building Blocks of Medical Abstracts: Frequency, Functions and Structures of Lexical Bundles. The Asian ESP Journal 14: 82–110. Available online: https://www.asian-esp-journal.com/volume-14-issue-1-june-2018/ (accessed on 18 August 2024).
- Anderson, Kenneth, and Joan Maclean. 1997. A Genre Analysis Study of 80 Medical Abstracts. Edinburgh Working Papers in Applied Linguistics 8: 1–23. [Google Scholar]
- Anthony, Laurence. 2023. AntConc (Version 4.2.4) [Computer Software]. Tokyo: Waseda University. Available online: https://www.laurenceanthony.net/software (accessed on 11 April 2024).
- Anthony, Lawrence. 2024. AntWordProfiler. Available online: https://www.laurenceanthony.net/software/antwordprofiler/ (accessed on 1 August 2024).
- Asano, Motoko, and Miho Fujieda. 2024. A Vocabulary Study for Enhancing Learners’ Experiences: English-Language Medical Research Abstracts. English Corpus Studies 31: 1–22. [Google Scholar]
- Asano, Motoko, Megumi Nakano, Yoshinori Miyazaki, and Miho Fujieda. 2022a. Introducing a Bilingual Corpus Database System of Medical Abstracts for Exploring Academic Connotations of Words: A Case Study of First-Year Medical Students. Journal of Medical English Education 21: 18–26. [Google Scholar]
- Asano, Motoko, Megumi Nakano, Yoshinori Miyazaki, Tomoko Wakasa, and Miho Fujieda. 2022b. Use of Authentic Translation in Helping Students Decipher English-Language Randomised Control Trial Abstracts. Journal of Medical English Education 21: 87–93. [Google Scholar]
- Ballance, Oliver, and Averil Coxhead. 2022. What can Corpora Tell us About English for Academic Purposes? In The Routledge Handbook of Corpus Linguistics, 2nd ed. Edited by Anne O’Keeffe and Michael J. McCarthy. London: Routledge, pp. 405–15. [Google Scholar]
- Belcher, Diane D. 2016. English for Professional Academic Purposes. In The Routledge Handbook of English for Academic Purposes. Edited by Ken Hyland and Philip Shaw. Oxon: Routledge, pp. 502–14. [Google Scholar]
- Bestgen, Yves. 2020. Comparing Lexical Bundles across Corpora of Different Sizes: The Zipfian Problem. Journal of Quantitative Linguistics 27: 272–90. [Google Scholar] [CrossRef]
- Bhatia, Vijay K. 2019. Genre as Interdiscursive Performance in English for Professional Communication. In Specialised English. Edited by Ken Hyland and Lillian L. C. Wong. Oxon: Routledge, pp. 36–49. [Google Scholar]
- Biber, Douglas, Stig Johansson, Geoffrey Leech, Susan Conrad, and Edward Finegan. 1999. The Longman Grammar of Spoken and Written English. Essex: Longman. [Google Scholar]
- Biber, Douglas, Susan Conrad, and Viviana Cortes. 2004. If You Look at …: Lexical Bundles in University Teaching and Textbooks. Applied Linguistics 25: 371–405. [Google Scholar] [CrossRef]
- Boers, Frank. 2020. Factors affecting the learning of multiword items. In The Routledge Handbook of Vocabulary Studies. Edited by Stuart Webb. Oxon: Routledge, pp. 143–57. [Google Scholar]
- Brooks, Gavin, Jon Clenton, and Simon Fraser. 2023. Exploring the Importance of Vocabulary for English as an Additional Language Learners’ Reading Comprehension. In EAL Research for the Classroom. Edited by Gavin Brooks, Jon Clenton and Simon Fraser. Oxon: Routledge, pp. 35–58. [Google Scholar]
- Browne, Charles. 2013. The New General Service List: Celebrating 60 Years of Vocabulary Learning. The Language Teacher 37: 13–16. [Google Scholar]
- Browne, Charles. 2021. The NGSL Project: Building Wordlists and Resources to help EFL Learners (and Teachers) to Succeed. Paper Presented at JALTCALL 2020 Conference, Online, June 6–7; pp. 1–18. [Google Scholar] [CrossRef]
- Browne, Charles, Brent Culligan, and Joseph Phillips. 2013. New Academic Word List. Available online: https://www.newgeneralservicelist.com/new-general-service-list-1 (accessed on 1 August 2024).
