Abstract
The objective of this study was to deepen our comprehension of how children develop understanding in the field of science, particularly in chemistry. Using the framework theory as a theoretical lens enabled a focus on emergence as a dynamic change and transition. According to the framework theory, children’s science learning involves a wide range of intuitive and counterintuitive scientific concepts related to ontological and epistemological perspectives. How children transition from everyday to scientific thinking during their early years of education is influenced by ontological and epistemological stances. The objective of this study is to introduce science content—including chemical concepts to preschool children—by utilizing a play-based learning approach in a longitudinal study. The exploration of verbal and non-verbal material, specifically pertaining to chemical content and individual differences, involved implementing educational experiments and real-life or animated zooming-in videos. The results indicated a well-established physical ontological framework utilized for the systematic interpretation of submicroscopic phenomena.
1. Introduction
In the past decade, there has been a growing emphasis on incorporating natural science into preschool education. Both educational researchers and practitioners have shown increased interest in using play-based learning and conceptual play to introduce science concepts. This early exposure to science offers numerous benefits. Firstly, high-quality preschool education is widely recognized as crucial for future academic success [1]. The preschool years also have the potential to shape lifelong learning and are seen as an important part of our cultural heritage [2], making this period an integral aspect of education. Science education in preschool settings serves not only the goal of teaching science but also contributes to various developmental domains. It helps foster social development [3], lays the foundation for language and conceptual understanding [4,5,6,7,8,9,10], enhances motor skills [3], and supports problem-solving abilities [11,12]. Additionally, there are equity goals in science education that aim to empower children with knowledge and enable them to make informed decisions for a sustainable society [13]. Early exposure to science also aims to create positive associations with the subject, catering to children’s curiosity and fostering their interest and positive attitudes toward science in their early education [14,15,16,17,18,19]. These positive associations are considered crucial not only for future formal learning but also for later informal science education [20].
1.1. Chemistry in Preschool
Research studies in natural science didactics have primarily focused on the fields of physics and biology. This is because phenomena within these subjects are naturally embedded in children’s everyday lives and easily pique their curiosity. Many biological and physical phenomena can be directly experienced, and their causes can often be deduced and predicted. However, chemistry faces a unique challenge as it offers a relatively limited number of everyday encounters, which are not always apparent. Furthermore, understanding the causes behind chemical phenomena is not easily attainable. While some chemical transformations, like phase transitions and combustions, can be experienced, many ongoing processes are so slow that they are difficult to witness, such as the transportation of matter through natural cycles. One practical way to experience chemistry is in the kitchen, where activities like mixing, stirring, dissolving, and tasting provide opportunities to explore chemical phenomena. Unfortunately, the scientific explanation behind these processes, or the reasons behind chemical phenomena, are often ignored or too abstract to explain. This is mainly because understanding these explanations requires comprehending submicroscopic particles and their relative size. In fact, size is a key factor in understanding chemistry. This project investigates how children develop the concept of smallness.
How can we describe the sizes of submicroscopic particles? Since there is no specific language to describe them, the scale of a femtometer (the radius of an atomic nucleus) can only be understood in relation to something else. Unfortunately, the objects we use to determine size quickly become insignificant because we have no real experience with their actual size. If we use numbers to explain the atomic size, most scientific textbooks typically refer to the radii of neutral atoms, considered in their relative isolation. These typically range between 30 and 300 pm (trillionths of a meter). The radii of neutral atoms can also be measured in ångströms, which is a unit for measuring the length of submicroscopic entities. The radii of neutral atoms are between 0.3 and 3 ångströms (10−10 m). This means that the radius of an atom is more than 10,000 times larger than its nucleus [21]. If one cannot fathom the magnitude of 30 trillionths of a meter or something 10,000 times smaller than an atom, numbers quickly become devoid of their true significance. However, the general understanding that atoms are incredibly minuscule is usually enough to grasp most chemical concepts.
Teaching the concept of smallness, as mentioned before, is challenging. Similarly, understanding how we grasp the idea of smallness becomes equally difficult when we rely on our everyday language, which predominantly focuses on macroscopic objects. Describing something as small can be achieved by comparing it to other tiny items, like being smaller than an ant. Alternatively, we can convey the idea by emphasizing that these things are so minuscule that they exist within everything. Another approach is to use intensifiers such as “very small” or, for instance, repeatedly emphasizing the word “tiny” like “tiny, tiny, tiny little ones”.
When asking children what the smallest thing imaginable is, the answers may well be “insect babies”. A grain of sugar viewed through a microscope becomes “dust” and toadstools become “fish”. When a magnifying glass is used to look at a grain of salt amplifying its size, children might describe it as an ice block. This provides some indication about how size is perceived, with the result that the magnifying glass is seen as an item that makes things large instead of as a way to explore small things. When children imagine what something really small looks like, it often results in representations of continuous matter, where they simply see the smaller parts as smaller pieces of the item at hand [22]. In fact, research suggests that children’s sub-microscopic perception can be enhanced with the aid of visual experience that supports their imagination [23]. This previous experience aids their imagination by providing a much firmer basis. The focus of this study is to examine how young children learn the concept of smallness when visual experience is provided and to explore the broader consequences of this learning on their understanding of natural phenomena, like evaporation.
1.2. Conceptual Development and Emergent Science
The process of learning scientific concepts has been extensively studied and is considered a transition from everyday concepts to formally introduced scientific concepts. Intuitive concepts, which are synonymous with everyday concepts, often originate from children’s sense-based observations of their surrounding environment. Through the impact of education, intuitive ideas are influenced by formally introduced concepts. This transition involves expanding the content of concepts and incorporating scientific ideas, all within the context of language and the child’s surrounding culture [24]. This progression is referred to as “emergent science” at the preschool level. Understanding how formally learned knowledge and intuitive ideas interact provides insights into the learning progression [25]. Emergent science emphasizes children’s personal reflections regarding science, without assessing the accuracy of children’s concepts from a strictly scientific standpoint. The emphasis is on the actual process of subject-specific learning. The development of scientific progress depends on various factors, including individual stances and sociocultural characteristics. Motivations, emotional connections, and interests are crucial elements in acquiring knowledge and learning.
