An Interval-Simplex Approach to Determine Technological Parameters from Experimental Data
Abstract
:1. Introduction
- (1)
- Thermodynamic factors: constants of chemical and phase equilibrium. This group of factors determines the reaction direction and technological parameters and affects the rate and selectivity of the entire process [25];
- (2)
- (3)
- (4)
- Heat exchange factors: heat transfer coefficients within phases and between the medium and heat exchange devices, as well as the external heat exchange surface size [15];
- (5)
2. Materials and Methods
- (1)
- For the value of the argument ; and
- (2)
- For the value of the argument .
3. Results
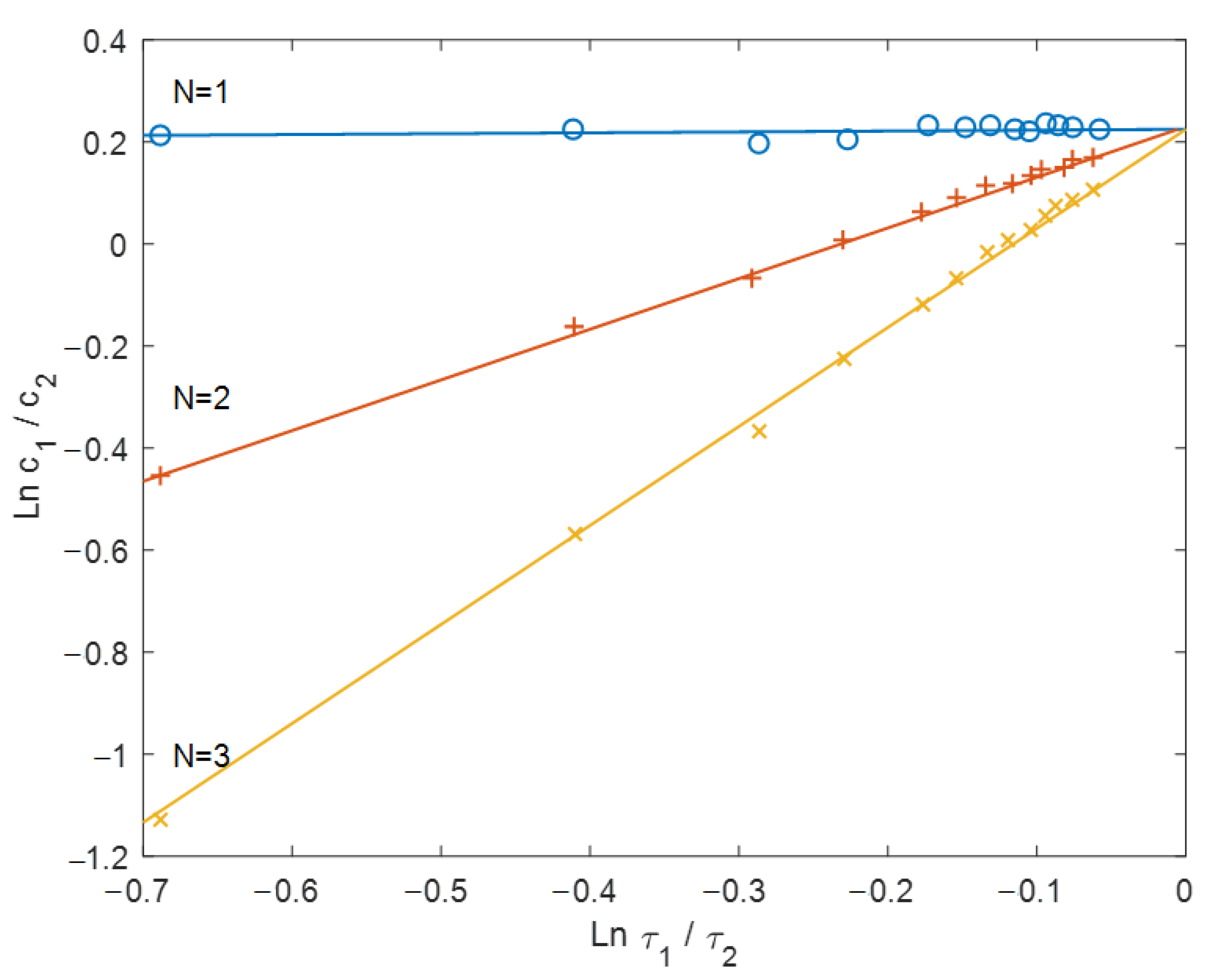
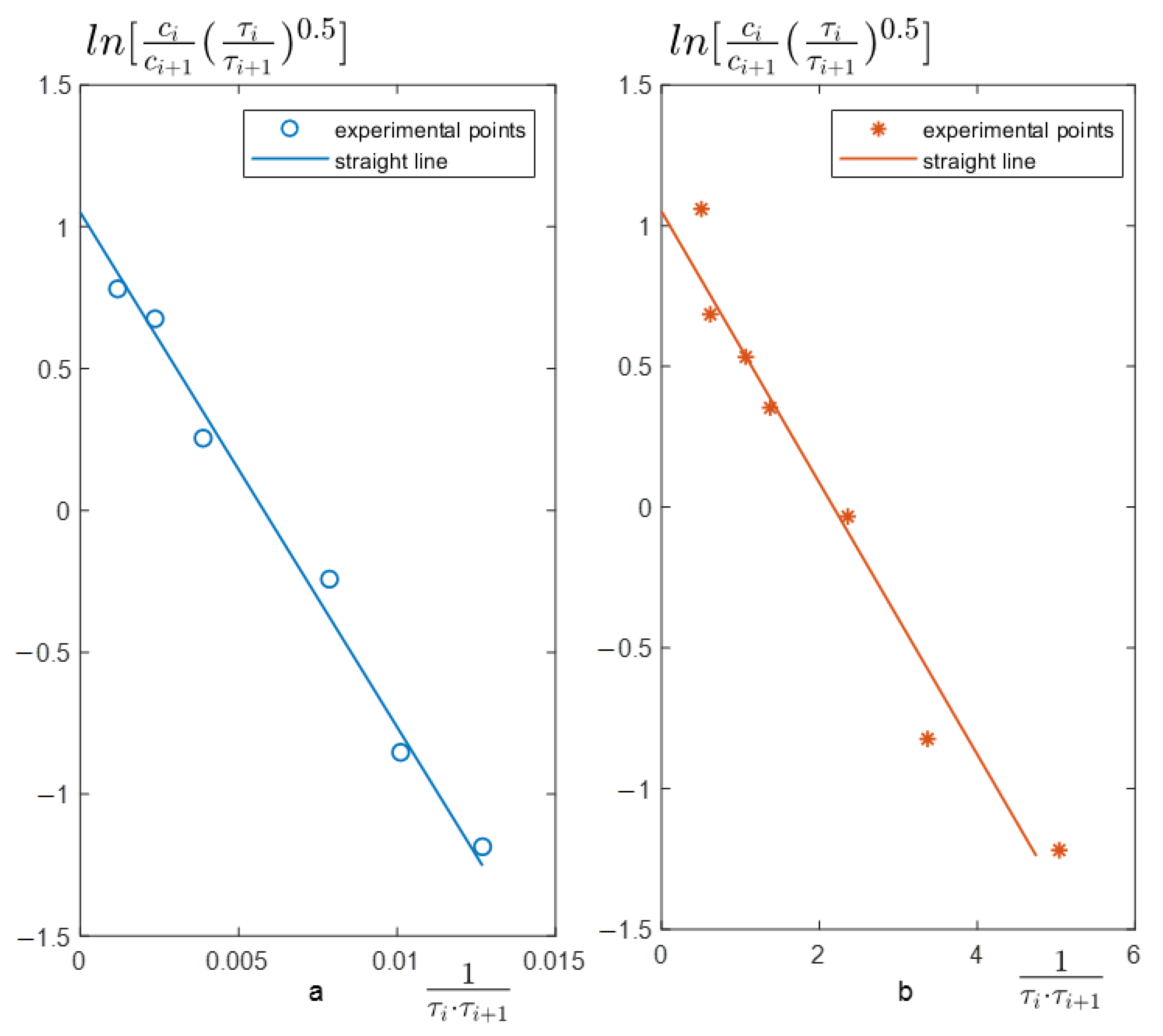
3.1. Simplex-Interval Method Examples
3.2. Mathematical Modeling Examples
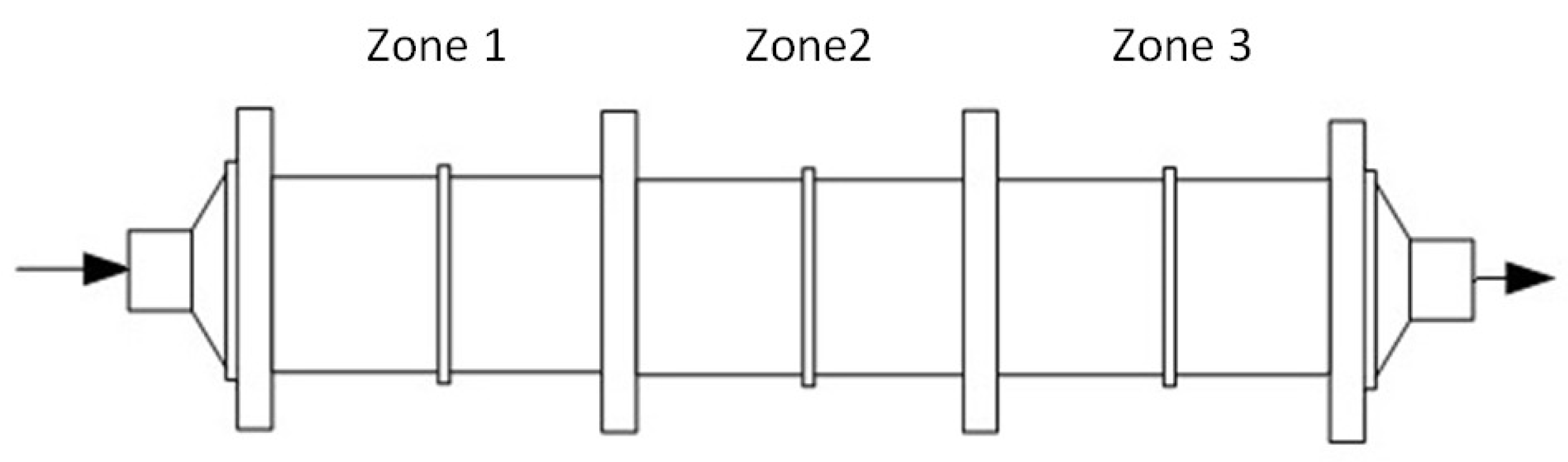
3.2.1. CSTR Model
3.2.2. Dispersion Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carlson, R.; Carlson, J.E. Design and Optimization in Organic Synthesis: Second Revised and Enlarged Edition; Elsevier Science: Amsterdam, The Netherlands, 2005; ISBN 9780080455273. [Google Scholar]
- Bonvin, D.; Georgakis, C.; Pantelides, C.C.; Barolo, M.; Grover, M.A.; Rodrigues, D.; Schneider, R.; Dochain, D. Linking Models and Experiments. Ind. Eng. Chem. Res. 2016, 55, 6891–6903. [Google Scholar] [CrossRef]
- Li, S. Reaction Engineering. In Handbook of Heterogeneous Catalysis; Wiley: Hoboken, NJ, USA, 2017; pp. 1–664. [Google Scholar] [CrossRef]
- Carr, R.W. Modeling of Chemical Reactions. In Comprehensive Chemical Kinetics; Elsevier: Amsterdam, The Netherlands, 2007; p. 297. [Google Scholar]
- Bueno, M.P.; Kojovic, T.; Powell, M.S.; Shi, F. Multi-Component AG/SAG Mill Model. Miner. Eng. 2013, 43–44, 12–21. [Google Scholar] [CrossRef]
- Cameron, I.; Gani, R. Product and Process Modelling; Elsevier: Amsterdam, The Netherlands, 2011; p. 571. [Google Scholar] [CrossRef]
