A New Incommensurate Fractional-Order COVID 19: Modelling and Dynamical Analysis
Abstract
:1. Introduction
2. Preliminaries
3. Existence and Uniqueness Results
4. Stability Analysis of the Model
4.1. Disease-Free Equilibrium (DFE)
4.2. Basic Reproduction Number
4.3. Stability Analysis
4.3.1. Local Stability of DFE
4.3.2. Global Stability of DFE
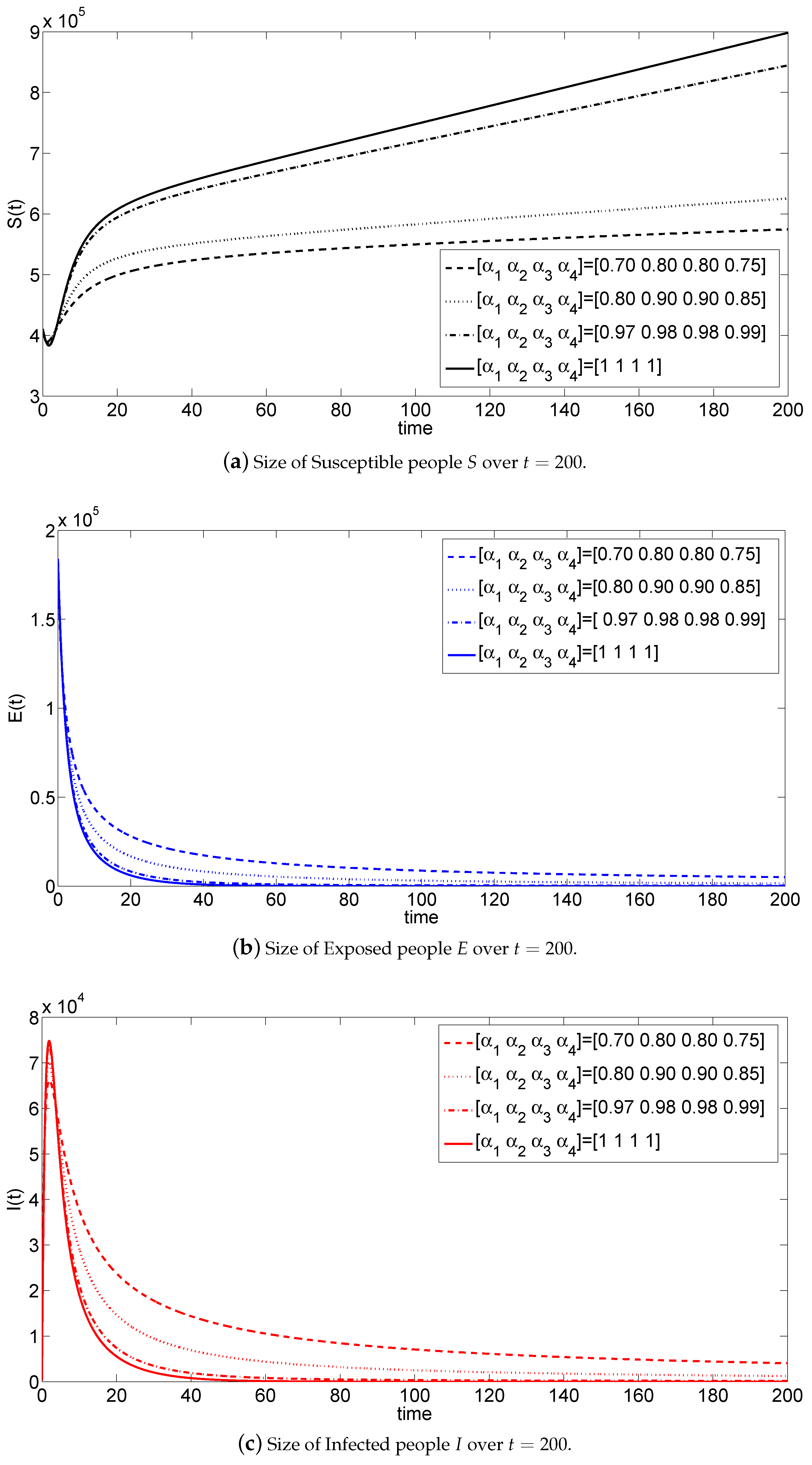
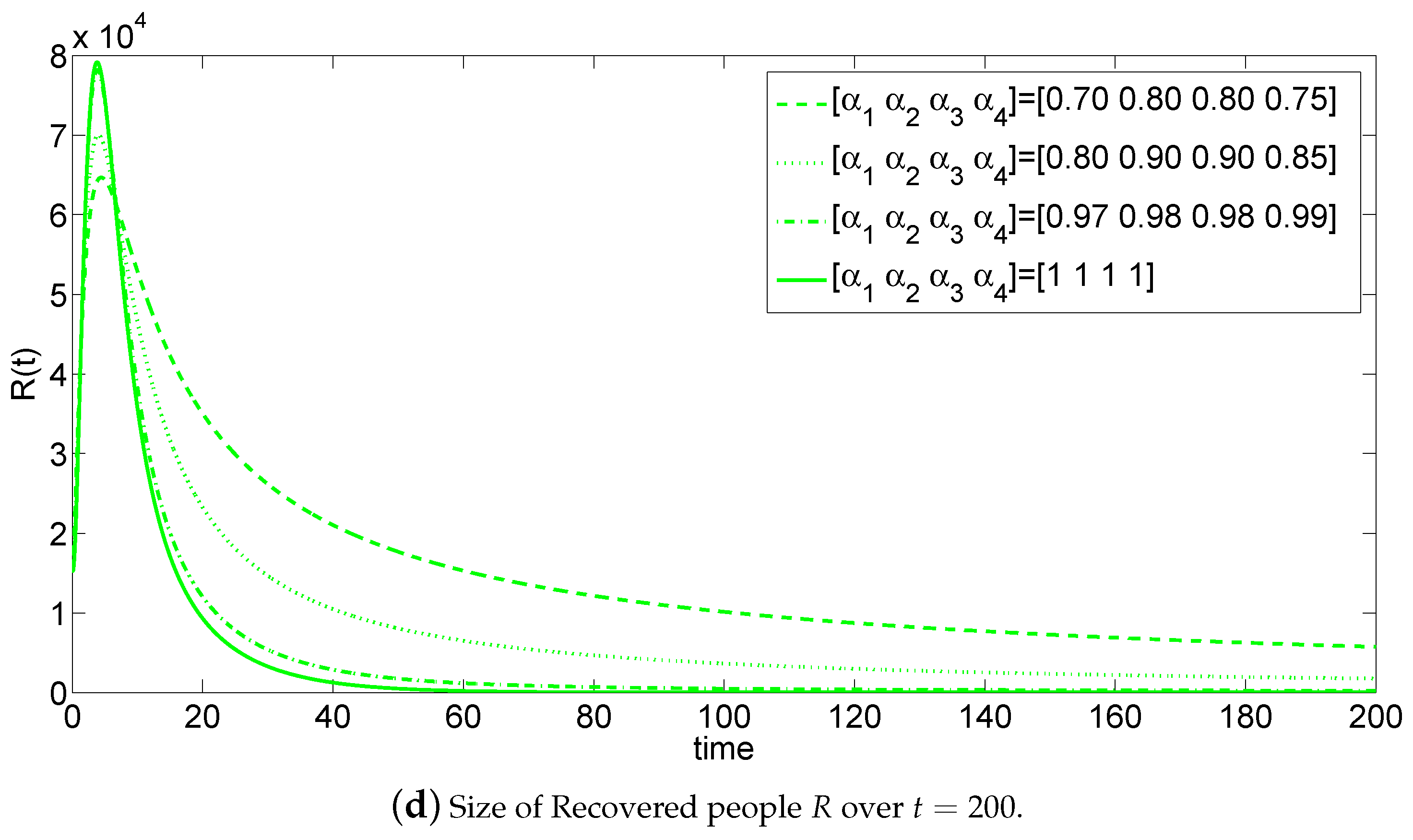
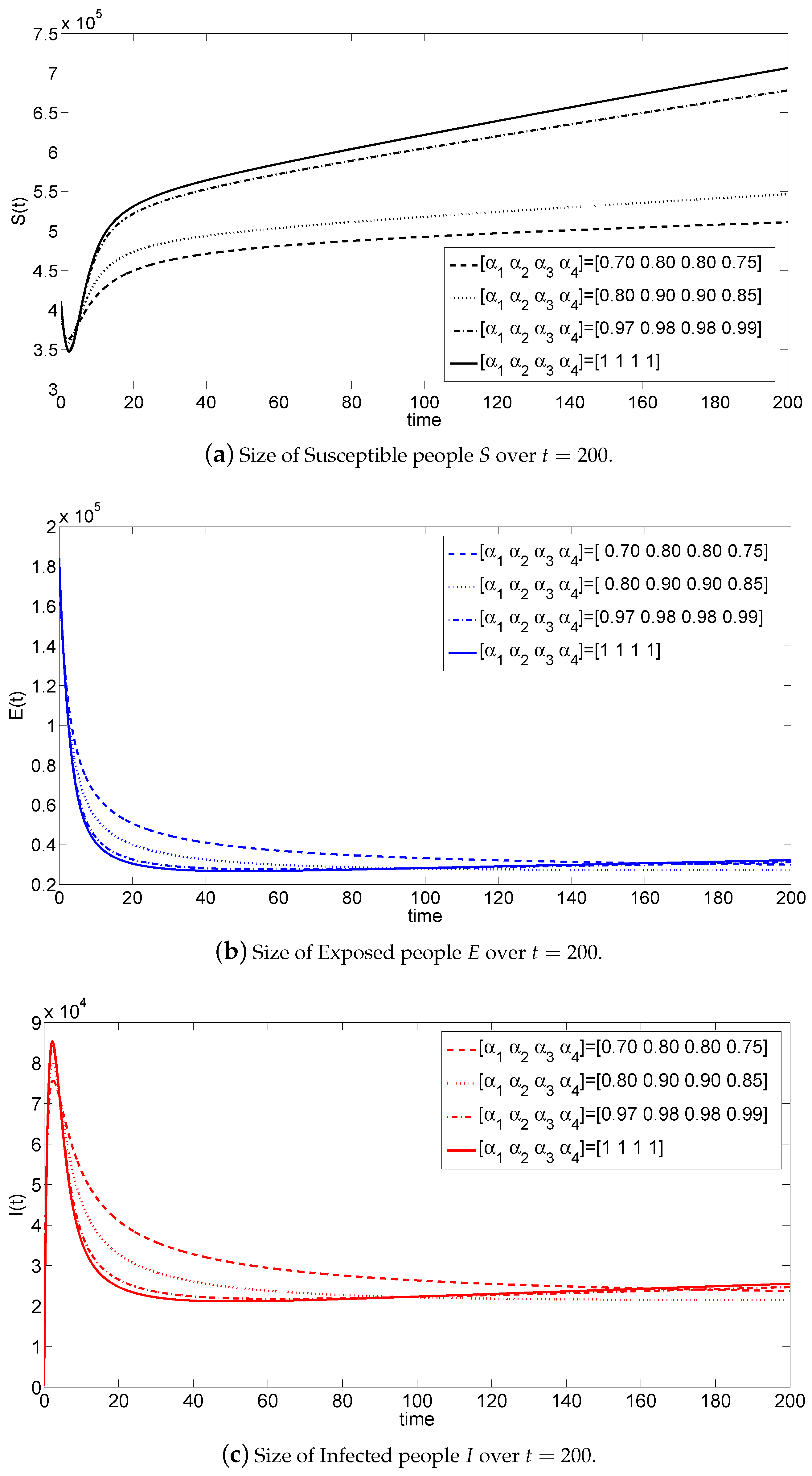
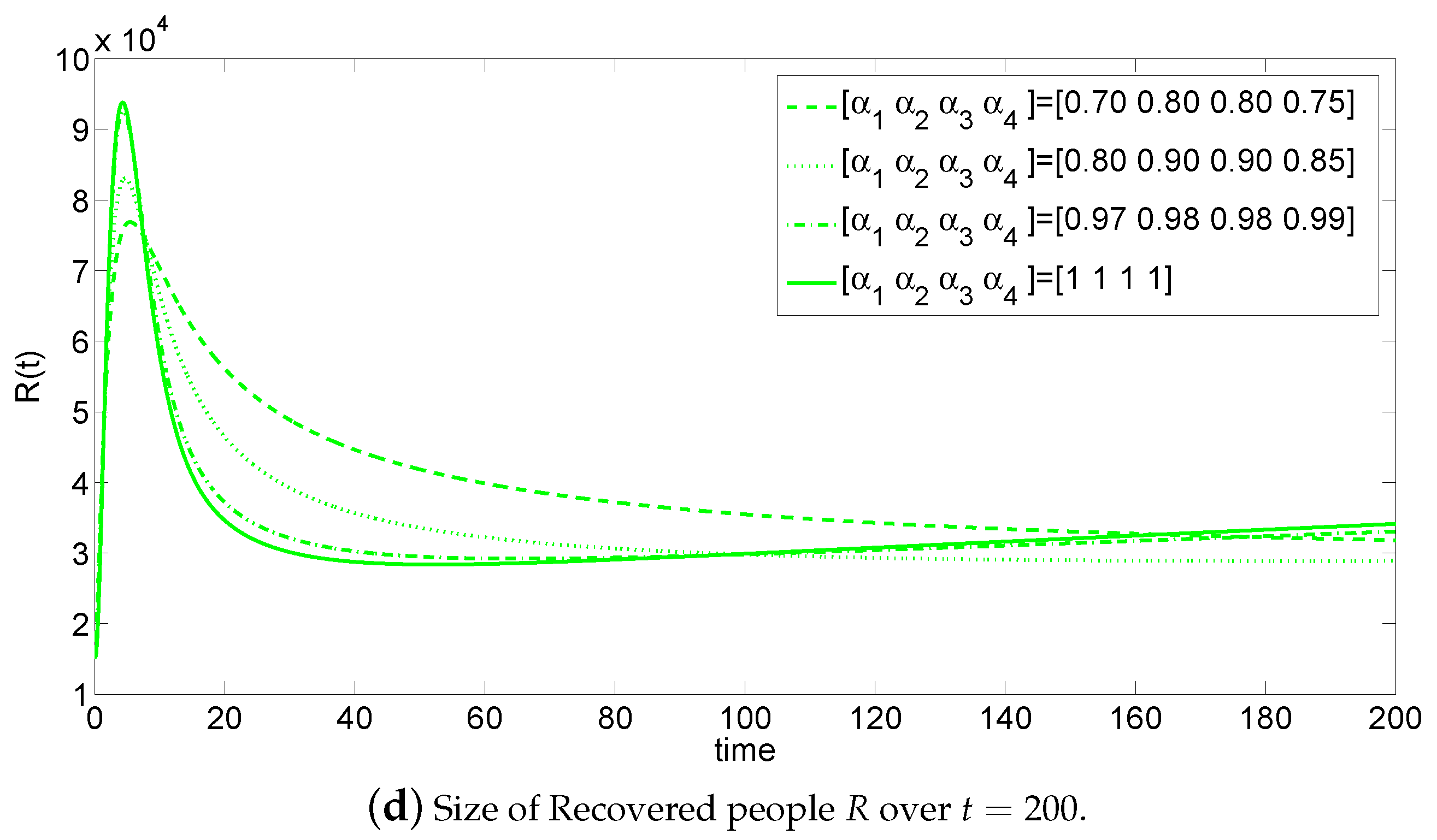
5. Numerical Simulations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wintachai, P.; Prathom, K. Stability analysis of SEIR model related to efficiency of vaccines for COVID-19 situation. Heliyon 2021, 7, e06812. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of Covid-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Shen, Q.; Guo, W.; He, W.; Li, J.; Zhang, Y.; Wang, Y.; Zhou, Z.; Deng, D.; Ouyang, X.; et al. Clinical characteristics of elderly patients with Covid-19 in Hunan province, China: A multicenter, retrospective study. Gerontology 2020, 66, 467–475. [Google Scholar] [CrossRef]