Framework Theory
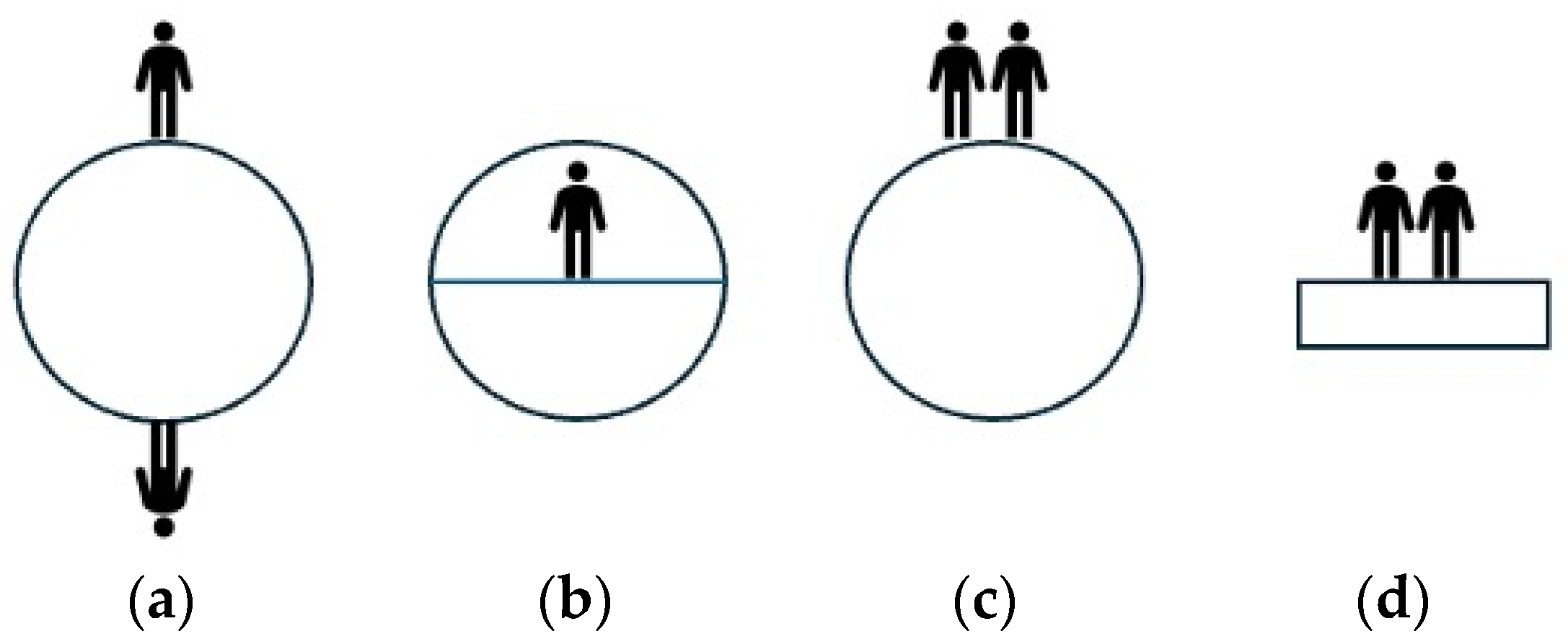
The analysis of the actual changes in emerging science can be approached from different perspectives. In this case, the framework theory is utilized to examine the development of children’s understanding of science. The term “emerging science” is employed to emphasize the focus on the process of change. According to the framework theory, scientific learning arises from intuitive experiences in our everyday lives, which are primarily based on sense-based observations. These intuitive concepts are not separate entities but rather interconnected within frameworks and models that encompass ontological and epistemological concepts. Ontology involves our interpretations of the fundamental nature of reality, while epistemology pertains to our interpretations of the causal mechanisms used to explain a phenomenon. An example of the interrelation between an intuitive concept and its ontological and epistemological aspects can be observed in how children perceive the Earth and its connection to the intuitive concept of up/down gravity. When children are asked to draw the planet, the result is often various interpretations, as depicted in Figure 1.
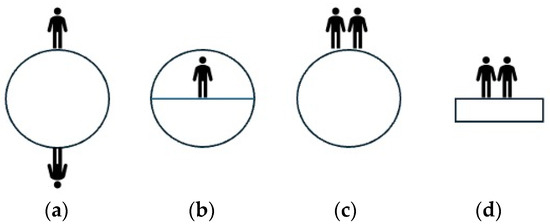
Figure 1.
Some examples of children viewing the Earth as an object with up/down gravity; (a) people live on a ball-shaped Earth, (b) people live on flat parts of the Earth, (c) people live on top of the ball-shaped Earth, (d) people live on a flat Earth [26].
Research on children’s initial ontology is an ongoing discussion, with researchers making different proposals regarding the number of initial ontological groups [27,28,29,30]. Table 1 presents one of these proposals, which states that children’s early ontology is distinguished by four main ontological frameworks. In the psychological framework, the cause of an object’s change is attributed to intentionality, whereas numbers and words are categorized as two separate elements of reality.

Table 1.
Examples of early ontological and epistemological stances included in intuitive models [28].
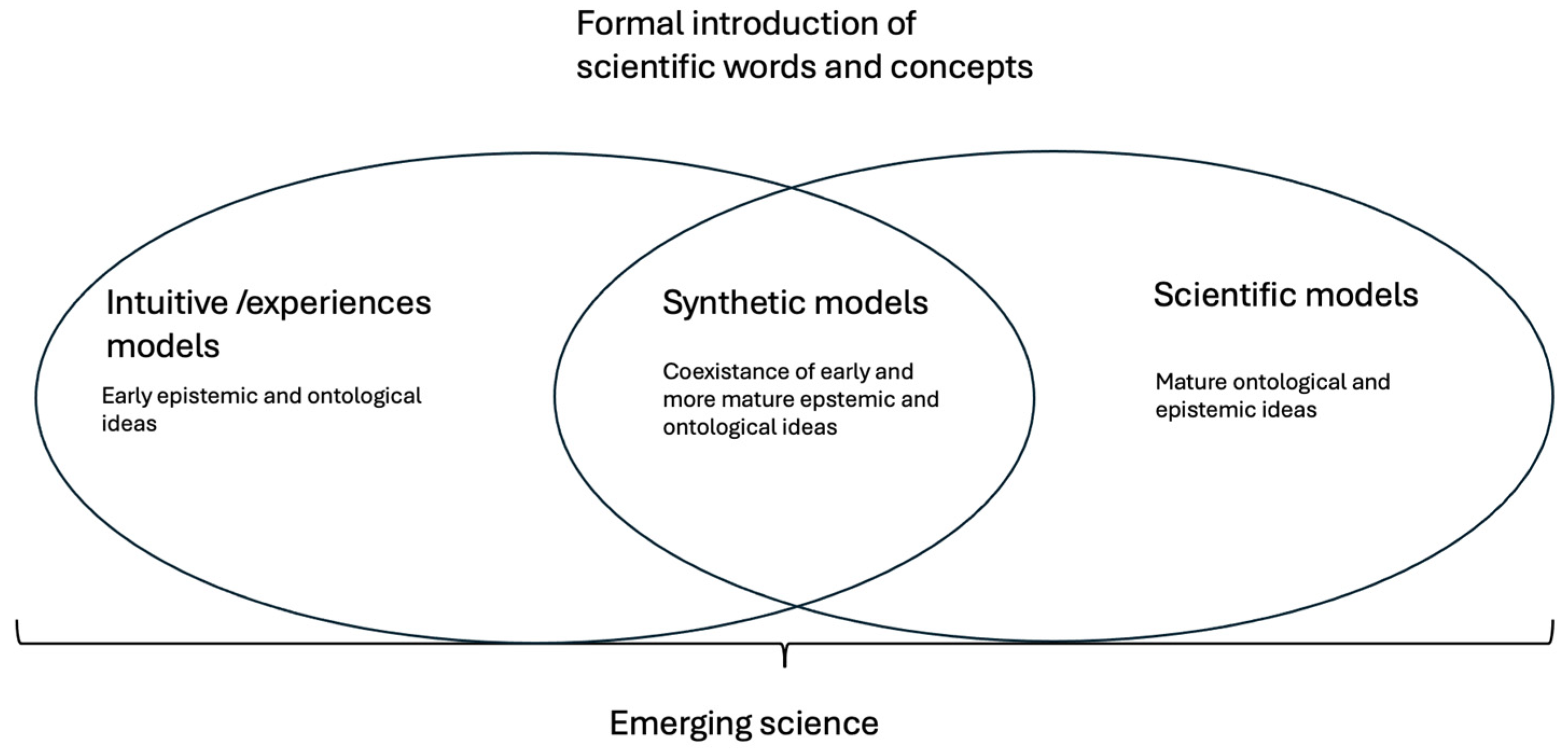
Introducing scientific concepts in a formal manner poses a challenge for children as they try to reconcile their sensory-based ideas with more abstract explanations of their surrounding phenomena. This procedure stimulates broader tectonic shifts in the epistemic and ontological principles of children [31]. As children commence their acquisition of scientific knowledge, the development of synthetic models becomes apparent. These models are a creative blend of intuitive and counterintuitive concepts (see Figure 2 and Table 2). In the framework theory, this change can be described as a progression from intuitive models to synthetic models and eventually to scientific models.
