Current State of SLC and ABC Transporters in the Skin and Their Relation to Sweat Metabolites and Skin Diseases
Abstract
:1. Introduction
2. The Role of Proteomics in the Identification of Skin SLC and ABC Transporters
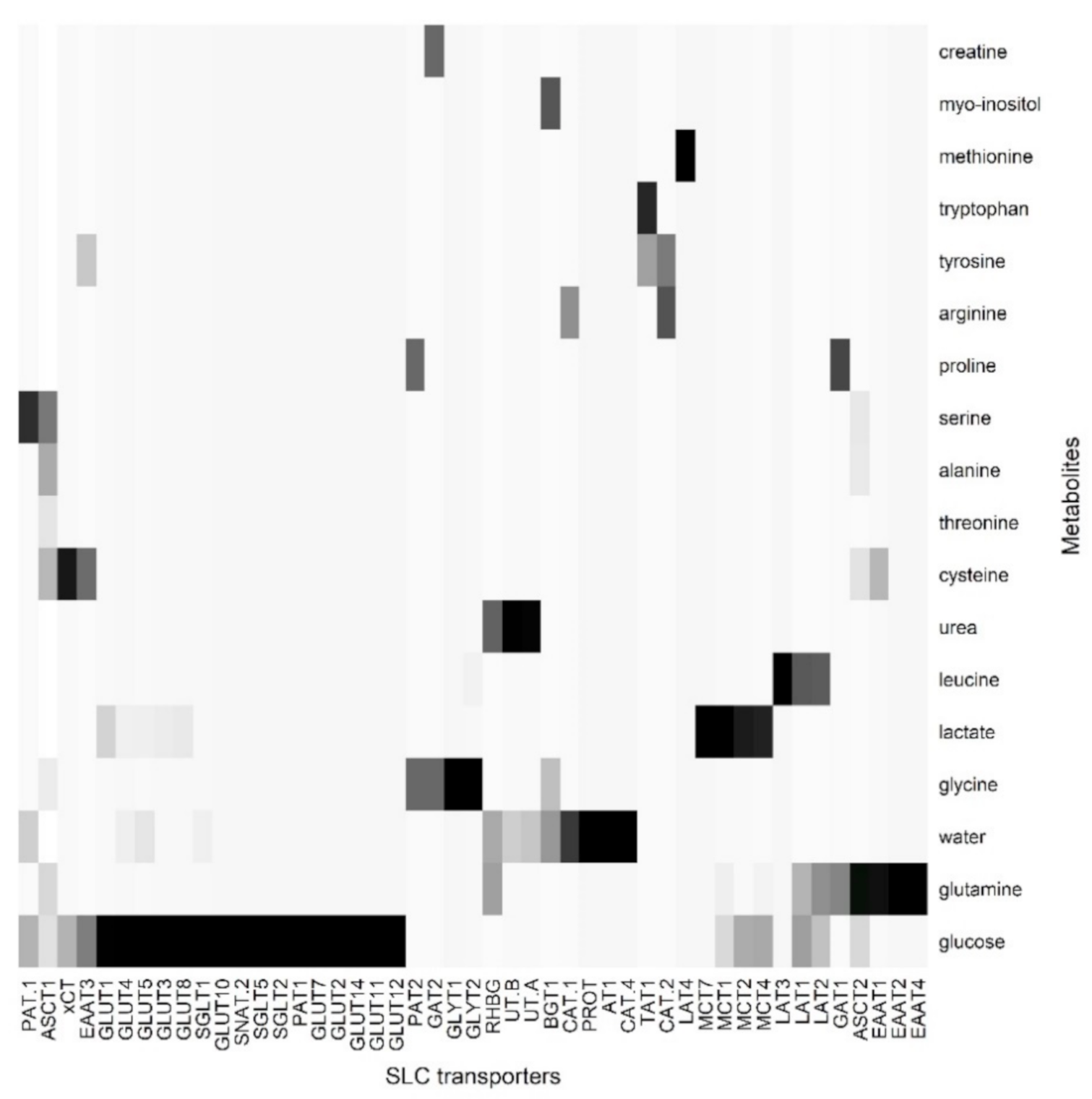
3. Correlation between Sweat Metabolome and Skin SLC and ABC Transporters
4. The Implication of SLC and ABC Transporters in the Skin and Related Diseases
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tsuruta, D.; Green, K.J.; Getsios, S.; Jones, J.C.R. The barrier function of skin: How to keep a tight lid on water loss. Trends Cell Biol. 2002, 12, 355–357. [Google Scholar] [CrossRef]
- Wilke, K.; Martin, A.; Terstegen, L.; Biel, S.S. A short history of sweat gland biology. Int. J. Cosmet. Sci. 2007, 29, 169–179. [Google Scholar] [CrossRef]
- Groscurth, P. Anatomy of sweat glands. Curr. Probl. Dermatol. 2002, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hodge, B.D.; Sanvictores, T.; Brodell, R.T. Anatomy, Skin Sweat Glands; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sato, K.; Leidal, R.; Sato, F. Morphology and development of an apoeccrine sweat gland in human axillae. Am. J. Physiol. 1987, 252, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sato, F. Sweat secretion by human axillary apoeccrine sweat gland in vitro. Am. J. Physiol. 1987, 252, 181–187. [Google Scholar] [CrossRef]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Kang, W.H.; Saga, K.; Sato, K.T. Biology of sweat glands and their disorders. I. Normal sweat gland function. J. Am. Acad. Dermatol. 1989, 20, 537–563. [Google Scholar] [CrossRef]
- Jadoon, S.; Karim, S.; Akram, M.R.; Kalsoom Khan, A.; Zia, M.A.; Siddiqi, A.R.; Murtaza, G. Recent developments in sweat analysis and its applications. Int. J. Anal. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Saint-Criq, V.; Gray, M.A. Role of CFTR in epithelial physiology. Cell. Mol. Life Sci. 2017, 74, 93–115. [Google Scholar] [CrossRef] [Green Version]
- Kell, D.B. What would be the observable consequences if phospholipid bilayer diffusion of drugs into cells is negligible? Trends Pharm. Sci. 2015, 36, 15–21. [Google Scholar] [CrossRef]
- Kell, D.B. The transporter-mediated cellular uptake of pharmaceutical drugs is based on their metabolite-likeness and not on their bulk biophysical properties: Towards a systems pharmacology. Perspect. Sci. 2015, 6, 66–83. [Google Scholar] [CrossRef]
