Mathematical Modeling and the Use of Network Models as Epidemiological Tools
Abstract
1. Introduction
Main Epidemiological Models
2. Materials and Methods
3. Results
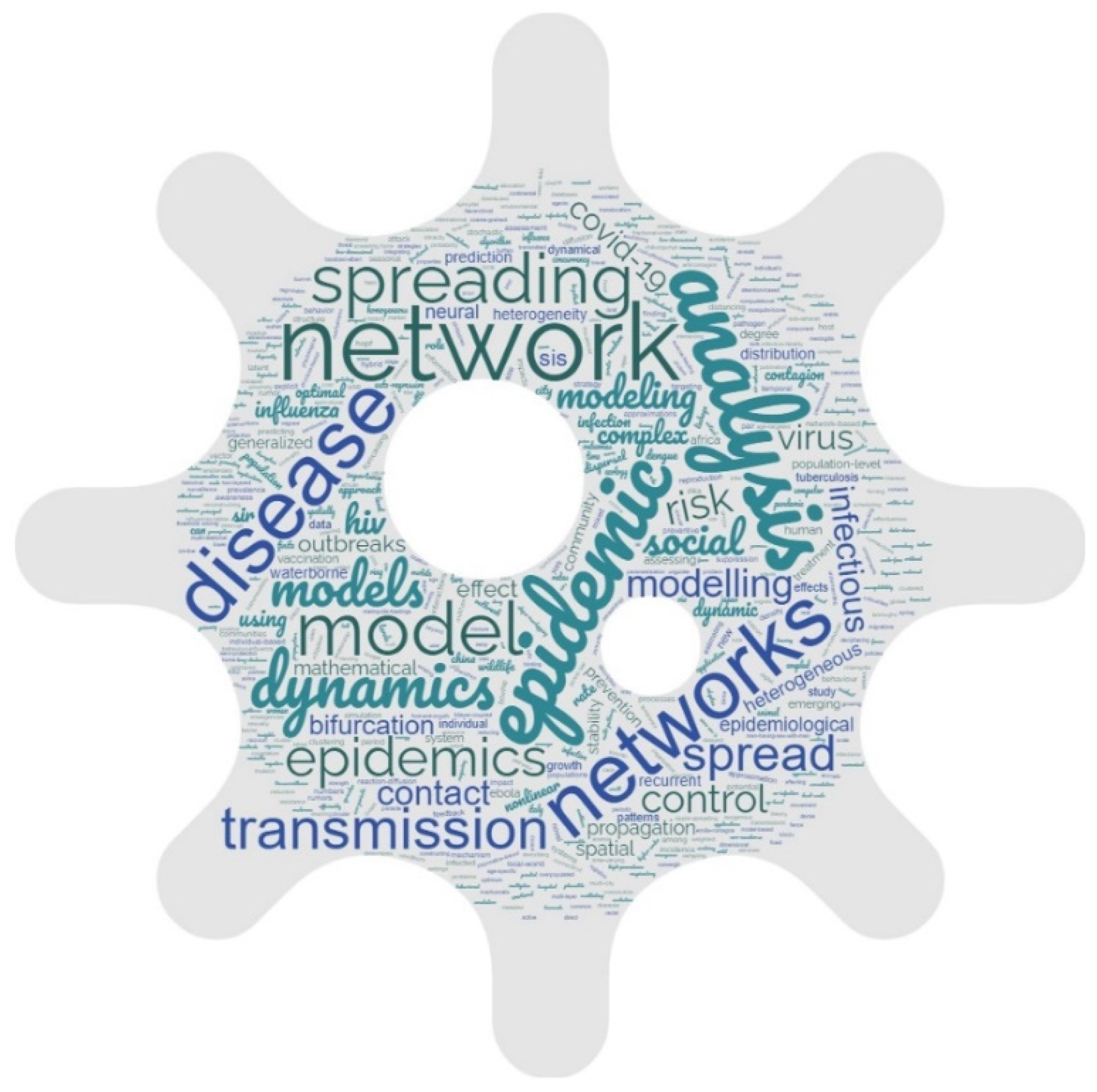
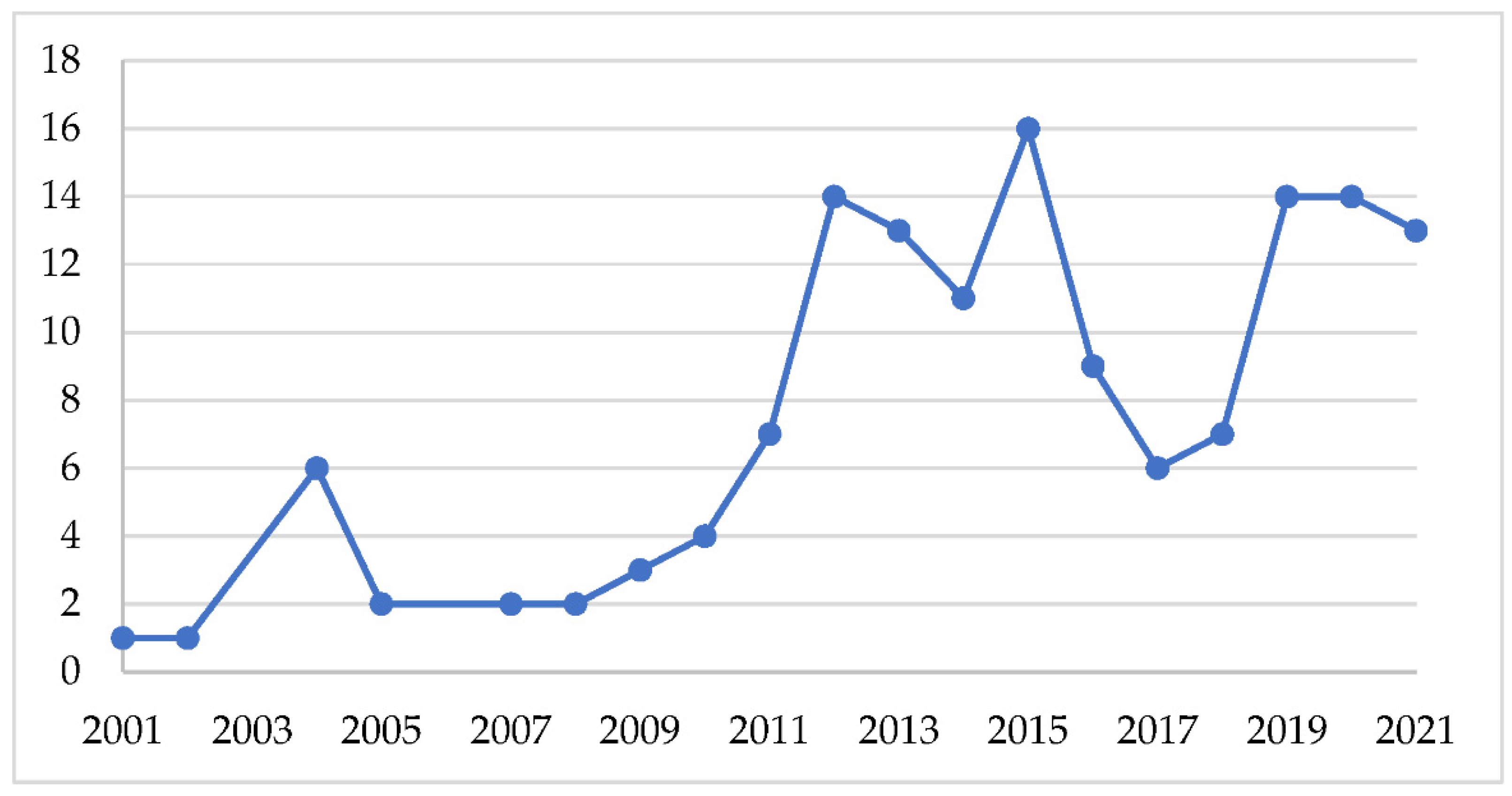
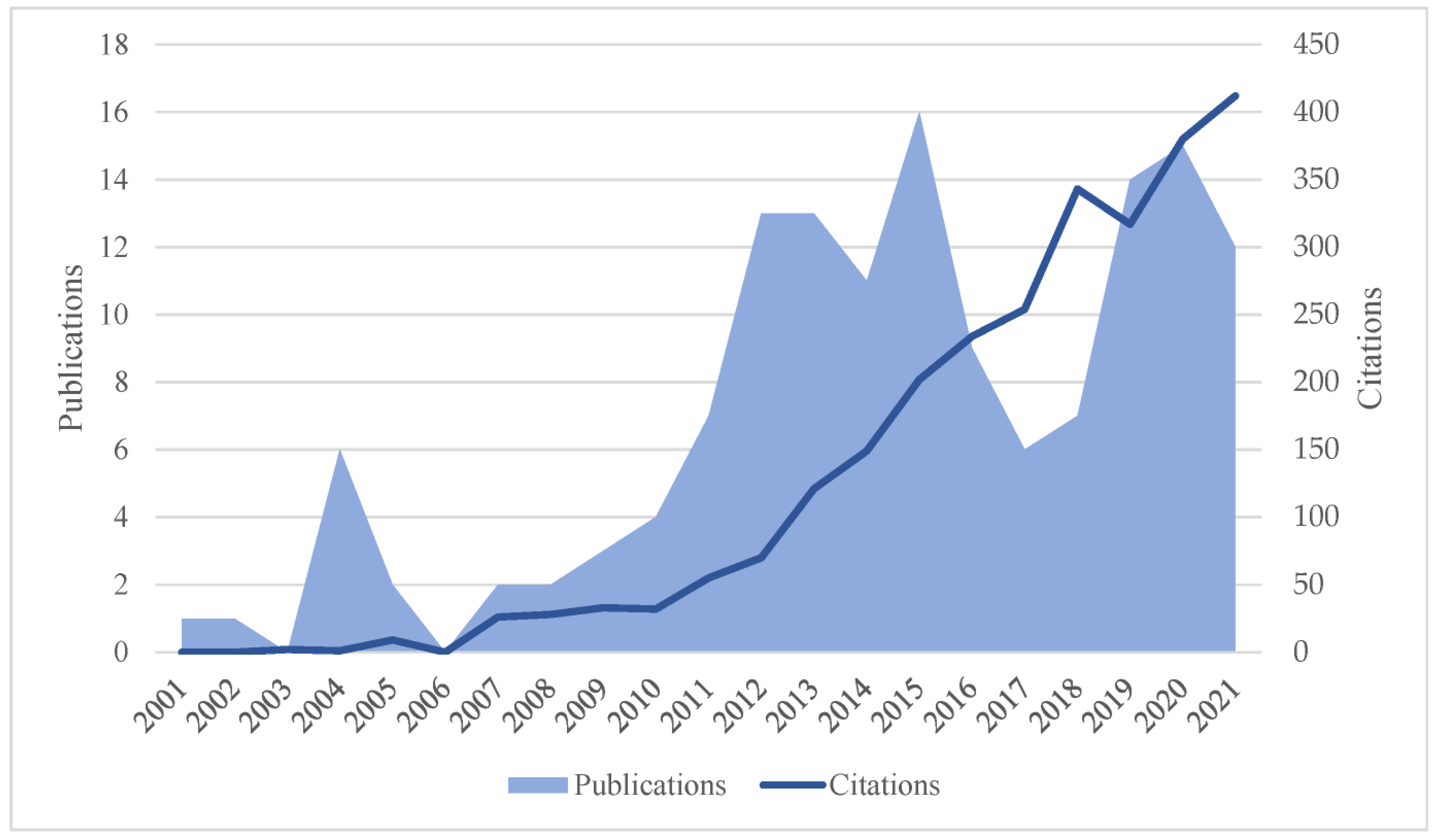
3.1. Growth Trajectory and Distribution of Publications
3.2. Influential Authors, Documents and Journals
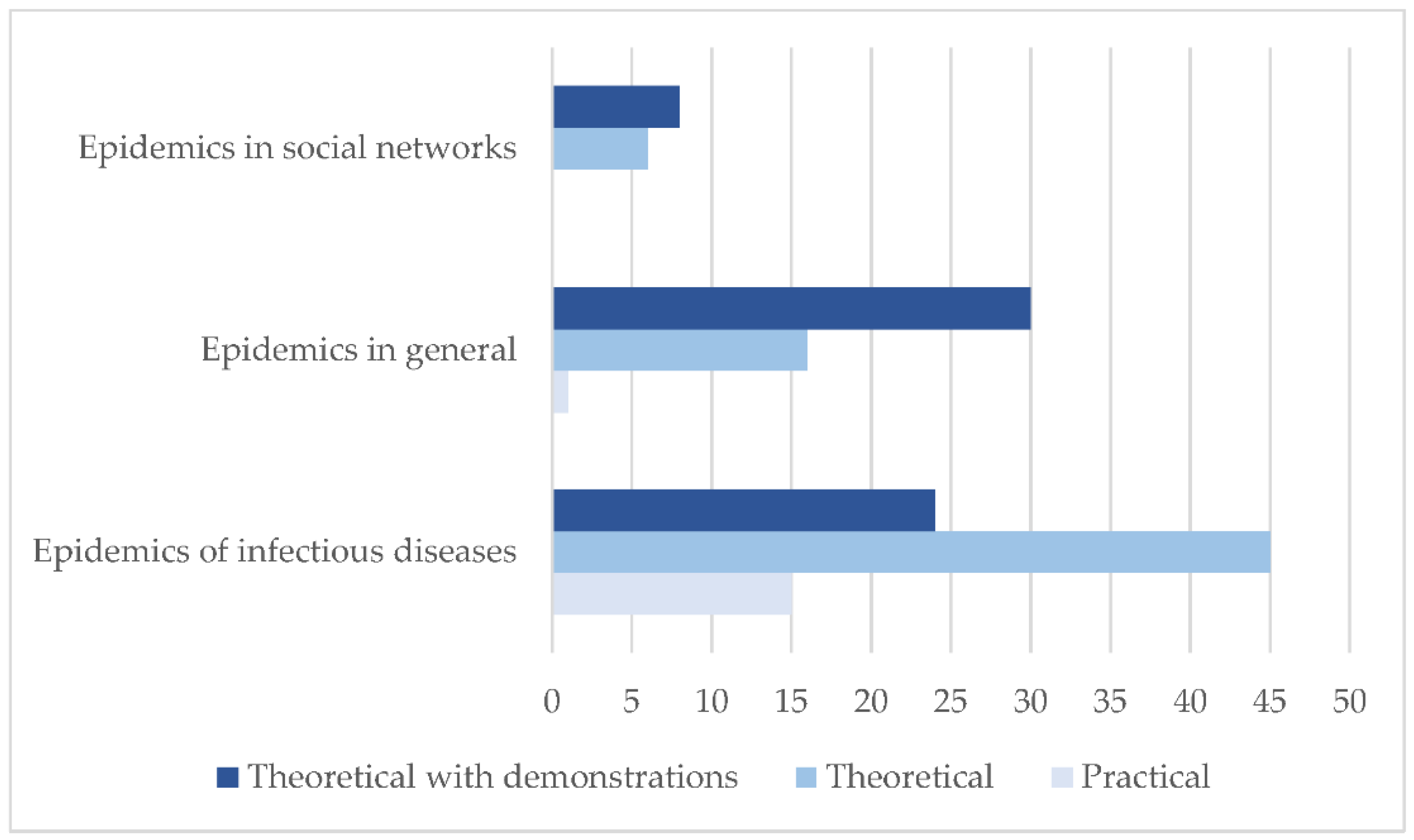
3.3. Epidemiological Models Developed
3.4. Analysis of Citations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brauer, F.; Castillo-Chavez, C.; Feng, Z. Mathematical Models in Epidemiology; Springer: New York, NY, USA, 2019; Volume 32. [Google Scholar]