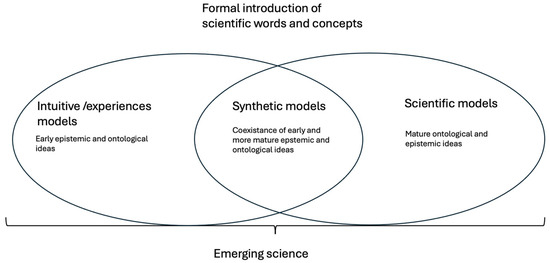
Figure 2.
A summary of development from the aspects of the framework theory.

Table 2.
Definitions of core concepts in the framework theory.
Considerable research has been undertaken on subject-specific learning, and the findings support that initial intuitive concepts are rooted in sensory experiences and vary in their development, depending on the learner and the topic being studied. Moving from intuitive to synthetic models represents both the specific learning pathway in a scientific field and scientific emergence in general. Various learning pathways have been proposed for different scientific subjects as well as for the scientific process itself. One such pathway involves systematic observations that start with the connection between the body and the five senses and then progress toward making predictions, and finally the verification of those predictions [30,32]. This particular learning pathway can serve as a useful tool for moving away from intuitive explanations and toward scientific concepts, including changes in epistemology and ontology [33]. These findings highlight the importance of engaging in scientific activities, acquiring scientific vocabulary, and enabling children to express different aspects of scientific explanations. The developmental process must allow for transferability across different contexts and extended periods of time [30]. This conclusion applies to the development of all subject-specific concepts.
1.3. Emerging Chemistry
There have been suggestions regarding learning pathways or trajectories for various chemistry topics, such as matter or the water cycle. At a broad level, understanding the fundamental aspects of matter involves understanding: (a) structure and composition; (b) physical properties and change; (c) chemical reactions; and (d) conservation [34]. A 2013 study outlined an overarching learning progression for the concept of matter, suggesting that children initially have a continuous understanding of matter, with no identification of its submicroscopic structure. Considering their exposure to education, children afterward reach an intermediate stage in which they recognize the presence of particles but project them with macroscopic attributes, for example, believing that the tiniest parts of a substance hold all of its macroscopic properties such as taste and color [35]. Finally, children recognize that particles make up the substance without displaying macroscopic properties. In this discussion, we will explore the first step in greater detail: particles existing within the continuous substance.
Research findings on children’s understanding of chemical concepts align with the framework theory, emphasizing the importance of sensory-based intuitive concepts. Matter is deeply ingrained in individuals’ lived experiences, and certain research suggests that children’s ideas about the environment are implicit and unquestioned [36,37].
Research studies show that initially, children tend to categorize solid matter based on sensory-motor aspects like color, shininess, and softness [38]. These aspects are also connected to everyday generalizations, such as the belief that a hard, smooth, and transparent object will break when dropped. This sensory-based perception views matter as static, continuous, and uniform, with no empty space between particles, and its identity remains constant [39]. This leads to the conclusion that when an object changes appearance, it does not transform into a different form, but a completely different entity appears. In other words, there is no concept of transformation of matter.
An object’s initial, external characteristics, like its boundedness, solidity, and enduring and distinct properties and functions, become the basis of its identification [40]. Other intuitive concepts related to the properties of matter include mass, volume, and weight, with weight being evaluated based on the feeling of heaviness, while length and volume are assessed based on size. These assumptions lead to the association of mass, volume, and weight οnly with large and visible objects. Simultaneously, objects of small size, like a small piece of plasticine, are not perceived as having mass, volume, or weight [41,42]. The attribute of solidity for objects is determined using everyday criteria such as hardness, durability, and resistance to cracking [43]. Additional challenges arise in children’s identification of atypical solids such as dust, powders, or pliable and brittle materials [44].
The different states of matter represent one part of chemistry that can be experienced and defined on a macroscopic level using shape and volume. If a solid object is placed in a container, it maintains its shape and volume. If a liquid is placed in a container, it maintains its volume but assumes the shape of the container. If a gas is placed in a container, it assumes the volume and shape of the container. By following the observable general features of the different states of matter, children start to acquire stable signs as a way to identify or distinguish between the different states.
Similarly, appearance and actions such as spillability, colorlessness, and odorlessness [45] are used as properties to identify liquids. The most common liquid is water, and it can easily become a prototype for all kinds of liquids, due to liquids having external similarity. This conclusion is further supported by results that show that viscous liquids are not classified as liquids, because they do not appear like water. The gaseous state is a state that is not easily visualized and is often seen as non-material [41,42], resulting in the notion that liquids vanish during evaporation. The gaseous state is often associated with various phenomena, including heat, electricity, and everyday gases like soda [46,47].
To gain a comprehensive understanding of matter, it is essential to explore the transitions between its different states. One particular aspect that has been extensively studied is the water cycle [48]. To grasp the water cycle, at this educational level, it is necessary to consider a few key concepts: the different states of matter (solid, liquid, and gas), as well as the phenomena of evaporation and condensation caused by heating or cooling and the conservation of water during these processes. In another study, four distinct intuitive concepts regarding evaporation were identified [49]. These include the notion that water simply disappears, the belief that water can penetrate either the floor or a solid object, or that water is scattered in the air.