- Oreshkova, N.; Molenaar, R.J.; Vreman, S.; Harders, F.; Munnink, B.B.O.; der Honing, R.W.H.; Gerhards, N.; Tolsma, P.; Bouwstra, R.; Sikkema, R.S.; et al. SARS-CoV-2infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance 2020, 25, 2001005. [Google Scholar] [CrossRef]
- Schlottau, K.; Rissmann, M.; Graaf, A.; Schön, J.; Sehl, J.; Wylezich, C.; Höper, D.; Mettenleiter, T.C.; Balkema-Buschmann, A.; Harder, T.; et al. Sars-CoV-2 in fruit bats, ferrets, pigs, and chickens: An experimental transmission study. Lancet Microbe 2020, 1, e218–e225. [Google Scholar] [CrossRef]
- Kermack, W.O.; McKendrick, A.G. A contribution to the mathematical theory of epidemics. Proc. Roy. Soc. A 1927, 115, 700–721. [Google Scholar]
- Pastor-Satorras, R.; Vespignani, A. Epidemic dynamics and endemic states in complex networks. Proc. Rev. E 2001, 63, 66117. [Google Scholar] [CrossRef] [Green Version]
- Soper, H.E. The interpretation of periodicity in disease prevalence. J. R. Stat. Soc. 1929, 92, 34. [Google Scholar] [CrossRef]