- Tang, Y.; Serdan, T.D.A.; Masi, L.N.; Tang, S.; Gorjao, R.; Hirabara, S.M. Epidemiology of COVID-19 in Brazil: Using a mathematical model to estimate the outbreak peak and temporal evolution. Emerg. Microbes Infect. 2020, 9, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Bekiros, S.; Kouloumpou, D. SBDiEM: A new mathematical model of infectious disease dynamics. Chaos Solitons Fractals 2020, 136, 109828. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Jing, S.; Miller, J.C.; Sun, W.; Li, H.; Estrada-Franco, J.G.; Hyman, J.M.; Zhu, H. A data-driven network model for the emerging COVID-19 epidemics in Wuhan, Toronto and Italy. Math. Biosci. 2020, 326, 108391. [Google Scholar] [CrossRef]
- Panovska-Griffiths, J. Can mathematical modelling solve the current Covid-19 crisis? BMC Public Health 2020, 20, 551. [Google Scholar] [CrossRef]
- Adiga, A.; Dubhashi, D.; Lewis, B.; Marathe, M.; Venkatramanan, S.; Vullikanti, A. Mathematical models for covid-19 pandemic: A comparative analysis. J. Indian Inst. Sci. 2020, 100, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Cheng, G.; Yang, R.; Zhang, Y.; Lin, Y.; Ding, Y. Prediction on the number of confirmed Covid-19 with the FUDAN-CCDC mathematical model and its epidemiology, clinical manifestations, and prevention and treatment effects. Results Phys. 2021, 20, 103618. [Google Scholar] [CrossRef]
- Olivares, A.; Staffetti, E. Uncertainty quantification of a mathematical model of COVID-19 transmission dynamics with mass vaccination strategy. Chaos Solitons Fractals 2021, 146, 110895. [Google Scholar] [CrossRef]
- Allen, L.J.; Burgin, A.M. Comparison of deterministic and stochastic SIS and SIR models in discrete time. Math. Biosci. 2000, 163, 1–33. [Google Scholar] [CrossRef]
- Champagne, C.; Cazelles, B. Comparison of stochastic and deterministic frameworks in dengue modelling. Math. Biosci. 2019, 310, 1–12. [Google Scholar] [CrossRef]
- Cifuentes-Faura, J. Factors influencing the COVID-19 mortality rate in the European Union: Importance of medical professionals. Public Health 2021, 200, 1–3. [Google Scholar] [CrossRef]
- Abellán García, A.; Aceituno Nieto, P.; Allende, A.; de Andrés, A.; Arenillas, A.; Bartomeus, F.; Bastolla, U.; Benavides, J.; Cabal, B.; Castillo Belmonte, A.B.; et al. Una Visión Global de la Pandemia COVID-19: Qué Sabemos y Qué Estamos Investigando Desde el CSIC; CSIC: Madrid, Spain, 2021.