Transitioning from everyday experiences to a more subject-specific chemical perspective necessitates specific modifications. This change involves shifting from a macroscopic, sense-based perspective of the world to a submicroscopic worldview. In a sub-microscopic perspective, the properties of objects are conceptualized based on their internal composition. This change is difficult for learners of all ages [47] as it includes epistemic changes, notably understanding an object through multiple representations [50]. Considering these findings, learning pathways for matter should commence from discovering a child’s everyday world, with an emphasis on the identification of various forms of matter [35]. The correlation between properties and color or shininess implies that learning could be enhanced by recognizing differences in properties among samples of matter with similar colors and sizes. These actions could lead to a deeper understanding of the intrinsic and fundamental variations in matter. Research has shown that sensory-based perception plays a crucial role in supporting the imagination at the submicroscopic level. In particular, providing visual experiences can effectively bridge the gap between the macroscopic and submicroscopic levels of matter [23,51]. This is particularly important in encouraging the development of children’s understanding of concepts in more abstract domains.
Research Questions
The objective of this study is to examine the fundamental principles of children’s emergent chemistry, focusing specifically on their comprehension of the concepts of smallness and evaporation. Smallness was chosen because it represents one of the core aspects of chemical knowledge. Vaporization was also included in this study because the phase transition between the liquid and gaseous state reflects children’s understanding of the transition between visual and non-visual matter. The first goal of this research was to examine the process by which children generate everyday, synthetic, and scientific models of these concepts.
2. Materials and Methods
The purpose of this study was to explore the fundamental principles of emergent chemistry in children, focusing specifically on their understanding of smallness and evaporation. The research design took the form of a longitudinal study, designed as an educational experiment [52]. The present educational experiments followed a cyclical pattern, where each activity was promptly analyzed, and the resulting analysis served as the basis for planning the next activity. The activities were specially designed as play-based learning interventions. These play-based learning activities were carefully tailored to match the individual interests of the children, encouraging their ongoing engagement in the educational process [53]. In this way, scientific concept formation became a deliberate and thoughtful process led by the early childhood teacher [54]. Play-based learning environments both challenge children’s broader understanding of the world and facilitate their acquisition of scientific knowledge. Other important aspects of this learning context include intersubjectivity and sustained shared thinking. Sustained shared thinking refers to an extended situation where the educator and the children engage in shared understanding and discourse [25].
2.1. Design of Activities
The interventions implemented in the play-based approach consisted of experimental activities and real-life or animated zooming-in videos, enabling children to grasp fundamental chemical concepts through play (See Table 3). The primary theme of the first five activities revolved around the concept of smallness and the last two revolved around evaporation. A detailed record of the activities was documented, and individual recall interviews were conducted to explore each child’s understanding. The collected data were analyzed, with a specific focus on the children’s conversations, body language, and gestures, in order to gain a deeper understanding of their engagement with the activity content.

Table 3.
Description and time frame of the activities.
The preschool environment in Greece is currently undergoing a transformation, incorporating contemporary educational practices and drawing inspiration from international experiences. The latest Greek curriculum outlines the essential abilities, skills, and attitudes that children should acquire upon completing their preschool education. This includes the field of natural sciences, which involves understanding living organisms and the properties of matter, as well as studying the Earth, space, and planetary systems. Instructions should be structured around everyday occurrences to facilitate exploratory inquiries on these topics. Specifically, teachers should support children in asking appropriate questions for investigation and encourage them to use their imagination and creativity to conduct experiments and acquire new knowledge.
Data were collected in Greece from participants in two middle-class public schools. These students had limited exposure to natural science experiments and did not participate in any organized play-based learning programs with natural science materials. This study included five groups, each consisting of five children aged 5 to 6 years old. Each play session lasted 20–25 min. Video recordings were made to capture the interactions and communication within each group, and additional material was collected through individual interviews. Research ethics approvals were granted by both the university and the Greek Ministry of Education.
2.1.1. The Teacher’s Role
The teacher/researcher adopted a scaffolding stance toward the children. This involved being supportive of questions while not providing definite answers but rather scaffolding the children’s thinking process. This also included gradually providing less support as the children showed improvement. The notions of microbes and molecules were never introduced by the researcher.
2.1.2. Data Analysis
Data were analyzed in the following steps:
- Data were collected.
- Relevant vignettes (smallness in the first experiment and evaporation in the last two) were selected.
- Key objects and topics that reflected children’s ideas about smallness and evaporation were identified. For example, the leaf, the ants, and the magnifying glass were central objects that reflected these ideas. The question “What is the smallest thing you can imagine” serves as a paradigm for this topic. The vignettes were organized based on these key objects and topics.
- Criteria to distinguish intuitive and counterintuitive conceptions regarding smallness and evaporation were formalized.
- Intuitive and counterintuitive vignettes, as well as synthetic models of smallness and evaporation, were categorized
- General characteristics of intuitive smallness, counterintuitive ideas about smallness, and synthetic models of smallness were analyzed.
The participation of children in the experiments sparked the emergence of concrete ideas regarding smallness and evaporation. These ideas were further examined and categorized as intuitive, synthetic, and scientific models, based on how the children conceptualized crucial materials and processes (see Table 4).

Table 4.
A summary of the analytical basis for categorization into intuitive, counterintuitive, synthetic concepts, and scientific concepts.
3. Results
3.1. Intuitive Model of Macroscopic Smallness
The focus of the first five activities was children’s conceptualization of smallness, explored through how they made sense of the main objects in each activity. Characteristic examples of using these words were presented in the form of vignettes. The children’s intuitive model of smallness was defined by its macroscopic characteristics. In other words, smallness only refers to visually accessible small things.
When children were asked what the smallest thing was, they typically provided a number of concrete objects, which were categorized as intuitive concepts. The tiniest conceivable entities were derived from objects that were readily observable, such as specks of dust, miniature LEGO pieces, ants, baby flies, turtles, stars, butterflies, snakes, snails, caterpillars, mice, camera lenses, bread crumbs, keys, and small buttons. Quite often, the children were oriented toward their immediate environment using their hands to portray or show something small or define smallness negatively, such as something that was not big.
Vignette 1
Researcher: What would it look like if we cut them into very small pieces? If we close our little eyes and think of sugar being cut into little tiny little pieces, what do we get?
Anastasis: It will melt, melt, melt, melt if we do (he claps his hands as if to show he’s melting the sugar) and then... gone.
Athina: If I do it too much like this with the knife (pretends to cut something with her hands) it will cut and it will become like a little tiny baby.
Researcher: Like a little baby, huh? What if we cut it even smaller?
Marianna: It will get so much smaller (she puts her fingers together).
Children’s comprehension of smallness was also articulated in terms of comparable objects. When attempting to imagine microscopic aspects of salt and sugar, children would liken them to small balls, stones, small medicine, ants, snow, glass, or gold dust. In the two zooming-in sessions, the microscopic elements were reinterpreted as real physical objects such as animals or flowers. Water molecules were described as little spores, bubbles, stones, little balls of water, a Mickey Mouse head, a rabbit’s, or a human head. Geometric vocabulary was also used, as the molecules were viewed as circles that were tied to each other. These vignettes were categorized as intuitive concepts.