- Bestehorn, M.; Michelitsch, T.M.; Collet, B.A.; Riascos, A.P.; Nowakowski, A.F. Simple model of epidemic dynamics with memory effects. Phys. Rev. E 2022, 105, 24205. [Google Scholar] [CrossRef]
- Ng, T.W.; Turinici, G.; Danchin, A. A double epidemic model for the Sars propagation. BMC Infect. Dis. 2003, 3, 19. [Google Scholar] [CrossRef]
- Han, X.-N.; Vlas, S.J.D.; Fang, L.-Q.; Feng, D.; Cao, W.-C.; Habbema, J.D.F. Mathemat-ical modelling of Sars and other infectious diseases in China: A review. Trop. Med. Int. Health 2009, 14, 92–100. [Google Scholar] [CrossRef]
- Kwon, C.-M.; Jung, J.U. Applying discrete SEIR model to characterizing MERSspread in Korea. Int. J. Model. Simul. Sci. Comput. 2016, 7, 1643003. [Google Scholar] [CrossRef]
- Manaqib, M.; Fauziah, I.; Mujiyanti, M. Mathematical model for MERS-COVdisease transmission with medical mask usage and vaccination. InPrime Indones. J. Pure Appl. Math. 2019, 1, 30–42. [Google Scholar] [CrossRef]
- Diaz, P.; Constantine, P.; Kalmbach, K.; Jones, E.; Pankavich, S. A modified SEIRmodel for the spread of Ebola in western Africa and metrics for resource allocation. Appl. Math. Comput. 2018, 324, 141–155. [Google Scholar]