- Kell, D.B. Transport—Linked Phosphorylation: Problems and Prospects. Curr. Top. Cell. Regul. 1992, 289, 1–12. [Google Scholar] [CrossRef]
- Kell, D.B.; Dobson, P.D.; Bilsland, E.; Oliver, S.G. The promiscuous binding of pharmaceutical drugs and their transporter-mediated uptake into cells: What we (need to) know and how we can do so. Drug Discov. Today 2013, 18, 218–239. [Google Scholar] [CrossRef]
- Kell, D.B.; Dobson, P.D.; Oliver, S.G. Pharmaceutical drug transport: The issues and the implications that it is essentially carrier-mediated only. Drug Discov. Today 2011, 16, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Oliver, S.G. How drugs get into cells: Tested and testable predictions to help discriminate between transporter-mediated uptake and lipoidal bilayer diffusion. Front. Pharm. 2014, 5, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Alexander, S.P.H.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Transporters. Br. J. Pharm. 2019, 176, S397–S493. [Google Scholar] [CrossRef]
- Babcock, J.J.; Li, M. Deorphanizing the human transmembrane genome: A landscape of uncharacterized membrane proteins. Acta Pharm. Sin. 2014, 35, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Dean, M.; Hamon, Y.; Chimini, G. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001, 42, 1007–1017. [Google Scholar] [CrossRef]
- Hediger, M.A.; Romero, M.F.; Peng, J.-B.; Rolfs, A.; Takanaga, H.; Bruford, E.A. The ABCs of solute carriers: Physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 2004, 447, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; Zelcer, N.; Van Helvoort, A. ABC transporters in lipid transport. Biochim. Biophys Acta. 2000, 1486, 128–144. [Google Scholar] [CrossRef]
- Ruetz, S.; Gros, P. Phosphatidylcholine translocase: A physiological role for the mdr2 gene. Cell 1994, 77, 1071–1081. [Google Scholar] [CrossRef]
- Schmitz, G.; Kaminski, W.E.; Orsó, E. ABC transporters in cellular lipid trafficking. Curr. Opin. Lipidol. 2000, 11, 493–501. [Google Scholar] [CrossRef]
- Schmitz, G.; Langmann, T.; Heimerl, S. Role of ABCG1 and other ABCG family members in lipid metabolism. J. Lipid. Res. 2001, 42, 1513–1520. [Google Scholar] [CrossRef]