- Casal, J. Elliott, and Matt Kessler. 2020. Form and Rhetorical Function of Phrase-frames in Promotional Writing: A Corpus- and Genre-based Analysis. System 95: 102370. [Google Scholar] [CrossRef]
- Chen, Yu-Hua, and Paul Baker. 2010. Lexical Bundles in L1 and L2 Academic Writing. Language Learning & Technology 14: 30–49. [Google Scholar]
- Cook, Guy. 1998. The Uses of Reality: A Reply to Ronald Carter. ELT Journal 52: 57–63. [Google Scholar] [CrossRef]
- Cook, Guy. 2010. Translation in Language Teaching: An Argument for Reassessment. Oxford: Oxford University Press. [Google Scholar]
- Cortes, Viviana. 2004. Lexical Bundles in Published and Student Disciplinary Writing: Examples from History and Biology. English for Specific Purposes 23: 397–423. [Google Scholar] [CrossRef]
- Cortes, Viviana. 2013. The Purpose of This Study is to: Connecting Lexical Bundles and Moves in Research Article Introductions. English for Specific Purposes 12: 33–43. [Google Scholar] [CrossRef]
- Cortes, Viviana. 2015. Situating Lexical Bundles in the Formulaic Language Spectrum: Origins and Functional Analysis Development. In Corpus-Based Research in Applied Linguistics: Studies in Honor of Doug Biber. Edited by Viviana Cortes and Eniko Csomay. Amsterdam: John Benjamins, pp. 197–218. [Google Scholar]
- Cortes, Viviana. 2024. Lexical Bundles and Phrase Frames. In Conducting Genre-Based Research in Applied Linguistics. Edited by Matt Kessler and Charlene Polio. New York: Routledge, pp. 105–26. [Google Scholar]
- Coxhead, Averil. 2000. A New Academic Word List. TESOL Quarterly 34: 213–38. [Google Scholar] [CrossRef]
- Coxhead, Averil. 2016. Acquiring Academic and Disciplinary Vocabulary. In The Routledge Handbook of English for Academic Purposes. Edited by Ken Hyland and Paul Shaw. Oxon: Routledge, pp. 177–90. [Google Scholar]
- Coxhead, Averil. 2020. Academic Vocabulary. In The Routledge Handbook of Vocabulary Studies. Edited by Stuart Webb. Oxon: Routledge, pp. 97–110. [Google Scholar]
- Coxhead, Averil, and David Hirsh. 2007. A Pilot Science-specific Word List. Revue Française de Linguistique Appliquée 2: 65–78. [Google Scholar]
- Coxhead, Averil, and Thi Ngoc Yen Dang. 2019. Vocabulary in University Tutorials and Laboratories. In Specialised English. Edited by Ken Hyland and Lillian L. C. Wong. Oxon: Routledge, pp. 120–34. [Google Scholar]
- Culligan, Brent A. 2019. Evaluating Corpora with Word Lists and Word Difficulty. Vocabulary Learning and Instruction 8: 29–38. [Google Scholar] [CrossRef]
- Dang, Thi Ngoc Yen. 2020. The Potential for Learning Specialized Vocabulary of University Lectures and Seminars through Watching Discipline-related TV Programs. TESOL Quarterly 54: 436–59. [Google Scholar] [CrossRef]
- Dang, Thi Ngoc Yen, and Stuart Webb. 2016. Evaluating Lists of High-frequency Words. International Journal of Applied Linguistics 167: 132–58. [Google Scholar] [CrossRef]
- Dang, Thi Ngoc Yen, Averil Coxhead, and Stuart Webb. 2017. The Academic Spoken Word List. Language Learning 67: 959–97. [Google Scholar] [CrossRef]
- Durrant, Philip. 2017. Lexical Bundles and Disciplinary Variation in University Students’ Writing: Mapping the Territories. Applied Linguistics 38: 165–93. [Google Scholar] [CrossRef]
- EQUATOR Network. 2024. Reporting Guidelines. Available online: https://www.equator-network.org/ (accessed on 30 April 2024).