In other words, children perceived the smaller particles of salt and sugar, as well as the real-life and animated elements in the zooming-in videos, and the water molecules, as concrete, real-life physical objects. Their identification of similarities between these objects illustrates that children observed a sense of commonality between the items. These objects are physical entities, exemplifying the application of a physical ontological framework. The usage of this framework may create barriers in trying to understand the counterintuitive properties of submicroscopic elements.
Moreover, less frequently encountered examples of smallness were exemplified in terms of depth. The children viewed the animated zooming-in videos as a journey into matter, with the progressively smaller levels being perceived as moving deeper in. A few vignettes defined smallness as an age category, suggesting that smaller ants are younger.
3.2. Synthetic Conceptions of Smallness: Invisibility Does Not Mean Non-Existence
Counterintuitive concepts of smallness were predominantly centered around the notion of the microbe. This notion functioned as a general category, which children used to represent all kinds of characteristics in relation to smallness. In meeting 1, parts of the leaves were defined as microbes. In meeting 3, the children used the same notion to describe the various forms and colors that they saw in the animated zooming-in video. The idea of a microbe was employed in an unspecified way, encompassing meanings such as exceedingly small elements or circles of very small sizes. In many instances, children reported that microbes were the fundamental components of various objects, suggesting that the world is made out of tiny, round entities that constitute all sorts of things. In these vignettes, microbes were perceived as being so small that they could only be viewed under a microscope. These counterintuitive ideas suggest the emergence of a synthetic model of smallness, in which things may exist, even though they cannot be seen with the eye. This model designates the emergence of an atomic, molecular perspective of the world.
As an example, the children described how a leaf, an orange, and dust would all appear to consist of microbes when observed under a microscope. Within the context of these vignettes, the children considered microbes to be of such diminutive proportions that their visibility could only be achieved through microscopic examination, thereby the children acknowledged the existence of microbes despite their invisibility to the naked eye. This finding represents a generalization of the zooming-in animation, in which the molecular structure of water was depicted as a model of little balls connected with lines. This emerging understanding of the microscopic level does not necessarily mean that children understand that all things are made of small particles, as children often interpret them as real physical objects.
In one vignette, a child reimagined the microscopic structure of a leaf that he saw in an animated zooming-in video as a fight between two sides—the illness-producing and curing elements. The child used his imagination to understand the microscopic level in a way that made sense to him.
The application of the notion of microbes is termed as counterintuitive because it includes the counterintuitive notion that things are made of really small elements, thus opposing the understanding of matter as continuous. Even though microbes were used to denote visually accessible small structures, their usage contributed to the emergence of a scientific perspective toward smallness.
Vignette 2
Anastasia: That they are some tiny little creatures.
Researcher: Some tiny little things... And what are these tiny little things? What do they look like?
Areti: Microbes.
Researcher: And what do they look like?
Anna: There is a so and so (forms a circle in the air); there is a so and so and so and so microbe (draws it in the air).
Researcher: Show the group.
Anna (makes circles with her hand in the air): One like this, one like this, one like this, and one like this, many many, many, and circles and like this...
Researcher: No, you don’t have to. Well, tell us what we saw in the videos.
Areti: Microbes, just microbes.
Anna: I had seen some microbes that were making some sounds; there were so many of them in the leaf... They were such little circles, little circles, little circles.
Researcher: Have you ever seen something so small before?
Anna and Areti: No.
Researcher: Is this the first time you’ve seen it?
Anastasia: I saw it.
Researcher: What did you see?
Anastasia: I saw some small, very small things that I saw... round... I saw inside there in the tree... Those round things were ants... No, they weren’t ants... They were microbes.
In counterintuitive concepts of salt and sugar, the children reported that cutting salt into small pieces would have made the small pieces invisible to the naked eye, but they could still exist. Similar conceptions were expressed with microorganisms, with children stating that objects were full of these small germs that could only be seen through the microscope. In counterintuitive concepts regarding the smallest thing that they could think of, some children acknowledged the existence of objects invisible to the eye, which they referred to as “very microscopic”. They recognized that these objects exist at a great depth and appear different from the same objects at the macroscopic level. In a counterintuitive concept regarding ants, ants were understood as having nonvisible internal organs, like a heart and bones.
Vignette 3
Researcher: Let me ask you. If this salt was cut into smaller pieces?
Anastasis: Yes.
Researcher: And we cut it so small that we couldn’t see it, would there still be salt?
Anastasis: Yes.
Researcher: There would be, wouldn’t there? I mean, is it possible for something to be so invisible to the eye but still exist?
Anastasis: Yes, but wouldn’t we see it?
Researcher: What would it take for us to see it?
Anastasis: Microscope.
In counterintuitive concepts related to water molecules, some children were able to generalize the idea that all objects consisted of imperceptible tiny spheres, similar to the ones depicted in the videos on water molecules. The concept highlighted in this idea indicates the emergence of the concept of molecules. Alongside this comprehension, several additional scientific fragments emerged, including the notion that these formations are held together through some forces. The usage of vocabulary such as “electrical current” or “microscopic laser” served to communicate the idea that the particles bonded together like tiny magnets. The same children also replicated other scientific fragments, such as the discovery that water maintains an identical atomic structure in all three forms, regardless of its external appearance. This indicates that scientific knowledge does not appear as a set of disparate fragments but as an interconnected network of concepts. In fact, the counterintuitive idea that matter’s behavior relies on electrical currents can be more easily understood in the context of a molecular depiction of matter.
Vignette 4
Researcher: Anna, do you remember what we did last week?
Anna: We watched some videos. There was something round that had made them, particles, and the current had joined them.
Researcher: The current had connected them? Okay, what were these balls that were connected to the current?
Anna: Particles.
3.3. Intuitive Conceptions of Evaporation
Many children participating in the vaporization experiment perceived water, bubbles, fire, and smoke as distinct elements, failing to recognize their interconnectedness. Specifically, each element was perceived intuitively, for example, stating that “water is making some bouncing bubbles, that it is boiling, goes up and down and getting out, like a wave, was turning, was becoming white, appeared like milk, and that it made a strange sound”. The bubbles were described as follows: some little white balls, rumbling water, shampoo, some oil, a hole (which) opens in the water, and growing water. They also used the metaphors of a pool, a whirlpool, a tornado, and a volcano. The water vapor above the surface was not seen as a result of water condensation, but instead, it was identified as smoke produced by the stove.