- Lu, K.; Lee, M.H.; Patel, S.B. Dietary cholesterol absorption; more than just bile. Trends Endocrinol. Metab. 2001, 12, 314–320. [Google Scholar] [CrossRef]
- Seguin, A.; Ward, D.M. Mitochondrial ABC Transporters and Iron Metabolism. Int. J. Clin. Exp. Pathol. 2018, 08, 1–5. [Google Scholar] [CrossRef]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genom. 2009, 3, 281. [Google Scholar] [CrossRef]
- Glavinas, H.; Krajcsi, P.; Cserepes, J.; Sarkadi, B. The Role of ABC Transporters in Drug Resistance, Metabolism and Toxicity. Curr. Drug Deliv. 2005, 1, 27–42. [Google Scholar] [CrossRef]
- Perland, E.; Fredriksson, R. Classification Systems of Secondary Active Transporters. Trends Pharm. Sci. 2017, 38, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yee, S.W.; Kim, R.B.; Giacomini, K.M. SLC transporters as therapeutic targets: Emerging opportunities. Nat. Rev. Drug Discov. 2015, 14, 543–560. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, Y.; Sun, K.; Meng, Z.; Chen, L. The SLC transporter in nutrient and metabolic sensing, regulation, and drug development. J. Mol. Cell Biol. 2018, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- El-Awady, R.; Saleh, E.; Hashim, A.; Soliman, N.; Dallah, A.; Elrasheed, A.; Elakraa, G. The Role of Eukaryotic and Prokaryotic ABC Transporter Family in Failure of Chemotherapy. Front. Pharm. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottra, G.; Daniel, H. Bidirectional electrogenic transport of peptides by the proton-coupled carrier PEPT1 in Xenopus laevis oocytes: Its asymmetry and symmetry. J. Physiol. 2001, 536, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vazquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, 608–617. [Google Scholar] [CrossRef]
- Takechi, T.; Hirota, T.; Sakai, T.; Maeda, N.; Kobayashi, D.; Ieiri, I. Interindividual differences in the expression of atp-binding cassette and solute carrier family transporters in human skin: Dna methylation regulates transcriptional activity of the human abcc3 gene. Drug Metab. Dispos. 2018, 46, 628–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takenaka, S.; Itoh, T.; Fujiwara, R. Expression pattern of human ATP-binding cassette transporters in skin. Pharm. Res. Perspect. 2013, 1, e00005. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, R.; Takenaka, S.; Hashimoto, M.; Narawa, T.; Itoh, T. Expression of human solute carrier family transporters in skin: Possible contributor to drug-induced skin disorders. Sci. Rep. 2014, 4, 5251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Majdoub, Z.M.; Achour, B.; Couto, N.; Howard, M.; Elmorsi, Y.; Scotcher, D.; Alrubia, S.; El-Khateeb, E.; Vasilogianni, A.-M.; Alohali, N.; et al. Mass spectrometry-based abundance atlas of ABC transporters in human liver, gut, kidney, brain and skin. FEBS Lett. 2020, 594, 4134–4150. [Google Scholar] [CrossRef]
- Aebersold, R.; Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 2016, 537, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Larance, M.; Lamond, A.I. Multidimensional proteomics for cell biology. Nat. Rev. Mol. Cell Biol. 2015, 16, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyring-Andersen, B.; Løvendorf, M.B.; Coscia, F.; Santos, A.; Møller, L.B.P.; Colaço, A.R.; Niu, L.; Bzorek, M.; Doll, S.; Andersen, J.L.; et al. Spatially and cell-type resolved quantitative proteomic atlas of healthy human skin. Nat. Commun. 2020, 11, 5587. [Google Scholar] [CrossRef]
- Wang, D.; Eraslan, B.; Wieland, T.; Hallström, B.; Hopf, T.; Zolg, D.P.; Zecha, J.; Asplund, A.; Li, L.-h.; Meng, C.; et al. A deep proteome and transcriptome abundance atlas of 29 healthy human tissues. Mol. Syst. Biol. 2019, 15, e8503. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Newton, J.R.A.; Russo, C.; Karunakaran, E.; Achour, B.; Al-Majdoub, Z.M.; Sidaway, J.; Rostami-Hodjegan, A.; Clench, M.R.; Barber, J. Label-free Quantitative Proteomics and Substrate Based Mass Spectrometry Imaging of Xenobiotic Metabolizing Enzymes in ex Vivo Human Skin and a Human Living Skin Equivalent Model. Drug Metab. Dispos. 2020, 49, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Bliss, E.; Heywood, W.E.; Benatti, M.; Sebire, N.J.; Mills, K. An optimised method for the proteomic profiling of full thickness human skin. Biol. Proced. Online 2016, 18, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, C.S.; Foehr, S.; Garfield, D.A.; Furlong, E.E.; Steinmetz, L.M.; Krijgsveld, J. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol. 2014, 10, 757. [Google Scholar] [CrossRef]