- Flowerdew, John. 2000. Discourse community, Legitimate Peripheral Participation, and the Nonnative-English Speaking Scholar. TESOL Quarterly 34: 127–50. [Google Scholar] [CrossRef]
- Flowerdew, Lynn. 2012. Corpora and Language Education. Hampshire: Palgrave MacMillan. [Google Scholar]
- Fraser, Simon. 2007. Providing ESP Learners with the Vocabulary They Need: Corpora and the Creation of Specialized Word Lists. Hiroshima Studies in Language and Language Education 10: 127–43. [Google Scholar] [CrossRef]
- Gardner, Dee, and Mark Davies. 2014. A New Academic Vocabulary List. Applied Linguistics 35: 305–27. [Google Scholar] [CrossRef]
- Gilmore, Alexander, and Neil Millar. 2018. The Language of Civil Engineering Research Articles: A Corpus-based Approach. English for Specific Purposes 51: 1–17. [Google Scholar] [CrossRef]
- Gilner, Leah. 2011. A Primer on the General Service List. Reading in a Foreign Language 23: 65–83. [Google Scholar]
- Golparvar, Seyyed Ehsan, and Elyas Barabadi. 2020. Key Phrase Frames in the Discussion Section of Research Articles of Higher Education. Lingua 236: 102804. [Google Scholar] [CrossRef]
- Graetz, Naomi. 1982. Teaching EFL Students to Extract Structural Information from Abstracts. Paper Presented at the International Symposium on Language for Special Purposes, Eindhoven, The Netherlands, August 2–4. [Google Scholar]
- Gray, Bethany, Elena Cotos, and Jordan Smith. 2020. Combining Rhetorical Move Analysis with Multi-dimensional Analysis. In Advances in Corpus-based Research on Academic Writing. Edited by Ute Römer, Viviana Cortes and Eric Friginal. Amsterdam: John Benjamins, pp. 137–68. [Google Scholar]
- Green, Clarence, and James Lambert. 2018. Advancing Disciplinary Literacy through English for Academic Purposes: Discipline-specific Wordlists, Collocations and Word Families for Eight Secondary Subjects. English for Specific Purposes 35: 105–15. [Google Scholar] [CrossRef]
- Handford, Michael. 2010. What Can a Corpus Tell us About Specialist Genres? In Routledge Handbook of Corpus Linguistics. Edited by Anne O’Keeffe and Michael McCarthy. Oxon: Routledge, pp. 255–69. [Google Scholar]
- Hanidar, Sharifah. 2016. Rhetorical Patterns, Verb Tense, and Voice in Cross Disciplinary Research Article Abstract. Humaniora 28: 12–27. [Google Scholar] [CrossRef]
- Hartley, James. 1993. Recalling Structured Text: Does What Goes in Determine What Comes Out? British Journal of Educational Tehnology 24: 359–64. [Google Scholar] [CrossRef]
- Hartley, James. 1999. Applying Ergonomics to Applied Ergonomics: Using Structured Abstracts. Applied Ergonomics 30: 535–41. [Google Scholar] [CrossRef]
- Hartley, James. 2003. Improving the Clarity of Journal Abstracts in Psychology: The Case for Structure. Science Communication 24: 366–79. [Google Scholar] [CrossRef]
- Huckin, Thomas N., and Leslie A. Olsen. 1983. English for Science and Technology: A Handbook for Nonnative Speakers. New York: McGraw-Hill. [Google Scholar]
- Hyland, Ken. 2000. Disciplinary Discourses: Social Interactions in Academic Writing. London: Longman. [Google Scholar]
- Hyland, Ken. 2008a. As Can Be Seen: Lexical Bundles and Disciplinary Variation. English for Specific Purposes 27: 4–21. [Google Scholar] [CrossRef]