- Ram, V.; Schaposnik, L.P. A modified age-structured SIR model for COVID-19 type viruses. Sci. Rep. 2021, 11, 15194. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lu, P.E.; Chang, C.S.; Liu, T.H. A time-dependent SIR model for COVID-19 with undetectable infected persons. IEEE Trans. Netw. Sci. Eng. 2020, 7, 3279–3294. [Google Scholar] [CrossRef]
- Cooper, I.; Mondal, A.; Antonopoulos, C.G. A SIR model assumption for the spread of COVID-19 in different communities. Chaos Solitons Fractals 2020, 139, 110057. [Google Scholar] [CrossRef] [PubMed]
- Kudryashov, N.A.; Chmykhov, M.A.; Vigdorowitsch, M. Analytical features of the SIR model and their applications to COVID-19. Appl. Math. Model. 2021, 90, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Atkeson, A. On using SIR models to model disease scenarios for COVID-19. Q. Rev. 2020, 41, 1–35. [Google Scholar] [CrossRef]
- Muñoz-Fernández, G.A.; Seoane, J.M.; Seoane-Sepúlveda, J.B. A SIR-type model describing the successive waves of COVID-19. Chaos Solitons Fractals 2021, 144, 110682. [Google Scholar] [CrossRef]
- Rǎdulescu, A.; Williams, C.; Cavanagh, K. Management strategies in a SEIR-type model of COVID 19 community spread. Sci. Rep. 2020, 10, 21256. [Google Scholar] [CrossRef]
- He, S.; Peng, Y.; Sun, K. SEIR modeling of the COVID-19 and its dynamics. Nonlinear Dyn. 2020, 101, 1667–1680. [Google Scholar] [CrossRef]
- Annas, S.; Pratama, M.I.; Rifandi, M.; Sanusi, W.; Side, S. Stability analysis and numerical simulation of SEIR model for pandemic COVID-19 spread in Indonesia. Chaos Solitons Fractals 2020, 139, 110072. [Google Scholar] [CrossRef]
- Kosmidis, K.; Macheras, P. A fractal kinetics SI model can explain the dynamics of COVID-19 epidemics. PLoS ONE 2020, 15, e0237304. [Google Scholar] [CrossRef]
- Demongeot, J.; Griette, Q.; Magal, P. SI epidemic model applied to COVID-19 data in mainland China. R. Soc. Open Sci. 2020, 7, 201878. [Google Scholar] [CrossRef] [PubMed]
- Otunuga, O.M. Time-dependent probability distribution for number of infection in a stochastic SIS model: Case study COVID-19. Chaos Solitons Fractals 2021, 147, 110983. [Google Scholar] [CrossRef]
- Brusset, X.; Davari, M.; Kinra, A.; Torre, D.L. Modelling COVID-19 Ripple Effect and Global Supply Chain Productivity Impacts Using a Reaction-Diffusion Time-Space SIS Model. In IFIP International Conference on Advances in Production Management Systems; Springer: Cham, Switzerland, 2021; pp. 3–12. [Google Scholar]
- Allen, L.J. Some discrete-time SI, SIR, and SIS epidemic models. Math. Biosci. 1994, 124, 83–105. [Google Scholar] [CrossRef]
- Allen, L.J.S.; Jones, M.A.; Martin, C.F. A discrete-time model with vaccination for a measles epidemic. Math. Biosci. 1991, 105, 111–131. [Google Scholar] [CrossRef]
- Elazzouzi, A.; Alaoui, A.L.; Tilioua, M.; Tridane, A. Global stability analysis for a generalized delayed SIR model with vaccination and treatment. Adv. Differ. Equ. 2019, 1, 532. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, Z.; Brauer, F. Global analysis of discrete-time SI and SIS epidemic models. Math. Biosci. Eng. 2007, 4, 699. [Google Scholar] [PubMed]
- Hassouna, M.; Ouhadan, A.; El Kinani, E.H. On the solution of fractional order SIS epidemic model. Chaos Solitons Fractals 2018, 117, 168–174. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Shen, H. Dynamical analysis of a discrete-time SIS epidemic model on complex networks. Appl. Math. Lett. 2019, 94, 292–299. [Google Scholar] [CrossRef]
- Mena-Lorcat, J.; Hethcote, H.W. Dynamic models of infectious diseases as regulators of population sizes. J. Math. Biol. 1992, 30, 693–716. [Google Scholar] [CrossRef]
- Harko, T.; Lobo, F.S.; Mak, M. Exact analytical solutions of the Susceptible-Infected-Recovered (SIR) epidemic model and of the SIR model with equal death and birth rates. Appl. Math. Comput. 2014, 236, 184–194. [Google Scholar] [CrossRef]