- Kaleja, P.; Emmert, H.; Gerstel, U.; Weidinger, S.; Tholey, A. Evaluation and improvement of protein extraction methods for analysis of skin proteome by noninvasive tape stripping. J. Proteom. 2020, 217, 103678. [Google Scholar] [CrossRef]
- O’Hagan, S.; Kell, D.B. Software review: The KNIME workflow environment and its applications in genetic programming and machine learning. Genet. Program. Evolvable. Mach. 2015, 16, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Fillbrunn, A.; Dietz, C.; Pfeuffer, J.; Rahn, R.; Landrum, G.A.; Berthold, M.R. KNIME for reproducible cross-domain analysis of life science data. J. Biotechnol. 2017, 261, 149–156. [Google Scholar] [CrossRef]
- Osman-Ponchet, H.; Boulai, A.; Kouidhi, M.; Sevin, K.; Alriquet, M.; Gaborit, A.; Bertino, B.; Comby, P.; Ruty, B. Characterization of ABC transporters in human skin. Drug Metabol. Drug Interact. 2014, 29, 91–100. [Google Scholar] [CrossRef]
- Okamura, N.; Sakaeda, T.; Okumura, K. Pharmacogenomics of MDR and MRP subfamilies. Pers. Med. 2004, 1, 85–104. [Google Scholar] [CrossRef]
- Hendig, D.; Langmann, T.; Fau-Kocken, S.; Kocken, S.; Fau-Zarbock, R.; Zarbock, R.; Fau-Szliska, C.; Szliska, C.; Fau-Schmitz, G.; Schmitz, G.; et al. Gene expression profiling of ABC transporters in dermal fibroblasts of pseudoxanthoma elasticum patients identifies new candidates involved in PXE pathogenesis. Lab. Investig. 2008, 88, 1303–1315. [Google Scholar] [CrossRef] [Green Version]
- Boll, M.; Daniel, H.; Gasnier, B. The SLC36 family: Proton-coupled transporters for the absorption of selected amino acids from extracellular and intracellular proteolysis. Pflug. Arch. 2004, 447, 776–779. [Google Scholar] [CrossRef]
- Shen, L.; Qian, C.; Cao, H.; Wang, Z.; Luo, T.; Liang, C. Upregulation of the solute carrier family 7 genes is indicative of poor prognosis in papillary thyroid carcinoma. World J. Surg. Oncol. 2018, 16, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, E.M. Glucose transport families SLC5 and SLC50. Mol. Asp. Med. 2013, 34, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Turk, E. The sodium/glucose cotransport family SLC5. Pflugers Arch. 2004. [Google Scholar] [CrossRef] [Green Version]
- Romero, M.F.; Fulton, C.M.; Boron, W.F. The SLC4 family of HCO3- transporters. Mol. Asp. Med. 2004, 447, 495–509. [Google Scholar] [CrossRef]