- Hyland, Ken. 2008b. Academic Clusters: Text Patterning in Published and Postgraduate Writing. International Journal of Applied Linguistics 18: 41–62. [Google Scholar] [CrossRef]
- Hyland, Ken. 2012. Disciplinary Specificity: Discourse, Context, and ESP. In New Directions in English for Specific Purposes Research. Edited by Diane Belcher, Ann M. Johns and Brian Paltridge. Ann Arbor: The University of Michigan Press, pp. 6–24. [Google Scholar]
- Hyland, Ken. 2016. General and Specific EAP. In The Routledge Handbook of English for Academic Purposes. Edited by Ken Hyland and Philip Shaw. Oxon: Routledge, pp. 17–29. [Google Scholar]
- Hyland, Ken, and Feng (Kevin) Jiang. 2019. Academic Discourse and Global Publishing: Disciplinary Persuasion in Changing Times. London: Routledge. [Google Scholar]
- Hyon, Sunny. 2018. Introducing Genre and English for Specific Purposes. London: Routledge. [Google Scholar]
- ICMJE. 2024. Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. Available online: https://www.icmje.org/icmje-recommendations.pdf (accessed on 30 April 2024).
- Imao, Yasuhiro. 2024. CasualConc (Version 3.0.8). [Computer Software]. Available online: https://sites.google.com/site/casualconcj/ (accessed on 9 July 2024).
- Jego, Eric Hajime. 2012. Corpus Analysis Demonstrates That Scientific Writing Uses the Structure “<Disease> was Diagnosed…” More Than “<Person> was Diagnosed…”. The Journal of Medical English Education 11: 50–58. [Google Scholar]
- Kanoksilapatham, Budsaba. 2005. Rhetorical Structure of Biochemistry Research Articles. English for Specific Purposes 24: 269–92. [Google Scholar] [CrossRef]
- Kanoksilapatham, Budsaba. 2009. Generic Structure of Research Article Abstracts in Sciences. Journal of English Studies 4: 95–111. Available online: https://so04.tci-thaijo.org/index.php/jsel/article/view/22067 (accessed on 18 August 2024).
- Lake, William Michael, and Viviana Cortes. 2020. Lexical Bundles as Reflections of Disciplinary Norms in Spanish and English Literary Criticism, History, and Psychology Research. In Advances in Corpus-Based Research on Academic Writing. Edited by Ute Römer, Viviana Cortes and Eric Friginal. Amsterdam: John Benjamins, pp. 183–203. [Google Scholar]
- Li, Liang, Margaret Franken, and Shaoqun Wu. 2020. Bundle-driven Move Analysis: Sentence Initial Lexical Bundles in PhD Abstracts. English for Specific Purposes 60: 85–97. [Google Scholar] [CrossRef]
- Liu, Chen-Yu, and Hao-Jan Howard Chen. 2022. A Phraseological Exploration of University Lectures through Phrase Frames. Journal of English for Academic Purposes 58: 101135. [Google Scholar] [CrossRef]
- Luo, Na, and Ken Hyland. 2019. “I Won’t Publish in Chinese Now”: Publishing, Translation and the Non-English Speaking Academic. Journal of English for Academic Purposes 39: 37–47. [Google Scholar] [CrossRef]
- MacDonald, Ewen. 2019. An Analysis of Vocabulary Level in Reading Passages of the National Center Test. Shiken 23: 19–27. [Google Scholar]
- Maswana, Sayoko, Toshiyuki Kanamaru, and Akira Tajino. 2015. Move Analysis of Research Articles Across Five Engineering Fields: What They Share and What They Do Not. Ampersand 2: 1–11. [Google Scholar] [CrossRef]
- Millar, Neil, Brian Budgell, and Keith Fuller. 2012. ‘Use the Active Voice Whenever Possible’: The Impact of Style Guidelines in Medical Journals. Applied Linguistics 34: 393–414. [Google Scholar] [CrossRef]