- Quaglio, M.; Roberts, L.; bin Jaapar, M.S.; Fraga, E.S.; Dua, V.; Galvanin, F. An Artificial Neural Network Approach to Recognise Kinetic Models from Experimental Data. Comput. Chem. Eng. 2020, 135, 106759. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Wang, S.; Zhu, B. Optimization of LPDC Process Parameters Using the Combination of Artificial Neural Network and Genetic Algorithm Method. J. Mater. Eng. Perform. 2012, 21, 492–499. [Google Scholar] [CrossRef]
- Chi, C.; Janiga, G.; Thévenin, D. On-the-Fly Artificial Neural Network for Chemical Kinetics in Direct Numerical Simulations of Premixed Combustion. Combust. Flame 2021, 226, 467–477. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A Simplex Method for Function Minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]
- Morgan, S.L.; Deming, S.N. Simplex Optimization of Analytical Chemical Methods. Anal. Chem. 1974, 46, 1170–1181. [Google Scholar] [CrossRef]
- Olsson, D.M.; Nelson, L.S. The Nelder-Mead Simplex Procedure for Function Minimization. Technometrics 1975, 17, 45–51. [Google Scholar] [CrossRef]
- Spendley, W.; Hext, G.R.; Himsworth, F.R. Sequential Application of Simplex Designs in Optimisation and Evolutionary Operation. Technometrics 1962, 4, 441. [Google Scholar] [CrossRef]
- Li, H.; Nalim, R.; Haldi, P.A. Thermal-Economic Optimization of a Distributed Multi-Generation Energy System—A Case Study of Beijing. Appl. Therm. Eng. 2006, 26, 709–719. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, Y.; Peng, H.; Ding, G.; Sun, S. Effect of Tube Diameter on Pressure Drop Characteristics of Refrigerant-Oil Mixture Flow Boiling inside Metal-Foam Filled Tubes. Appl. Therm. Eng. 2013, 61, 433–443. [Google Scholar] [CrossRef]
- Wang, J.J.; Jing, Y.Y.; Zhang, C.F. Optimization of Capacity and Operation for CCHP System by Genetic Algorithm. Appl. Energy 2010, 87, 1325–1335. [Google Scholar] [CrossRef]
- Fan, S.K.S.; Liang, Y.C.; Zahara, E. A Genetic Algorithm and a Particle Swarm Optimizer Hybridized with Nelder–Mead Simplex Search. Comput. Ind. Eng. 2006, 50, 401–425. [Google Scholar] [CrossRef]
- Lin, H.; Yamashita, K. Hybrid Simplex Genetic Algorithm for Blind Equalization Using RBF Networks. Math. Comput. Simul. 2002, 59, 293–304. [Google Scholar] [CrossRef]
- Li, S.; Xin, F.; Li, L. Fluidized Bed Reactor. In Reaction Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 369–403. [Google Scholar]