- Oh, C.S.; Hong, J.H.; Jin, S.N.; Lee, W.J.; Lee, Y.S.; Lee, E. Expression of glucose transporters in the developing rat skin. Anat. Cell Biol. 2017, 50, 214–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gherzi, R.; Melioli, G.; de Luca, M.; D’Agostino, A.; Distefano, G.; Guastella, M.; D’Anna, F.; Franzi, A.T.; Cancedda, R. “HepG2/erythroid/brain” type glucose transporter (GLUT1) is highly expressed in human epidermis: Keratinocyte differentiation affects glut1 levels in reconstituted epidermis. J. Cell. Physiol. 1992, 150, 463–474. [Google Scholar] [CrossRef]
- Bonen, A.; Heynen, M.; Hatta, H. Distribution of monocarboxylate transporters MCT1-MCT8 in rat tissues and human skeletal muscle. Appl. Physiol. Nutr. Metab. 2006, 31, 31–39. [Google Scholar] [CrossRef]
- Cibrian, D.; Castillo-González, R.; Fernández-Gallego, N.; de la Fuente, H.; Jorge, I.; Saiz, M.L.; Punzón, C.; Ramírez-Huesca, M.; Vicente-Manzanares, M.; Fresno, M.; et al. Targeting L-type amino acid transporter 1 in innate and adaptive T cells efficiently controls skin inflammation. J. Allergy Clin. Immunol. 2020, 145, 199–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnorr, O.; Suschek, C.V.; Kolb-Bachofen, V. The importance of cationic amino acid transporter expression in human skin. J. Investig. Derm. 2003, 120, 1016–1022. [Google Scholar] [CrossRef] [Green Version]
- Jensen, A.; Figueiredo-Larsen, M.; Holm, R.; Broberg, M.L.; Brodin, B.; Nielsen, C.U. PAT1 (SLC36A1) shows nuclear localization and affects growth of smooth muscle cells from rats. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E65–E74. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Jin, L.; Feng, J.; Lv, J. The expression of AQP5 and UTs in the sweat glands of uremic patients. BioMed Res. Int. 2017, 2017, 8629783. [Google Scholar] [CrossRef] [PubMed]
- Alriquet, M.; Sevin, K.; Gaborit, A.; Comby, P.; Ruty, B.; Osman-Ponchet, H. Characterization of SLC transporters in human skin. Admet Dmpk 2015, 3, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.; Brooks S Fau-Bellone, R.; Bellone R Fau-Bailey, E.; Bailey, E. Missense mutation in exon 2 of SLC36A1 responsible for champagne dilution in horses. PLoS Genet. 2008, 4, e1000195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, S.M.; Castorino, J.J.; Philp, N.J. Interaction of monocarboxylate transporter 4 with beta1-integrin and its role in cell migration. Am. J. Physiol. Cell Physiol. 2009, 296, C414–C421. [Google Scholar] [CrossRef] [Green Version]