- Millar, Neil, Françoise Salager-Meyer, and Brian Budgell. 2019. It Is Important to Reinforce the Importance of…”: ‘Hype’ in Reports of Randomized Controlled Trials. English for Specific Purposes 54: 139–51. [Google Scholar] [CrossRef]
- Mizumoto, Atsushi. 2015. Corpus-based Analysis of Lexical Bundles: Its Potential Applications in English Language Teaching. Journal of the Japan Society for Speech Sciences 16: 30–34. [Google Scholar]
- Mizumoto, Atsushi, Geoffrey G. Pinchbeck, and Stuart McLean. 2021. Comparisons of Word Lists on New Word Level Checker. Vocabulary Learning and Instruction 10: 30–41. [Google Scholar] [CrossRef]
- Mizumoto, Atsushi, Sawako Hamatani, and Yasuhiro Imao. 2017. Applying the Bundle–Move Connection Approach to the Development of an Online Writing Support Tool for Research Articles. Language Learning 67: 885–921. [Google Scholar] [CrossRef]
- Moreno, Ana I., and John M. Swales. 2018. Strengthening Move Analysis Methodology towards Bridging the Function-Form Gap. English for Specific Purposes 50: 40–63. [Google Scholar] [CrossRef]
- Morley, John. 2023. Academic Phrasebank. Available online: https://phrasebankresearch.net/ (accessed on 4 July 2024).
- Nam, Sejin, Senator Jeong, Sang-Kyun Kim, Hong-Gee Kim, Victoria Ngo, and Nansu Zong. 2016. Structuralizing Biomedical Abstracts with Discriminative Linguistic Features. Computers in Biology and Medicine 79: 276–85. [Google Scholar] [CrossRef]
- Nankodo. 2024. The New England Journal of Medicine Nihon Kokunai Ban [The Japanese Edition]. Available online: https://nejm.jp (accessed on 25 April 2024).
- Nation, Ian Stephen Paul. 2001. Learning Vocabulary in Another Language. Cambridge: Cambridge University Press. [Google Scholar]
- Nation, Ian Stephen Paul. 2005. Teaching and learning vocabulary. In Handbook of Research in Second Language Teaching and Learning. Edited by Eli Hinkel. Mahwah: Lawrence Erlbaum Associates, Inc., pp. 581–95. [Google Scholar]
- Nation, Ian Stephen Paul. 2022. Learning Vocabulary in Another Language, 3rd ed. Cambridge: Cambridge University Press. [Google Scholar]
- Noto, Hitoshi. 2015. The New England Journal of Medicine (NEJM) Saito no Katsuyo [The Effective Use of the New England Journal of Medicine Websites]. Hosupitaru Raiburarian [Hospital Librarian] 40: 159–61. (In Japanese). [Google Scholar]
- Nwogu, Kevin N. 1997. The Medical Research Paper: Structure and Functions. English for Specific Purposes 16: 119–38. [Google Scholar] [CrossRef]
- Ogawa, Ryuichi. 2014. Daigaku yakugakubu ni okeru kyoiku [Education in university pharmacy schools]. The Japanese Journal of Applied Therapeutics 6: 41–46. [Google Scholar] [CrossRef]
- Omidian, Taha, Hesamoddin Shahriari, and Anna Siyanova-Chanturia. 2018. A Cross-disciplinary Investigation of Multi-word Expressions in the Moves of Research Article Abstracts. Journal of English for Academic Purposes 36: 1–14. [Google Scholar] [CrossRef]
- Pho, Phuong Dzung. 2008. Research Article Abstracts in Applied Linguistics and Educational Technology: A Study of Linguistic Realizations of Rhetorical Structure and Authorial Stance. Discourse Studies 10: 231–35. [Google Scholar] [CrossRef]
- Qi, Hui, and Fan Pan. 2020. Lexical Bundle Variation Across Moves in Abstracts of Medical Research Articles. Southern African Linguistics and Applied Language Studies 38: 109–28. [Google Scholar] [CrossRef]