- Burton, K.W.C.; Nickless, G. Optimisation via Simplex. Part I. Background, Definitions and a Simple Application. Chemom. Intell. Lab. Syst. 1987, 1, 135–149. [Google Scholar] [CrossRef]
- Liotta, F.; Chatellier, P.; Esposito, G.; Fabbricino, M.; Van Hullebusch, E.D.; Lens, P.N.L. Hydrodynamic Mathematical Modelling of Aerobic Plug Flow and Nonideal Flow Reactors: A Critical and Historical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2642–2673. [Google Scholar] [CrossRef]
- Toson, P.; Doshi, P.; Jajcevic, D. Explicit Residence Time Distribution of a Generalised Cascade of Continuous Stirred Tank Reactors for a Description of Short Recirculation Time (Bypassing). Processes 2019, 7, 615. [Google Scholar] [CrossRef]
- Gorzalski, A.S.; Harrington, G.W.; Coronell, O. Modeling Water Treatment Reactor Hydraulics Using Reactor Networks. J. Am. Water Work. Assoc. 2018, 110, 13–29. [Google Scholar] [CrossRef]
- Sheoran, M.; Chandra, A.; Bhunia, H.; Bajpai, P.K.; Pant, H.J. Residence time distribution studies using radiotracers in chemical industry—A review. Chem. Eng. Commun. 2018, 205, 739–758. [Google Scholar] [CrossRef]
- Vyazovkin, S.V.; Goryachko, V.I.; Lesnikovich, A.I. An Approach to the Solution of the Inverse Kinetic Problem in the Case of Complex Processes. Part III. Parallel Independent Reactions. Thermochim. Acta 1992, 197, 41–51. [Google Scholar] [CrossRef]
- Braun, R.L.; Burnham, A.K. Analysis of Chemical Reaction Kinetics Using a Distribution of Activation Energies and Simpler Models. Energy Fuels 1987, 1, 153–161. [Google Scholar] [CrossRef]
- Levin, V.I. Optimization in terms of interval uncertainty: The determinization method. Autom. Control. Comput. Sci. 2012, 46, 157–163. [Google Scholar] [CrossRef]
- Gorlanov, E.S.; Brichkin, V.N. Polyakov Electrolytic production of aluminium: Review. part 1. conventional areas of development. Tsvetnye Met. 2020, 2, 36–41. [Google Scholar] [CrossRef]
- Savchenkov, S.A.; Bazhin, V.Y.; Brichkin, V.N.; Povarov, V.G.; Ugolkov, V.L.; Kasymova, D.R. Synthesis of magnesium-zinc-yttrium master alloy. Lett. Mater. 2019, 9, 339–343. [Google Scholar] [CrossRef]