- Gallagher-Colombo, S.; Maminishkis, A.; Fau-Tate, S.; Tate, S.; Fau-Grunwald, G.B.; Grunwald, G.B.; Fau-Philp, N.J.; Philp, N.J. Modulation of MCT3 expression during wound healing of the retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5343–5350. [Google Scholar] [CrossRef]
- Porporato, P.E.; Payen, V.L.; De Saedeleer, C.J.; Préat, V.; Thissen, J.-P.; Feron, O.; Sonveaux, P. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis 2012, 15, 581–592. [Google Scholar] [CrossRef]
- Zhang, Z.; Zi, Z.; Lee, E.E.; Zhao, J.; Contreras, D.C.; South, A.P.; Abel, E.D.; Chong, B.F.; Vandergriff, T.; Hosler, G.A.; et al. Differential glucose requirement in skin homeostasis and injury identifies a therapeutic target for psoriasis article. Nat. Med. 2018, 24, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.D.; Sands, J.M. Urea transport and clinical potential of urearetics. Curr. Opin. Nephrol. Hypertens 2016, 25, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Váradi, A.; Szabó, Z.; Pomozi, V.; de Boussac, H.; Fülöp, K.; Arányi, T. ABCC6 as a target in pseudoxanthoma elasticum. Curr. Drug Targets 2011, 12, 671–682. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.J.; Lau, E.; Singh, H.; Vergnes, L.; Tarling, E.J.; Mehrabian, M.; Mungrue, I.; Xiao, S.; Shih, D.; Castellani, L.; et al. ABCC6 localizes to the mitochondria-associated membrane. Circ. Res. 2012, 111, 516–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Teixeira, C.; Ferraz, R.; Prudêncio, C.; Gomes, P. Wound-Healing Peptides for Treatment of Chronic Diabetic Foot Ulcers and Other Infected Skin Injuries. Molecules 2017, 22, 1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witte, M.B.; Barbul, A. Arginine physiology and its implication for wound healing. Wound Repair Regen. 2003, 11, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-M.; Huang, W.-Y.; Lin, S.-J.; Huang, W.-C.; Shen, C.-R.; Mao, W.-Y.; Shen, C.-N. ABCG2 deficiency in skin impairs re-epithelialization in cutaneous wound healing. Exp. Derm. 2016, 25, 355–361. [Google Scholar] [CrossRef] [PubMed]
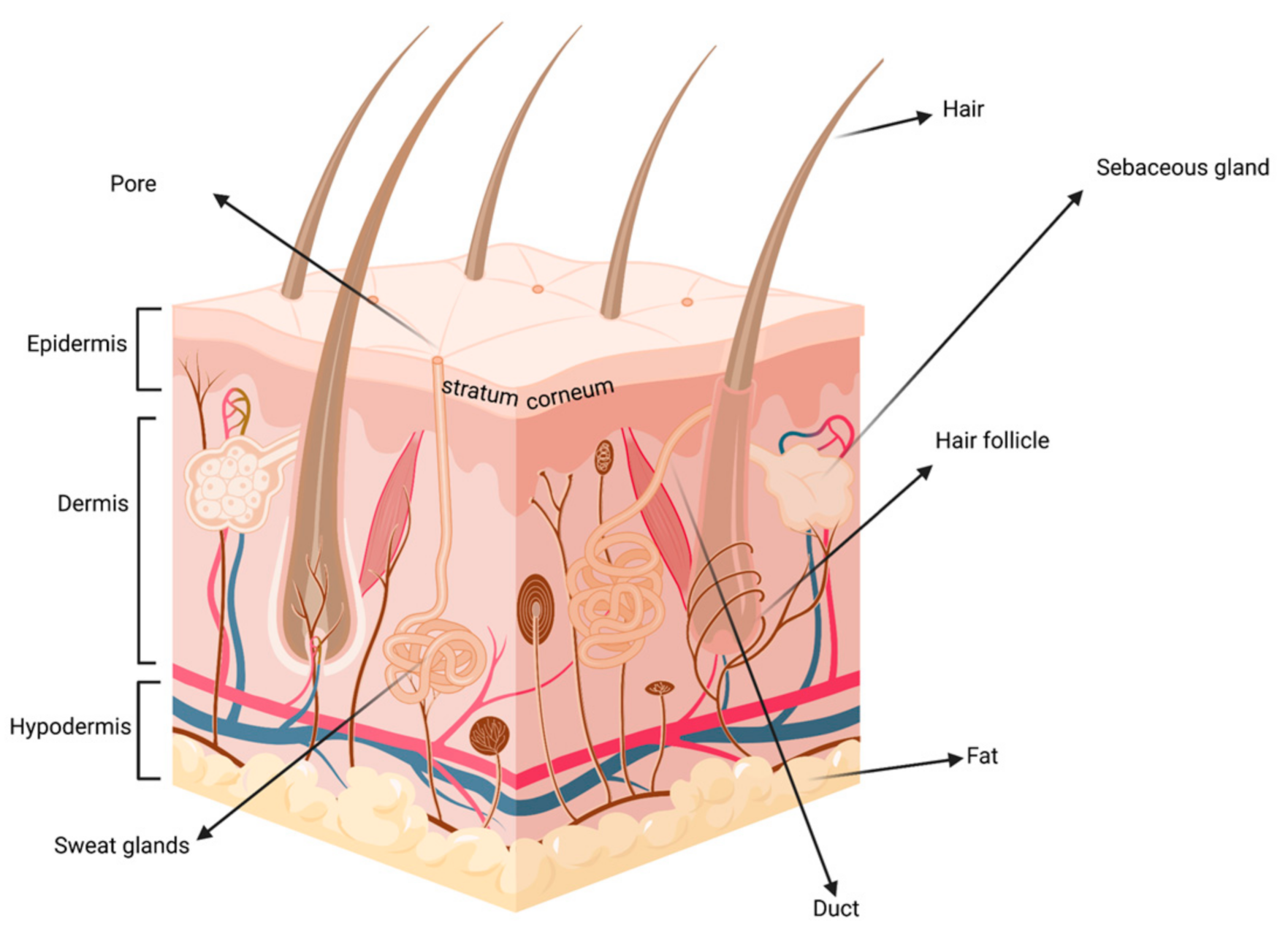
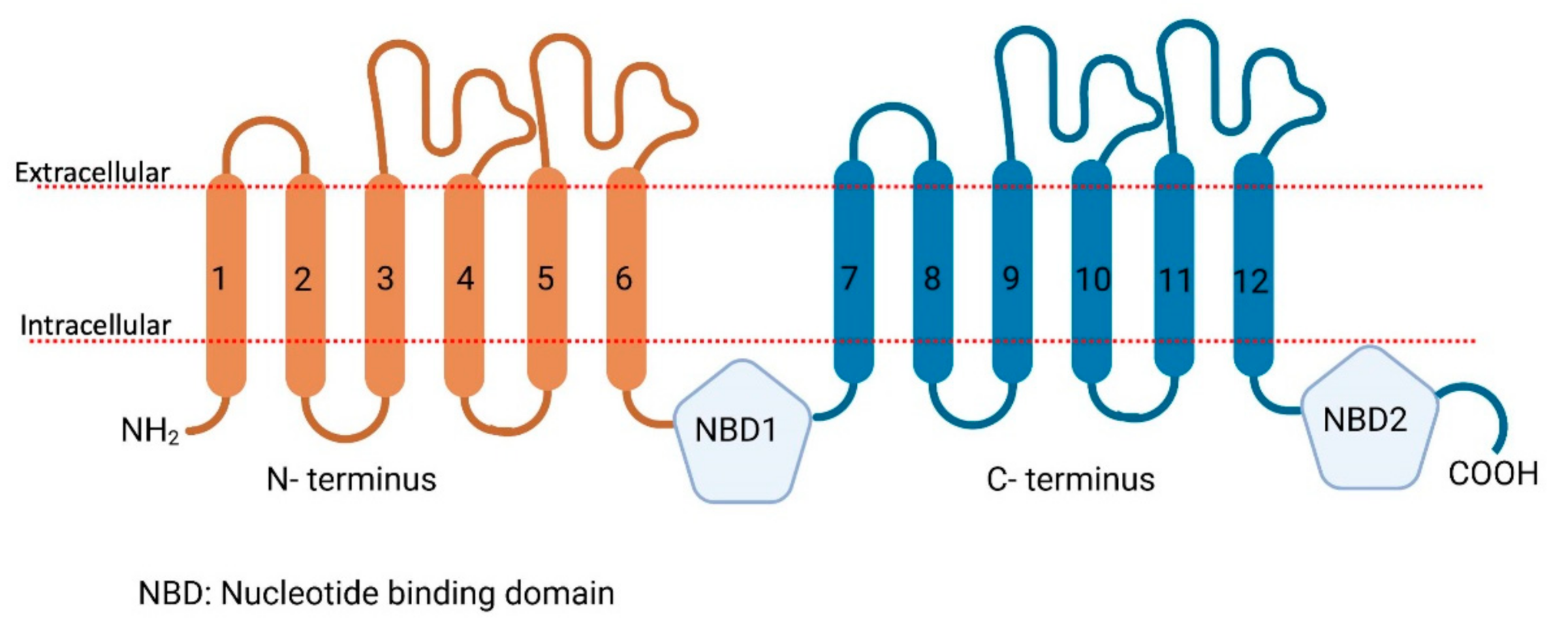


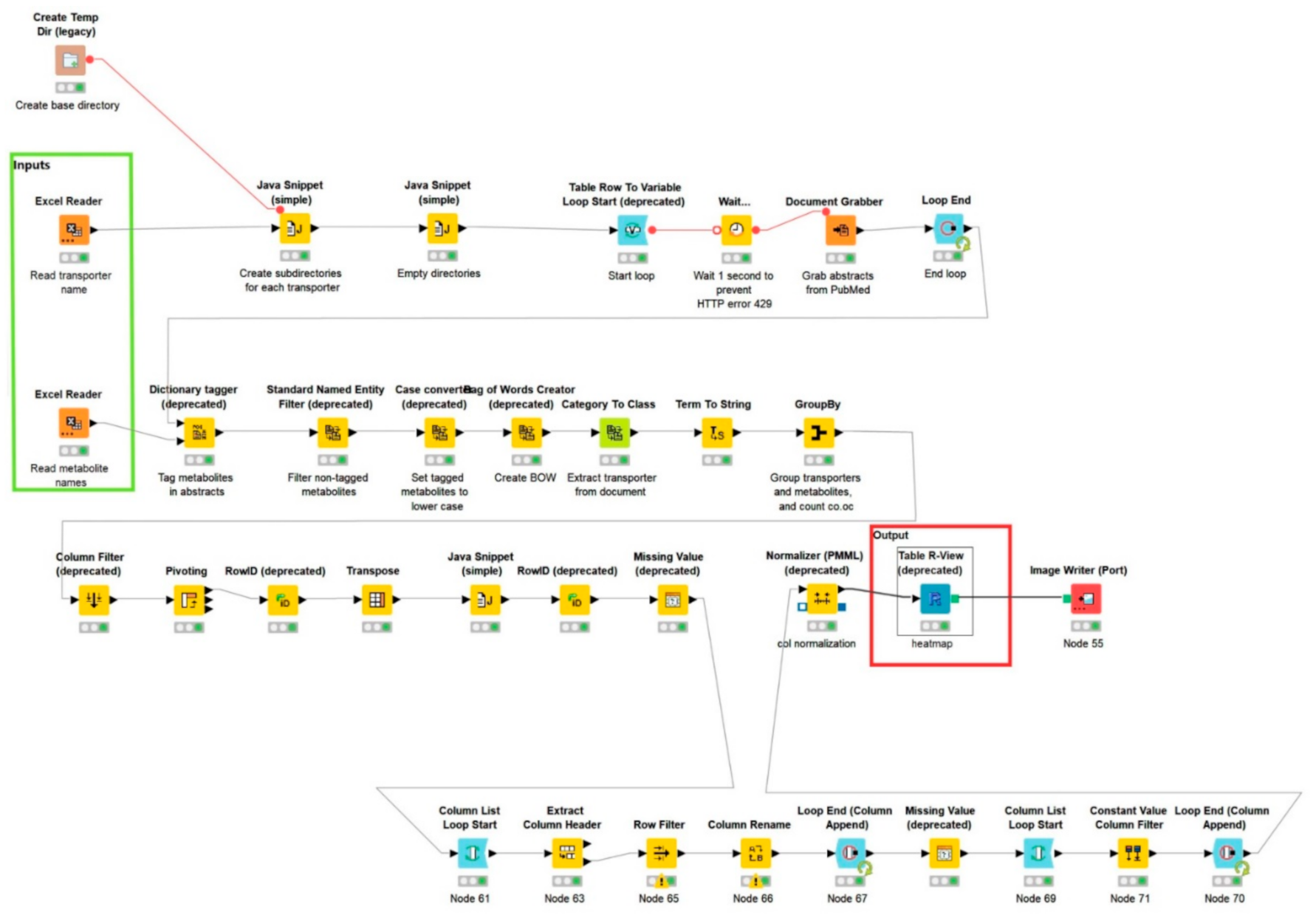
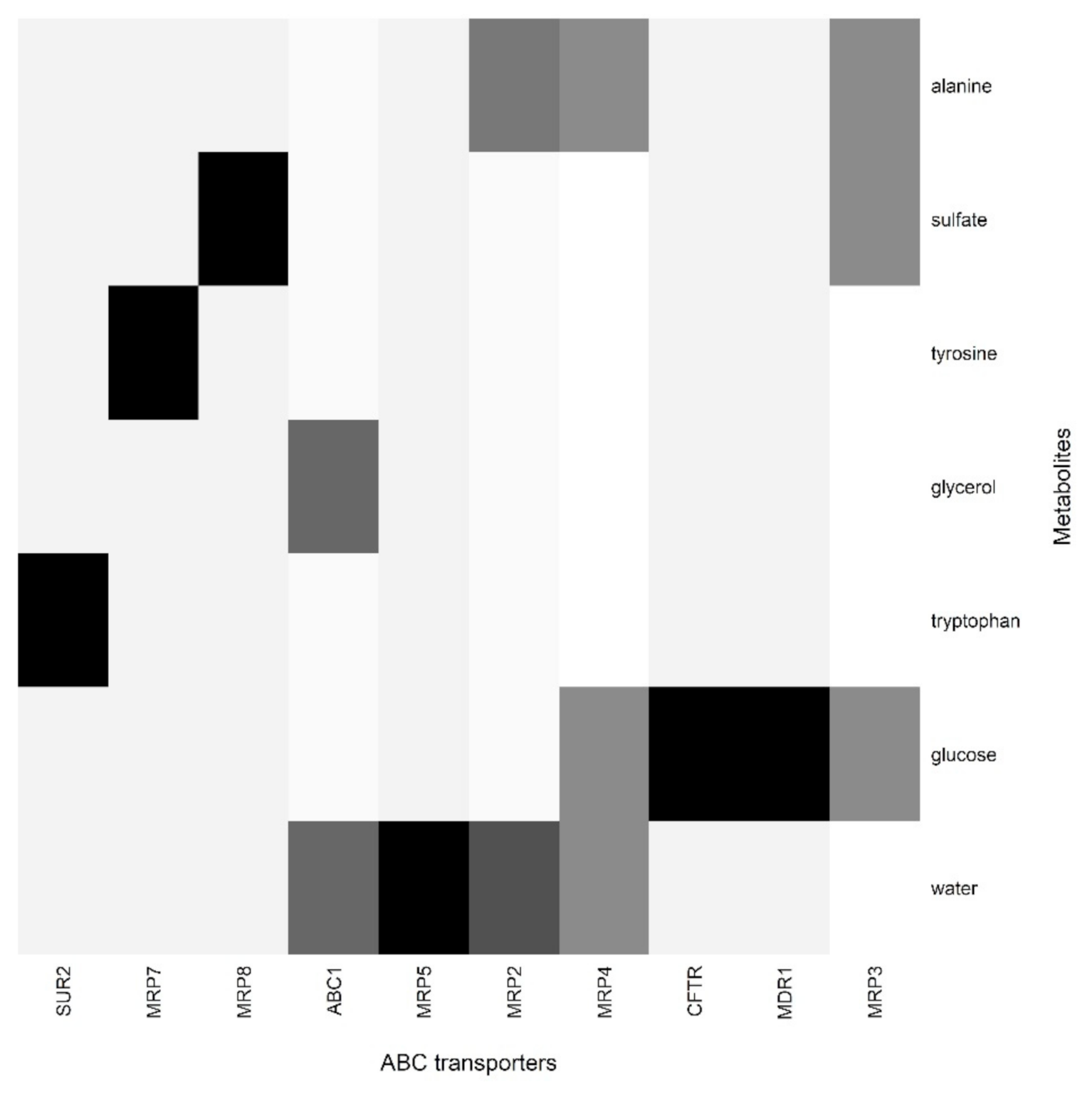