- Quero, Betsy, and Averil Coxhead. 2018. Using a Corpus-based Approach to Select Medical Vocabulary for an ESP Course: The Case for High-frequency Vocabulary. In Key Issues in English for Specific Purposes in Higher Education. Edited by Yasemin Kirkgöz and Kenan Dikilitaş. Berlin and Heidelberg: Springer, pp. 51–75. [Google Scholar]
- Rees, Geraint. 2022. Using Corpora to Write Dictionaries. In The Routledge Handbook of Corpus Linguistics, 2nd ed. Edited by Anne O’Keeffe and Michael J. McCarthy. London: Routledge, pp. 387–404. [Google Scholar]
- Reppen, Randi, and Shannon B. Olson. 2020. Lexical Bundles Across Disciplines. In Advances in Corpus-Based Research on Academic Writing. Edited by Ute Römer, Viviana Cortes and Eric Friginal. Amsterdam: John Benjamins, pp. 169–82. [Google Scholar]
- Robinson, Marin S., Fredricka L. Stoller, Molly S. Costanza-Robinson, and James K. Jones. 2008. Write Like a Chemist. Oxford: Oxford University Press. [Google Scholar]
- Salager-Meyer, Françoise. 1991. Medical English Abstracts: How Well Are They Structured? Journal of the American Society for Information Science 42: 528–31. [Google Scholar] [CrossRef]
- Salager-Meyer, Françoise. 1992. A Text-type and Move Analysis Study of Verb Tense and Modality Distribution in Medical English Abstracts. English for Specific Purposes 11: 93–113. [Google Scholar] [CrossRef]
- Salager-Meyer, Françoise, María Ángeles Alcaraz Ariza, and Beverly A. Lewin. 2014. Abstract Quality in Complementary and Alternative Medicine Papers: A Structural and Cross-Generic Analysis. In Abstracts in Academic Discourse. Edited by Marina Bondi and Rosa Lorés Sanz. Bern: Peter Lang, pp. 221–40. [Google Scholar]
- Samraj, Betty. 2016. Research Articles. In The Routledge Handbook of English for Academic Purposes. Edited by Ken Hyland and Philip Shaw. Oxon: Routledge, pp. 403–15. [Google Scholar]
- Sarica, Serhad, and Jianxi Luo. 2021. Stopwords in Technical Language Processing. PLoS ONE 16: e0254937. [Google Scholar] [CrossRef]
- Shimizu, Maki. 2019. Japanese medical students’ reading of English academic papers and an evaluation of their ability to put grammatical knowledge to practical use. Journal of Medical English Education 18: 82–86. [Google Scholar]
- Simpson, Alan. 2022. Medical Students’ English Needs and Curricular Developments. Journal of Medical English Education 21: 94–100. [Google Scholar]
- Simpson-Vlach, Rita, and Nick C. Ellis. 2010. An Academic Formulas List: New Methods in Phraseology Research. Applied Linguistics 31: 487–512. [Google Scholar] [CrossRef]
- Sinclair, John. 1991. Corpus, Concordance, Collocation. Oxford: The Oxford University Press. [Google Scholar]
- Stoller, Fredrica L., and Marin S. Robinson. 2013. Chemistry Journal Articles: An Interdisciplinary Approach to Move Analysis with Pedagogical Aims. English for Specific Purposes 32: 45–57. [Google Scholar] [CrossRef]
- Storch, Neomy, Janne Morton, and Celia Thomson. 2016. EAP Pedagogy in Undergraduate Contexts. In The Routledge Handbook of English for Academic Purposes. Edited by Ken Hyland and Philip Shaw. Oxon: Routledge, pp. 477–88. [Google Scholar]
- Stosic, Dragana. 2022. A Linguistic Construction of a Research Gap in Reports of Randomised Controlled Trials: A Systemic Functional Perspective. Journal of English for Academic Purposes 59: 101158. [Google Scholar] [CrossRef]