- Savchenkov, S.A.; Bazhin, V.Y.; Brichkin, V.N.; Kosov, Y.I.; Ugolkov, V.L. Production Features of Magnesium-Neodymium Master Alloy Synthesis. Metallurgist 2019, 63, 394–402. [Google Scholar] [CrossRef]
- Cleary, P.W.; Monaghan, J.J. Conduction Modelling Using Smoothed Particle Hydrodynamics. J. Comput. Phys. 1999, 148, 227–264. [Google Scholar] [CrossRef]
- Kondrasheva, N.K.; Rudko, V.A.; Ancheyta, J. Thermogravimetric Determination of the Kinetics of Petroleum Needle Coke Formation by Decantoil Thermolysis. ACS Omega 2020, 5, 29570–29576. [Google Scholar] [CrossRef]
- Beloglazov, I.N.; Beloglazov, N.K. The Simplex Method to Describe Hydrometallurgical Processes. Miner. Process. Extr. Metall. Rev. 1995, 15, 139. [Google Scholar] [CrossRef]
- Smol’nikov, A.D.; Sharikov, Y.V. Simulation of the Aluminum Electrolysis Process in a High-Current Electrolytic Cell in Modern Software. Metallurgist 2020, 63, 1313–1320. [Google Scholar] [CrossRef]
- Sharikov, F.Y.; Sharikov, Y.V.; Krylov, K.A. Selection of key parameters for green coke calcination in a tubular rotary kiln to produce anode petcoke. ARPN J. Eng. Appl. Sci. 2020, 15, 2904–2912. [Google Scholar]
- Cheremisina, O.V.; Cheremisina, E.A.; Ponomareva, M.A.; Fedorov, A.T. Sorption of rare earth coordination compounds. J. Min. Inst. 2020, 244, 474–481. [Google Scholar] [CrossRef]
- Cheremisina, E.; Cheremisina, O.; Ponomareva, M.; Bolotov, V.; Fedorov, A. Kinetic Features of the Hydrogen Sulfide Sorption on the Ferro-Manganese Material. Metals 2021, 11, 90. [Google Scholar] [CrossRef]
- Kondrasheva, N.K.; Rudko, V.A.; Nazarenko, M.Y.; Gabdulkhakov, R.R. Influence of parameters of delayed asphalt coking process on yield and quality of liquid and solid-phase products. J. Min. Inst. 2020, 241, 97. [Google Scholar] [CrossRef]
- Rehrl, J.; Kruisz, J.; Sacher, S.; Khinast, J.; Horn, M. Optimized continuous pharmaceutical manufacturing via model-predictive control. Int. J. Pharm. 2016, 510, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-H.; Fuh, C.-C.; Ho, J.-R.; Lin, C.-K. Design of Optimal Controllers for Unknown Dynamic Systems through the Nelder–Mead Simplex Method. Mathematics 2021, 9, 2013. [Google Scholar] [CrossRef]