The boiling water was also treated intuitively as a physical object. More specifically, the children often stated that the decreasing water level was attributed to the water going more down, or that it became shorter, moved toward the bottom, or became smaller and smaller, like any other physical object influenced by gravity. Another explanation provided was that it melted. Similar results were replicated with the juice, with the children stating that it went a little bit down, it was because it was boiled, it decreased, or even that it just disappeared. Another child described the process as ‘the mass became smaller and little.’ Another similar explanation was that ‘the juice got stuck at the bottom of the pot’. In this set of vignettes, the zooming-in videos of the previous sessions, which introduced children to the submicroscopic world, did not contribute to these interpretations of evaporation. In other words, children did not use this knowledge as a basis for explaining the transformation of macroscopic water to vapor.
Some children predicted that heating water would make it ‘bigger and longer’, or that it would stay the same. These answers are not entirely unjustified as water expands when boiling. Nevertheless, they are still deemed as intuitive conceptions because they do not depict the counterintuitive phenomenon of the change of state, even though they are in accordance with the facts when water boils. Other children also said that the researcher cheated, by spilling or drinking it while the children were not looking. All of these ideas are defined by the macroscopic understanding of the liquid.
During individual interviews, children were asked to generalize their findings. Vignettes in which children were unable to generalize vaporization with other liquids were evaluated as everyday conceptions. For example, children reported that heated water would not create smoke or vapors or that the juice would catch fire. In one vignette, a child reported that if you leave some water in the sun, the effect would be that the water would become “much thinner”. Also, in another vignette, children said that milk would not decrease, because it would not have bubbles. Findings demonstrated that the children had a combined set of intuitive ideas about the various aspects of the vaporization experiment.
3.4. Synthetic Models of Evaporation
In the synthetic model of vaporization through boiling, children managed to connect smoke with water and fire. Specifically, children started to understand that due to the thermal agent, water and juice became vapor. As the children realized that the vapor did not come from the fire, some children began to use more specific vocabulary, like steam, water vapor, or “cloud”. It was also interesting to see how two different children were also reminded of the cycle of water and used it to understand the experiment. One child expressed a very good understanding of evaporation, using the correct terminology, being able to predict what would happen if the boiling continued, what would happen with the vapor, and understanding that the boiled water continued to exist as vapor. In this set of vignettes, the zooming-in videos functioned as a preparatory step, which helped children reinterpret the disappearance of water through its transformation into vapor. In one vignette, a child interpreted vaporization by understanding fire and air as forces of activeness; the child reported that the fire and air push the juice upward, making it go up, and that the fire made the juice turn into smoke.
In synthetic vignettes of evaporation, children correctly predicted that the water and juice would decrease after boiling; often, they were able to explain why this was so and use the correct specified vocabulary. In one vignette, a child said that boiling would lead to the removal of vapors, which resembles the macroscopic idea that vapors are physically removed. The notion of burned water was also codified in synthetic conceptions, in that it shows the possible function as an intuitive expression reflecting the emergence of the scientific concept. Some children were also capable of generalizing, stating that other things, such as juice or tea, or even saliva and milk, could evaporate.
4. Discussion
The comparison between intuitive and synthetic models of smallness reveals numerous concrete, specific, and metacognitive changes that occur in children’s perception of the world. Intuitive concepts are largely characterized by macroscopic visual perception. The central objects of the experiments as well as the microscopic elements of the videos were perceived as tangible, physical objects. To give an example, in the zooming-in videos, the microscopic structures were regarded as physical entities. These children were limited to a visibly accessible representation of small objects and, thus, were not able to imagine that these objects also had submicroscopic structures.
In synthetic concepts, on the other hand, the children’s notions of smallness recognized the existence of invisible things, which can be viewed with the help of the microscope. The non-differentiated application of the term “microbes” included the scientific emergence of smallness through various unspecified characteristics. These notions strongly depended on immediate visual characteristics, while at the same time functioning as the basis for cultivating the idea that even smaller elements, which are not visually accessible, might exist and be the building blocks for various objects. The focus on small, invisible things called microbes may have initially been caused by a focus on illness due to the COVID pandemic and then reinforced through the animated zooming-in videos that showed small, round things. Nonetheless, these findings show that the conceptual framework for a more scientific emergence of the concept of smallness—as something smaller than what we can see—is indeed possible for preschool children, and this finding opens up the potential for the emergence of various chemical phenomena if transferable to other relatable experiences.
The existence of frameworks seems to be confirmed by another set of observations as well. Data indicate that children who used the notion of microbes were able to conceptualize leaves as part of a biological system of interconnected functions or to understand the electromagnetic properties of submicroscopic particles. In this sense, learning science is not a process limiting itself to subject-specific representational changes, but rather existing within a range of interconnected representations that are relevant to it. This possibility is significant for the emergence of chemistry, as it allows children to develop a submicroscopic perception of the world.
When comparing intuitive and counterintuitive concepts of evaporation, similar patterns emerge. Initially, children struggle to connect the different components of evaporation, leading to explanations based on observation rather than on an understanding of the underlying phenomenon of water loss. Conversely, the defining feature of the synthetic model was the children’s tendency to establish connections between the different elements of the experiment. The ability to create these connections represents a higher epistemic change toward understanding evaporation.
The present research identified a strong presence of the physical ontological framework where the children systematically interpreted submicroscopic elements as everyday physical objects. These data indicate that moving beyond a purely physical ontological framework is necessary to fully grasp the submicroscopic intricacies of the world. It is crucial to clarify that this does not imply the invalidation of the established principles governing physical objects; rather, it signifies a differentiation of the two ontological levels, shedding light on the behavior of submicroscopic constituents. As demonstrated by Vosniadou, a comparable phenomenon occurs in the comprehension of planets behaving distinctively from ordinary physical entities [55,56]. The results also indicated a prominent existence of a psychological framework among children and a tendency to interpret microscopic elements as living entities.
The analysis of the findings in both smallness and evaporation indicates a possible mutuality between the two conceptions. The scientific emergence of smallness is closely related to the understanding that non-visible objects, aspects, and traits exist. The children in the evaporation experiments made a similar discovery, leading them to the realization that water cannot disappear, but it exists in a different invisible form as vapor. By analyzing the interrelation between the different aspects of the experiments, the children arrived at an abstract result with no immediate concrete referents. This can be a guideline for how microscopic smallness generally emerges. Preschool-aged children who rely solely upon sensory observations often conclude that objects that are not visible no longer exist. But posing questions about everyday phenomena that cannot be answered through their immediate senses is a process that may lead them to unexpected results regarding the nature of their surrounding world. This process is also reflected in the cultivation of reasoning skills, like metacognition, which plays a crucial role in emergent science.
One limitation of the present study is the decision not to include any statistical analysis of the data. As a result, the findings have an indicative character, mainly aiming to understand how intuitive and synthetic conceptualizations of smallness and evaporation might appear. Further analyses should be undertaken, aiming to provide more certain and generalizable data for the future. More importantly, understanding the extent and age-specificity of children’s adoption of intuitive and counterintuitive ideas about smallness represents the next step for the current project.