- Nzokem, A.H. SIS epidemic model: Birth-and-death markov chain approach. arXiv 2021, arXiv:2102.08992. [Google Scholar] [CrossRef]
- Jardón-Kojakhmetov, H.; Kuehn, C.; Pugliese, A.; Sensi, M. A geometric analysis of the SIR, SIRS and SIRWS epidemiological models. Nonlinear Anal. Real World Appl. 2021, 58, 103220. [Google Scholar] [CrossRef]
- Kusmawati, I.S.; Chandra, T.D. Stability analysis of SIRS epidemic model on measles disease spreading with vaccination and migration. J. Phys. Conf. Ser. 2021, 1872, 012033. [Google Scholar] [CrossRef]
- Alonso-Quesada, S.; De la Sen, M.; Nistal, R. A SIRS Epidemic Model Supervised by a Control System for Vaccination and Treatment Actions Which Involve First-Order Dynamics and Vaccination of Newborns. Mathematics 2021, 10, 36. [Google Scholar] [CrossRef]
- Boutayeb, H.; Bidah, S.; Zakary, O.; Lhous, M.; Rachik, M. On the optimal vaccination and travel-restriction controls with a discrete multi-region SIRS epidemic model. Commun. Math. Biol. Neurosci. 2021, 2021, 31. [Google Scholar]
- Li, H.; Peng, R.; Wang, Z.A. On a diffusive SIS epidemic model with mass action mechanism and birth-death effect: Analysis, simulations and comparison with other mechanisms. arXiv 2018, arXiv:1807.03451. [Google Scholar]
- Keeling, M.J.; Eames, K.T. Networks and epidemic models. J. R. Soc. Interface 2005, 2, 295–307. [Google Scholar] [CrossRef]
- Keeling, M. The implications of network structure for epidemic dynamics. Theor. Popul. Biol. 2005, 67, 1–8. [Google Scholar] [CrossRef]
- Wang, W.; Tang, M.; Zhang, H.F.; Gao, H.; Do, Y.; Liu, Z.H. Epidemic spreading on complex networks with general degree and weight distributions. Phys. Rev. E 2014, 90, 042803. [Google Scholar] [CrossRef]
- Galante, G.; Rizzi, R.L.; Rizzi, C.B. Simulating epidemiological processes using community-structured scale-free networks. Rev. Bras. De Comput. Apl. 2015, 7, 82–96. [Google Scholar] [CrossRef]
- Pellis, L.; Ball, F.; Bansal, S.; Eames, K.; House, T.; Isham, V.; Trapman, P. Eight challenges for network epidemic models. Epidemics 2015, 10, 58–62. [Google Scholar] [CrossRef]
- Della Rossa, F.; Salzano, D.; Di Meglio, A.; De Lellis, F.; Coraggio, M.; Calabrese, C.; Guarino, A.; Cardona-Rivera, R.; De Lellis, P.; Liuzza, D.; et al. A network model of Italy shows that intermittent regional strategies can alleviate the COVID-19 epidemic. Nat. Commun. 2020, 11, 5106. [Google Scholar] [CrossRef] [PubMed]
- Britton, T. Epidemic models on social networks—With inference. Stat. Neerl. 2020, 74, 222–241. [Google Scholar] [CrossRef]
- Stahlschmidt, S.; Stephen, D. Comparison of Web of Science, Scopus and Dimensions Databases; German Centre for Higher Education Research and Science Studies (DZHW): Hannover, Germany, 2020. [Google Scholar]
- Qian, Z.; Tang, S.T.; Zhang, X.; Zheng, Z.M. The independent spreaders involved SIR Rumor model in complex networks. Phys. A Stat. Mech. Appl. 2015, 429, 95–102. [Google Scholar] [CrossRef]
- Kabir, K.M.; Kuga, K.; Tanimoto, J. Analysis of SIR epidemic model with information spreading of awareness. Chaos 2019, 119, 118–125. [Google Scholar] [CrossRef]
- Zhu, L.H.; Guan, G. Dynamical analysis of a rumor spreading model with self-discrimination and time delay in complex networks. Phys. A Stat. Mech. Appl. 2019, 533, 121953. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.M.; Guan, Z.H. Spreading dynamics of a SIQRS epidemic model on scale-free networks. Commun. Nonlinear Sci. Numer. Simul. 2014, 19, 686–692. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhu, J.J. Stability analysis of I2S2R rumor spreading model in complex networks. Phys. A Stat. Mech. Appl. 2018, 504, 862–881. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhu, J.J. Dynamic behavior of an I2S2R rumor propagation model on weighted contract networks. Phys. A Stat. Mech. Appl. 2019, 536, 120981. [Google Scholar] [CrossRef]