- Visuthirattanamanee, R.; Sinapiromsaran, K.; Boonperm, A. Self-Regulating Artificial-Free Linear Programming Solver Using a Jump and Simplex Method. Mathematics 2020, 8, 356. [Google Scholar] [CrossRef]
- Gutierrez, C.G.C.C.; Dias, E.F.T.S.; Gut, J.A.W. Residence time distribution in holding tubes using generalized convection model and numerical convolution for non-ideal tracer detection. J. Food Eng. 2010, 98, 248–256. [Google Scholar] [CrossRef]
- Islamov, S.R.; Bondarenko, A.V.; Mardashov, D.V. Substantiation of a Well Killing Technology for Fractured Carbonate Reservoirs. In Youth Technical Sessions Proceedings; CRC Press: Boca Raton, FL, USA, 2019; pp. 256–264. [Google Scholar] [CrossRef]
- Levenspiel, O.; Smith, W.K. Notes on the diffusion-type model for the longitudinal mixing of fluids in flow. Chem. Eng. Sci. 1957, 6, 227–235. [Google Scholar] [CrossRef]
- Dittrich, E.; Klincsik, M. Analysis of conservative tracer measurement results using the Frechet distribution at planted horizontal subsurface flow constructed wetlands filled with coarse gravel and showing the effect of clogging processes. Environ. Sci. Pollut. Res. 2015, 22, 17104–17122. [Google Scholar] [CrossRef]
- Braga, B.M.; Tavares, R.P. Description of a New Tundish Model for Treating RTD Data and Discussion of the Communication “New Insight into Combined Model and Revised Model for RTD Curves in a Multi-strand Tundish” by Lei. Met. Mater. Trans. A 2018, 49, 2128–2132. [Google Scholar] [CrossRef]
- Dryer, F.L.; Haas, F.M.; Santner, J.; Farouk, T.I.; Chaos, M. Interpreting chemical kinetics from complex reaction–advection–diffusion systems: Modeling of flow reactors and related experiments. Prog. Energy Combust. Sci. 2014, 44, 19–39. [Google Scholar] [CrossRef]
- Shestakov, A.K.; Sadykov, R.M.; Petrov, P.A. Multifunctional crust breaker for automatic alumina feeding system of aluminum reduction cell. E3S Web Conf. 2021, 266, 09002. [Google Scholar] [CrossRef]