Author Contributions
Conceptualization, N.C. and K.A.; methodology, N.C.; validation, N.C. and K.A.; formal analysis, N.C.; investigation, N.C.; resources, K.A.; writing—original draft, N.C.; writing—review & editing, K.A.; supervision, K.A.; project administration, K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the regional ethics committee in Linkoping, Sweden (protocol code: Dnr 2021-02075), and from the Greek Ministry of Education (protocol code: Φ15/71485/ΕΚ/91089/Δ1).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Measures were taken to enable informed consent for children, parents, and teachers. The consent obtained included specific provisions for ensuring the anonymity of the children and the school, as well as the commitment to publish this study’s results in research papers. The children’s names were altered and no details about the school’s whereabouts were given. The children were informed that participation in activities was optional and they had the choice to exit whenever they desired.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author due to privacy concerns.
Acknowledgments
The authors wish to thank the preschools and the children who participated in the project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Makles, A.; Schneider, K. Extracurricular Educational Programs and School Readiness: Evidence from a Quasi-Experiment with Preschool Children. Empir. Econ. 2017, 52, 1181–1204. [Google Scholar] [CrossRef]
- European Union Lifelong Learning Platform—European Civil Society for Education. Available online: http://lllplatform.eu/ (accessed on 10 December 2019).
- Hedegaard, M.; Fleer, M. Play, Learning, and Children’s Development: Everyday Life in Families and Transition to School, 1st ed.; Cambridge University Press: Cambridge, UK, 2013; ISBN 978-1-139-23674-4. [Google Scholar]
- Akerblom, A.; Anderberg, E.; Alvegard, C.; Svensson, L. Awareness of Language Use in Conceptualization: A Study of Children’s Understanding of Movement and Gravity. Scand. J. Educ. Res. 2011, 55, 255–271. [Google Scholar] [CrossRef]
- Clements, D.H.; Sarama, J.; Germeroth, C. Learning Executive Function and Early Mathematics: Directions of Causal Relations. Early Child. Res. Q. 2016, 36, 79–90. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Oerlemans, A.; Lao, S.; Wu, S.; Lew, M.S. Deep Learning for Visual Understanding: A Review. Neurocomputing 2016, 187, 27–48. [Google Scholar] [CrossRef]
- Henrichs, L.F.; Leseman, P.P.M. Early Science Instruction and Academic Language Development Can Go Hand in Hand. The Promising Effects of a Low-Intensity Teacher-Focused Intervention. Int. J. Sci. Educ. 2014, 36, 2978–2995. [Google Scholar] [CrossRef]
- Menninga, A. Language and Science in Young Learners: Intervening in the Balance between Challenging and Adapting; Rijksuniversiteit Groningen: Groningen, The Netherlands, 2017; ISBN 978-90-367-9910-2. [Google Scholar]
- Parsons, A.W.; Bryant, C.L. Deepening Kindergarteners’ Science Vocabulary: A Design Study. J. Educ. Res. 2016, 109, 375–390. [Google Scholar] [CrossRef]
- Spycher, P. Learning Academic Language through Science in Two Linguistically Diverse Kindergarten Classes. Elem. Sch. J. 2009, 109, 359–379. [Google Scholar] [CrossRef]
- Conezio, K.; French, L. Science in the Preschool Classroom—Capitalizing on Children’s Fascination with the Everyday World to Foster Language and Literacy Development. Young Child. 2002, 57, 12–18. [Google Scholar]
- Flannagan, J.S.; Rockenbaugh, L. Curiosity + Kindergarten = Future Scientists. Sci. Child. 2010, 48, 28–31. [Google Scholar]
- Caiman, C.; Lundegård, I. Pre-School Childrens Agency in Learning for Sustainable Development. Environ. Educ. Res. 2014, 20, 437–459. [Google Scholar] [CrossRef]
- Eshach, H.; Fried, M.N. Should Science Be Taught in Early Childhood? J. Sci. Educ. Technol. 2005, 14, 315–336. [Google Scholar] [CrossRef]
- Baruch, Y.K.; Spektor-Levy, O.; Mashal, N. Pre-Schoolers’ Verbal and Behavioral Responses as Indicators of Attitudes and Scientific Curiosity. Int. J. Sci. Math. Educ. 2016, 14, 125–148. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Paiva, J.; Grande, C. Hands-on Chemistry in Preschool Education: Experiments Executed by Little “Scientists” in Kindergarten. Comunicacoes 2017, 24, 99–112. [Google Scholar]
- Mantzicopoulos, P.; Patrick, H.; Samarapungavan, A. Young Children’s Motivational Beliefs about Learning Science. Early Child. Res. Q. 2008, 23, 378–394. [Google Scholar] [CrossRef]
- Pattison, S.A.; Dierking, L.D. Early Childhood Science Interest Development: Variation in Interest Patterns and Parent–Child Interactions among Low-income Families. Sci. Educ. 2019, 103, 362–388. [Google Scholar] [CrossRef]
- Ummanel, A. Metaphorical Perceptions of Preschool, Elementary and Secondary School Children About Science and Mathematics. EURASIA J. Math. Sci. Tech. Ed. 2017, 13, 4651–4668. [Google Scholar] [CrossRef]
- Alexander, J.M.; Johnson, K.E.; Kelley, K. Longitudinal Analysis of the Relations between Opportunities to Learn about Science and the Development of Interests Related to Science. Sci. Ed. 2012, 96, 763–786. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK; Boston, MA, USA, 1997; ISBN 978-0-7506-3365-9. [Google Scholar]
- Carey, S. Science Education as Conceptual Change. J. Appl. Dev. Psychol. 2000, 21, 13–19. [Google Scholar] [CrossRef]
- Adbo, K.; Vidal Carulla, C. Learning About Science in Preschool: Play-Based Activities to Support Children’s Understanding of Chemistry Concepts. Int. J. Early Child. 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Fleer, M. How Preschools Environments Afford Science Learning. In A Cultural-Historical Study of Children Learning Science: Foregrounding Affective Imagination in Play-Based Settings; Cultural Studies of Science Education; Fleer, M., Pramling, N., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 23–37. ISBN 978-94-017-9370-4. [Google Scholar]
- Siraj-Blatchford, I. Conceptualising Progression in the Pedagogy of Play and Sustained Shared Thinking in Early Childhood Education: A Vygotskian Perspective. Educ. Child Psychol. 2009, 26, 77–89. [Google Scholar] [CrossRef]