- Zan, Y.L. DSIR double-rumors spreading model in complex networks. Chaos Solitons Fractals 2018, 110, 191–202. [Google Scholar] [CrossRef]
- Yang, A.Z.; Huang, X.Y.; Cai, X.M.; Zhu, X.F.; Lu, L. ILSR rumor spreading model with degree in complex network. Phys. A Stat. Mech. Appl. 2019, 531, 121807. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Russo, L.; Tsakris, A.; Siettos, C. Data-based analysis, modelling and forecasting of the COVID-19 outbreak. PLoS ONE 2020, 15, e0230405. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Blanchini, F.; Bruno, R.; Colaneri, P.; Di Filippo, A.; Di Matteo, A.; Colaneri, M. Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy. Nat. Med. 2020, 26, 855–860. [Google Scholar] [CrossRef]
- Purkayastha, S.; Bhattacharyya, R.; Bhaduri, R.; Kundu, R.; Gu, X.; Salvatore, M.; Ray, D.; Mishra, S.; Mukherjee, B. A comparison of five epidemiological models for transmission of SARS-CoV-2 in India. BMC Infect. Dis. 2021, 21, 533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, K.; Chinazzi, M.; Pastore y Piontti, A.; Dean, N.E.; Rojas, D.P.; Merler, S.; Mistry, D.; Poletti, P.; Rossi, L.; et al. Spread of Zika virus in the Americas. Proc. Natl. Acad. Sci. USA 2017, 114, 4334–4343. [Google Scholar] [CrossRef]
- Craft, M.E. Infectious disease transmission and contact networks in wildlife and livestock. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140107. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Z.; Yang, Z.; Zhang, Z.K.; Zhou, T.; Sun, G.Q. Global analysis of an SIS model with an infective vector on complex networks. Nonlinear Anal. Real World Appl. 2012, 13, 543–557. [Google Scholar] [CrossRef]
- Cross, P.C.; Lloyd-Smith, J.O.; Bowers, J.A.; Hay, C.T.; Hofmeyr, M.; Getz, W.M. Integrating association data and disease dynamics in a social ungulate: Bovine tuberculosis in African buffalo in the Kruger National Park. Ann. Zool. Fenn. 2004, 41, 879–892. [Google Scholar]
- Cohen, T.; Colijn, C.; Finklea, B.; Murray, M. Exogenous re-infection and the dynamics of tuberculosis epidemics: Local effects in a network model of transmission. J. R. Soc. Interface 2007, 4, 523–531. [Google Scholar] [CrossRef]
- Godfrey, S.S. Networks and the ecology of parasite transmission: A framework for wildlife parasitology. Int. J. Parasitol. Parasites Wildl. 2013, 2, 235–245. [Google Scholar] [CrossRef]
- Ren, G.; Wang, X. Epidemic spreading in time-varying community networks. Chaos Interdiscip. J. Nonlinear Sci. 2014, 24, 023116. [Google Scholar] [CrossRef] [PubMed]
- Toutonji, O.A.; Yoo, S.M.; Park, M. Stability analysis of VEISV propagation modeling for network worm attack. Appl. Math. Model. 2012, 36, 2751–2761. [Google Scholar] [CrossRef]
- Craft, M.E.; Volz, E.; Packer, C.; Meyers, L.A. Distinguishing epidemic waves from disease spillover in a wildlife population. Proc. R. Soc. B Biol. Sci. 2009, 276, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, J.; Jin, Z.; Zhang, H.; Sun, G.Q. Impact of media coverage on epidemic spreading in complex networks. Phys. A Stat. Mech. Its Appl. 2013, 392, 5824–5835. [Google Scholar] [CrossRef] [PubMed]

| Countries | Percentage |
|---|---|
| People R. China | 25.51 |
| United States | 23.98 |
| England | 7.65 |
| Canada | 6.63 |
| India | 4.08 |
| Italy | 4.08 |
| Australia | 3.57 |
| Switzerland | 3.06 |
| Japan | 2.04 |
| Others | 19.39 |
| Author | Number of Publications |
|---|---|
| Jin, Zhen | 5 |
| Bertuzzo, Enrico | 4 |
| Casagrandi, Renato | 4 |
| Craft, Meggan E. | 4 |
| Gatto, Marino | 4 |
| Kiss, Istvan Z. | 4 |
| Mari, Lorenzo | 4 |
| Rinaldo, Andrea | 4 |
| Sun, Gui-Quan | 4 |
| Wang, Bing | 4 |
| Wang, Y. | 4 |
| Journal | Nº Articles | Quartile | Impact Factor 2020 |
|---|---|---|---|
| PLoS ONE | 8 | Q2 | 3.240 |
| Journal of Mathematical Biology | 5 | Q2 | 2.259 |