- Sharikov, Y.V.; Sharikov, F.Y.; Krylov, K.A. Mathematical Model of Optimum Control for Petroleum Coke Production in a Rotary Tube Kiln. Theor. Found. Chem. Eng. 2021, 55, 711–719. [Google Scholar] [CrossRef]
| Parameter, τ, s | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 | 130 | 140 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| τ/τ | 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | 1.2 | 1.4 | 1.6 | 1.8 | 2.0 | 2.2 | 2.4 | 2.6 | 2.8 |
| lnS | — | 0.693 | 0.405 | 0.288 | 0.223 | 0.176 | 0.154 | 0.134 | 0.118 | 0.105 | 0.094 | 0.088 | 0.070 | 0.074 | 0.069 |
| Ci (N = 1) | 1.000 | 0.219 | 0.676 | 0.649 | 0.449 | 0.0368 | 0.301 | 0.247 | 0.202 | 0.165 | 0.135 | 0.111 | 0.091 | 0.074 | 0.061 |
| lnSC | 1.222 | 1.222 | 1.222 | 1.222 | 1.222 | 1.222 | 1.222 | 1.222 | 1.222 | 1.221 | 1.223 | 1.222 | 1.222 | 1.222 | 1.222 |
| Ci (N = 2) | 0 | 0.164 | 0.268 | 0.329 | 0.360 | 0.368 | 0.361 | 0.345 | 0.323 | 0.298 | 0.224 | 0.274 | 0.218 | 0.193 | 0.170 |
| lnSC | — | −0.471 | −0.205 | −0.088 | −0.020 | +0.020 | +0.048 | +0.067 | +0.086 | +0.095 | +0.104 | +0.113 | +0.122 | +0.122 | 0.137 |
| Ci (N = 3) | 0 | 0.016 | 0.054 | 0.099 | 0.144 | 0.184 | 0.217 | 0.242 | 0.258 | 0.268 | 0.271 | 0.268 | 0.261 | 0.251 | 0.238 |
| lnSC | — | −1.184 | −0.612 | −0.375 | −0.246 | −0.165 | −0.067 | −0.037 | −0.037 | −0.011 | +0.010 | +0.030 | +0.040 | +0.049 | 0.050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beloglazov, I.; Krylov, K. An Interval-Simplex Approach to Determine Technological Parameters from Experimental Data. Mathematics 2022, 10, 2959. https://doi.org/10.3390/math10162959
Beloglazov I, Krylov K. An Interval-Simplex Approach to Determine Technological Parameters from Experimental Data. Mathematics. 2022; 10(16):2959. https://doi.org/10.3390/math10162959
Chicago/Turabian StyleBeloglazov, Ilia, and Kirill Krylov. 2022. "An Interval-Simplex Approach to Determine Technological Parameters from Experimental Data" Mathematics 10, no. 16: 2959. https://doi.org/10.3390/math10162959
APA StyleBeloglazov, I., & Krylov, K. (2022). An Interval-Simplex Approach to Determine Technological Parameters from Experimental Data. Mathematics, 10(16), 2959. https://doi.org/10.3390/math10162959