| S.No | Transporters (In Skin) | Detection | Refs | |
|---|---|---|---|---|
| mRNA | Proteomics | |||
| 1 | SLC28A3 | * | [37] | |
| SLCO2B1,SLCO1B1,SLCO1B2,SLCO1C1,SLCO3A1,SLCO4A1,SLCO4C1 | * | [37] | ||
| SLC16A1,SLC16A4 | * | [37] | ||
| SLC19A1 | * | [37] | ||
| 2 | ABCA5,ABCA2,ABCA6,ABCA9,ABCA10,ABCA12 | * | [36] | |
| ABCB3,ABCB2,ABCB4,ABCB6,ABCB7,ABCB8,ABCB10,ABCB11 | * | [36] | ||
| ABCC1,ABCC2,ABCC3,ABCC4,ABCC5,ABCC7,ABCC9,ABCC10,ABCC11 | * | [36] | ||
| ABCD1,ABCD2,ABCD3,ABCD4 | * | [36] | ||
| ABCE1 | * | [36] | ||
| ABCF1,ABCF2,ABCF3 | * | [36] | ||
| ABCG1,ABCG4 | * | [36] | ||
| 3 | ABCC1(DOMINANT) | * | [49] | |
| ABCG2,ABCB1,ABCC2 (relatively less) | * | [49] | ||
| 4 | ABCC3 (most abundant) | * | [35] | |
| ABCB1,ABCA7,ABCG2 (less abundant) | * | [35] | ||
| ABCA2,ABCC1 (moderately abundant) | * | [35] | ||
| SLC22A3(most abundant) | * | [35] | ||
| SLCO3A1,SLC16A7,SLCO2B1(moderately abundant) | * | [35] | ||
| 5 | ABCA6,ABCA8 | * | [38] | |
| ABCB2,ABCB3,ABCB6 | * | [38] | ||
| ABCC12 | * | [38] | ||
| ABCD3 | * | [38] | ||
| ABCE1 | * | [38] | ||
| 6 | SLC14 (1 and 2) | * | [63] | |
| 7 | SLC47A1 and SLC47A2 | * | [64] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, M.M.K.; Aryal, E.; Safari, E.; Mojsoska, B.; Jenssen, H.; Prabhala, B.K. Current State of SLC and ABC Transporters in the Skin and Their Relation to Sweat Metabolites and Skin Diseases. Proteomes 2021, 9, 23. https://doi.org/10.3390/proteomes9020023
Nielsen MMK, Aryal E, Safari E, Mojsoska B, Jenssen H, Prabhala BK. Current State of SLC and ABC Transporters in the Skin and Their Relation to Sweat Metabolites and Skin Diseases. Proteomes. 2021; 9(2):23. https://doi.org/10.3390/proteomes9020023
Chicago/Turabian StyleNielsen, Marcus M. K., Eva Aryal, Elnaz Safari, Biljana Mojsoska, Håvard Jenssen, and Bala Krishna Prabhala. 2021. "Current State of SLC and ABC Transporters in the Skin and Their Relation to Sweat Metabolites and Skin Diseases" Proteomes 9, no. 2: 23. https://doi.org/10.3390/proteomes9020023
APA StyleNielsen, M. M. K., Aryal, E., Safari, E., Mojsoska, B., Jenssen, H., & Prabhala, B. K. (2021). Current State of SLC and ABC Transporters in the Skin and Their Relation to Sweat Metabolites and Skin Diseases. Proteomes, 9(2), 23. https://doi.org/10.3390/proteomes9020023