- Boujakjian, H. Modeling the spread of Ebola with SEIRand optimal control. SIAM Undergrad. Res. Online 2016, 9, 299–310. [Google Scholar] [CrossRef]
- Syafruddin, S.; Noorani, M. SEIRmodel for transmission of Dengue fever in SelangorMalaysia. Int. J. Mod. Phys. Conf. Ser. 2012, 9, 380–389. [Google Scholar]
- Esteva, L.; Vargas, C. Analysis of a Dengue disease transmission model. Math. Biosci. 1998, 150, 131–151. [Google Scholar] [CrossRef]
- Sungchasit, R.; Pongsumpun, P. Mathematical model of Dengue virus with primary and secondary infection. Curr. Appl. Sci. Technol. 2019, 19, 154–176. [Google Scholar]
- Albadarneh, R.B.; Batiha, I.M.; Ouannas, A.; Momani, S. Modeling COVID-19 Pandemic Outbreak using Fractional-Order Systems. Int. J. Math. Comput. Sci. 2021, 16, 1405–1421. [Google Scholar]
- Almatroud, A.O.; Djenina, N.; Ouannas, A.; Grassi, G.; Al-sawalha, M.M. A novel discrete-time COVID-19 epidemic model including the compartment of vaccinated individuals. Math. Biosci. Eng. 2022, 19, 12387–12404. [Google Scholar] [CrossRef] [PubMed]