- Sneider, G.; Pulos, S. Children’s Cosmographies: Understanding the Earth’s Shape and Gravity. Sci. Educ. 1983, 67, 205–221. [Google Scholar] [CrossRef]
- Baillargeon, R.; Stavans, M.; Wu, D.; Gertner, Y.; Setoh, P.; Kittredge, A.K.; Bernard, A. Object Individuation and Physical Reasoning in Infancy: An Integrative Account. Lang. Learn. Dev. 2012, 8, 4–46. [Google Scholar] [CrossRef] [PubMed]
- Carey, S.; Spelke, E. Domain-Specific Knowledge and Conceptual Change. In Mapping the Mind: Domain Specificity in Cognition and Culture; Hirschfeld, L.A., Gelman, S.A., Eds.; Cambridge University Press: Cambridge, UK, 1994; pp. 169–200. ISBN 978-0-521-42993-1. [Google Scholar]
- Gelman, R.; Spelke, E.S.; Meck, E. What Preschoolers Know about Animate and Inanimate Objects. In The Acquisition of Symbolic Skills; Rogers, D., Sloboda, J.A., Eds.; Springer: Boston, MA, USA, 1983; pp. 297–326. ISBN 978-1-4613-3724-9. [Google Scholar]
- Gelman, S.A. Psychological Essentialism in Children. Trends Cogn. Sci. 2004, 8, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Vosniadou, S. (Ed.) International Handbook of Research on Conceptual Change, 2nd ed.; Routledge: New York, NY, USA, 2013; ISBN 978-0-203-15447-2. [Google Scholar]
- Brennan, M. Reflect, ‘Refract’ or Reveal: Sociocultural Explorations of the Place of Teacher Subjectivity in Infant Care. Int. J. Early Years Educ. 2017, 25, 156–170. [Google Scholar] [CrossRef]
- Christodoulakis, N.; Adbo, K. An Analysis of the Development of Preschoolers’ Natural Science Concepts from the Perspective of Framework Theory. Educ. Sci. 2024, 14, 126. [Google Scholar] [CrossRef]
- Hadenfeldt, J.; Neumann, K.; Bernholt, S.; Liu, X.; Parchmann, I. Students’ Progression in Understanding the Matter Concept. J. Res. Sci. Teach. 2016, 53, 683–708. [Google Scholar] [CrossRef]
- Nakhleh, M.; Samarapungavan, A.; Saglam, Y. Middle School Students’ Beliefs about Matter. J. Res. Sci. Teach. 2005, 42, 581–612. [Google Scholar] [CrossRef]
- Johnson, D.W.; Johnson, R.T. Cooperative, Competitive, and Individualistic Learning Environments. In International Guide to Student Achievement; Routledge: London, UK, 2012; ISBN 978-0-203-85039-8. [Google Scholar]
- Hellden, G. A Study of Core Development in Students’ Conceptions of Some Key Ecological Processes. Can. J. Sci. Math. Technol. Educ. 2004, 4, 59–76. [Google Scholar] [CrossRef]
- Smith, C.L.; Wiser, M. Learning and Teaching about Matter in the Elementary Grades; Routledge Handbooks Online: London, UK, 2013; ISBN 978-0-415-89882-9. [Google Scholar]
- Kouka, A. Το νερό στη χημική εκπαίδευση: έννοιες, παρανοήσεις, δυσκολίες στην κατανόηση [Water in Chemical Education: Concepts, Misconceptions, Difficulties in Understanding]. Ph.D. Thesis, University of Ioannina, Ioannina, Greece, 2000. [Google Scholar]
- Baillargeon, R. The Acquisition of Physical Knowledge in Infancy: A Summary in Eight Lessons. In Blackwell Handbook of Childhood Cognitive Development; Blackwell handbooks of developmental psychology; Blackwell Publishing: Malden, MA, USA, 2002; pp. 47–83. ISBN 978-0-631-21840-1. [Google Scholar]
- Hatzinikita, V.; Koulaidis, V. Pupils’ Ideas on Conservation during Changes in the State of Water. Res. Sci. Technol. Educ. 1997, 15, 53–70. [Google Scholar] [CrossRef]
- Hatzinikita, V. Oι αναπαραστασεις των μαθητων του δημοτικου για τις μεταβολες της υλης. Ειδη, αιτιακες σχεσεις και μηχανισμοι. [The Representations of Primary School Students about the Changes of the Material. Species, Causal Relations and Mechanisms]. Ph.D. Thesis, University of Patras, Patras, Greece, 1995. [Google Scholar]
- Johnson, P.; Tymms, P.; Roberts, S. Assessing Students’ Concept of a Substance; Full research report; ESRC Society Today: Bath, UK, 2008. [Google Scholar]
- Driver, R.; Asoko, H.; Leach, J.; Scott, P.; Mortimer, E. Constructing Scientific Knowledge in the Classroom. Educ. Res. 1994, 23, 5–12. [Google Scholar] [CrossRef]
- Stavy, R.; Stachel, D. Children’s Conception of Changes in the State of Matter: From Solid to Liquid. Arch. Psychol. 1985, 53, 331–344. [Google Scholar] [CrossRef]
- Carey, S. Knowledge Acquisition: Enrichment or Conceptual Change. In The Epigenesis of Mind: Essays on Biology and Cognition; The Jean Piaget Symposium series; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 1991; pp. 257–291. ISBN 0-8058-0438-2. [Google Scholar]
- Gikopoulou, O. Changing Students’ Perceptions of Matter through the Educational Model of Microcosm. Int. J. Sci. Res. 2017, 6, 848–855. [Google Scholar]
- Fragkiadaki, G.; Fleer, M.; Ravanis, K. A Cultural-Historical Study of the Development of Children’s Scientific Thinking about Clouds in Everyday Life. Res. Sci. Educ. 2019, 49, 1523–1545. [Google Scholar] [CrossRef]
- Bar, V.; Galili, I. Stages of Children’s Views about Evaporation. Int. J. Sci. Educ. 1994, 16, 157–174. [Google Scholar] [CrossRef]
- Vosniadou, S.; Skopeliti, I. Conceptual Change from the Framework Theory Side of the Fence. Sci. Educ. 2014, 23, 1427–1445. [Google Scholar] [CrossRef]
- Adbo, K.; Vidal Carulla, C. Designing Play-Based Learning Chemistry Activities in the Preschool Environment. Chem. Educ. Res. Pract. 2019, 20, 542–553. [Google Scholar] [CrossRef]
- Hedegaard, M. Principles for Interpreting Research Protocols. In Studying Children. A Cultural-Historical Approach; Open University Press: New York, NY, USA, 2008; pp. 46–64. [Google Scholar]
- Fleer, M. ‘Conceptual Play’: Foregrounding Imagination and Cognition during Concept Formation in Early Years Education. Contemp. Issues Early Child. 2011, 12, 224–240. [Google Scholar] [CrossRef]
- Fleer, M.; Hedegaard, M. Early Learning and Development: Cultural–Historical Concepts in Play; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0-511-84483-6. [Google Scholar]
- Vosniadou, S.; Skopeliti, I. Is It the Earth That Turns or the Sun That Goes behind the Mountains? Students’ Misconceptions about the Day/Night Cycle after Reading a Science Text. Int. J. Sci. Educ. 2017, 39, 2027–2051. [Google Scholar] [CrossRef]
- Vosniadou, S.; Brewer, W.F. Mental Models of the Earth: A Study of Conceptual Change in Childhood. Cogn. Psychol. 1992, 24, 535–585. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).