- Stubbs, Michael, and Isabel Barth. 2003. Using Recurrent Phrases as Text-Type Discriminators: A Quantitative Method and Some Findings. Functions of Languages 10: 65–108. [Google Scholar] [CrossRef]
- Swales, John. 1981. Aspects of Article Introductions. Birmingham: The Language Studies Unit, The University of Aston in Birmingham. [Google Scholar]
- Swales, John M. 1990. Genre Analysis. Cambridge: Cambridge University Press. [Google Scholar]
- Swales, John M. 2004. Research Genres. Cambridge: Cambridge University Press. [Google Scholar]
- Swales, John M. 2017. Standardisation and its Discontents. In Publishing Research in English as an Additional Language: Practices, Pathways, and Potentials. Edited by Margaret Cargill and Sally Burgess. Adelaide: University of Adelaide Press, pp. 239–54. [Google Scholar]
- Tang, Chiachieh, and Yeu-Ting Liu. 2019. Construction and Proposal of an Academic Word List for Medical Professionals at Different Developmental Stages. Journal of Medical English Education 18: 13–20. [Google Scholar]
- Tankó, Gyula. 2017. Literary Research Article Abstracts: An Analysis of Rhetorical Moves and Their Linguistic Realizations. Journal of English for Academic Purposes 27: 42–55. [Google Scholar] [CrossRef]
- Tardy, Christine M. 2009. Building Genre Knowledge. Anderson: Parlor Press. [Google Scholar]
- Tardy, Christine M., and Soomin Jwa. 2016. Composition Studies and EAP. In The Routledge Handbook of English for Academic Purposes. Edited by Ken Hyland and Philip Shaw. Oxon: Routledge, pp. 56–68. [Google Scholar]
- The New England Journal of Medicine. 2024. Available online: https://nejm.org/ (accessed on 18 August 2024).
- Thorndike, Edward Lee. 1921. The Teacher’s Word Book. New York: Teachers College, Columbia University. [Google Scholar]
- Thorndike, Edward Lee, and Irving Lorge. 1944. The Teacher’s Word Book of 30,000 Words; New York: Teachers College, Columbia University. Available online: https://books.google.com/books?id=FeJZAAAAMAAJ&q=The+Teacher%E2%80%99s+Word+Book+of+30,000+Words&dq=The+Teacher%E2%80%99s+Word+Book+of+30,000+Words&hl=ja&newbks=1&newbks_redir=1&printsec=frontcover&sa=X&ved=2ahUKEwjE9ZTW8IqIAxWkjK8BHTfBAI4Q6AF6BAgJEAI (accessed on 18 August 2024).
- Tribble, Christopher. 2017. ELFA vs. Genre: A New Paradigm War in EAP Writing Instruction? Journal of English for Academic Purposes 25: 30–44. [Google Scholar] [CrossRef]
- Viana, Vander, and Aisling O’Boyle. 2022. Corpus Linguistics for English for Academic Purposes. Oxon: Routledge. [Google Scholar]
- Wang, Jing, Shao-lan Liang, and Guan-chun Ge. 2008. Establishment of a Medical Academic Word List. English for Specific Purposes 27: 442–58. [Google Scholar] [CrossRef]
- West, Michael. 1953. A General Service List of English Words. London: Longman. [Google Scholar]
- Widdowson, Henry G. 1991. The Description and Prescription of Language. In Linguistics and Language Pedagogy: The State of the Art. Edited by James Alatis. Washington, DC: Georgetown University Press, pp. 11–24. [Google Scholar]
- Zhang, Chunfang, and Xueli Liu. 2011. Review of James Hartley’s Research on Structured Abstracts. Journal of Information Science 37: 570–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).