| Journal of The Royal Society Interface | 5 | Q2 | 4.118 |
| Journal of Theoretical Biology | 5 | Q2 | 2.691 |
| Physical Review E | 5 | Q1 | 2.529 |
| Applied Mathematical Modelling | 4 | Q1 | 5.129 |
| Mathematical Biosciences | 4 | Q3 | 2.144 |
| Physica A: Statistical Mechanics and its Applications | 4 | Q2 | 3.263 |
| Advances in Difference Equations | 3 | Q1 | 2.803 |
| Complexity | 3 | Q2 | 2.833 |
| Computational Biology | 3 | Q1 | 4.475 |
| Preventive Veterinary Medicine | 3 | Q1 | 2.670 |
| Proceedings of the Royal Society B: Biological Sciences | 3 | Q1 | 5.349 |
| Research Areas | Percentage |
|---|---|
| Mathematics | 14.83 |
| Science Technology Other Topics | 13.40 |
| Mathematical Computational Biology | 12.92 |
| Life Sciences Biomedicine Other Topics | 10.05 |
| Physics | 10.05 |
| Computer Science | 7.66 |
| Engineering | 7.18 |
| Infectious Diseases | 5.74 |
| Environmental Sciences Ecology | 3.83 |
| Mechanics | 3.35 |
| Public Environmental Occupational Health | 2.87 |
| Biochemistry Molecular Biology | 2.39 |
| Veterinary Sciences | 2.39 |
| Telecommunications | 1.91 |
| Evolutionary Biology | 1.44 |
| Scope | % of Publications |
|---|---|
| Epidemics of infectious diseases | 57.93 |
| Epidemics in general | 32.41 |
| Epidemics in social networks | 9.66 |
| Total Number of Citations | Average Number of Citations per Year | |
|---|---|---|
| Spread of Zika virus in the Americas [59] | 151 | 30.2 |
| Infectious disease transmission and contact networks in wildlife and livestock [60] | 140 | 20.0 |
| Global analysis of an SIS model with an infective vector on complex networks [61] | 111 | 11.1 |
| Integrating association data and disease dynamics in a social ungulate: bovine tuberculosis in African buffalo in the Kruger National Park [62] | 98 | 5.4 |
| Exogenous re-infection and the dynamics of tuberculosis epidemics: local effects in a network model of transmission [63] | 85 | 5.7 |
| Networks and the ecology of parasite transmission: A framework for wildlife parasitology [64] | 82 | 9.1 |
| Epidemic spreading in time-varying community networks [65] | 66 | 8.3 |
| Stability analysis of VEISV propagation modeling for network worm attack [66] | 66 | 6.6 |
| Distinguishing epidemic waves from disease spillover in a wildlife population [67] | 63 | 4.8 |
| Impact of media coverage on epidemic spreading in complex networks [68] | 60 | 6.7 |
| Journal | Percentage of Citations |
|---|---|
| PLoS ONE | 5.17 |
| Scientific Reports | 3.49 |
| Physica A: Statistical Mechanics and its Applications | 3.36 |
| Physical Review E | 1.94 |
| Journal of Theoretical Biology | 1.81 |
| PLoS Computational Biology | 1.64 |
| PLoS Neglected Tropical Diseases | 1.29 |
| Chaos Solitons Fractals | 1.21 |
| Epidemics | 1.16 |
| Applied Mathematics and Computation | 1.12 |
| Journal of The Royal Society Interface | 1.12 |
| Preventive Veterinary Medicine | 1.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifuentes-Faura, J.; Faura-Martínez, U.; Lafuente-Lechuga, M. Mathematical Modeling and the Use of Network Models as Epidemiological Tools. Mathematics 2022, 10, 3347. https://doi.org/10.3390/math10183347
Cifuentes-Faura J, Faura-Martínez U, Lafuente-Lechuga M. Mathematical Modeling and the Use of Network Models as Epidemiological Tools. Mathematics. 2022; 10(18):3347. https://doi.org/10.3390/math10183347
Chicago/Turabian StyleCifuentes-Faura, Javier, Ursula Faura-Martínez, and Matilde Lafuente-Lechuga. 2022. "Mathematical Modeling and the Use of Network Models as Epidemiological Tools" Mathematics 10, no. 18: 3347. https://doi.org/10.3390/math10183347
APA StyleCifuentes-Faura, J., Faura-Martínez, U., & Lafuente-Lechuga, M. (2022). Mathematical Modeling and the Use of Network Models as Epidemiological Tools. Mathematics, 10(18), 3347. https://doi.org/10.3390/math10183347