- Dababneh, A.; Djenina, N.; Ouannas, A.; Grassi, G.; Batiha, I.M. A New Incommensurate Fractional-Order Discrete COVID-19 Model with Vaccinated Individuals Compartment. Fractal Fract. 2022, 6, 456. [Google Scholar] [CrossRef]
- Abbes, A.; Ouannas, A.; Shawagfeh, N.; Jahanshahi, H. The fractional-order discrete COVID-19 pandemic model: Stability and chaos. Nonlinear Dyn. 2023, 111, 965–983. [Google Scholar] [CrossRef]
- Djenina, N.; Ouannas, A.; Batiha, I.M.; Grassi, G.; Oussaeif, T.E.; Momani, S. A novel fractional-order discrete SIR model for predicting COVID-19 behavior. Mathematics 2022, 10, 2224. [Google Scholar] [CrossRef]
- Momani, S.; Ouannas, A.; Momani, Z.; Hadid, S.B. Fractional-order COVID-19 pandemic outbreak: Modeling and stability analysis IM Batiha. Int. J. Biomath. 2022, 15, 2150090. [Google Scholar]
- Djenina, N.; Ouannas, A. The fractional discrete model of COVID-19: Solvability and simulation. Innov. J. Math. 2022, 1, 23–33. [Google Scholar] [CrossRef]
- Batiha, I.; Al-Nana, A.A.; Albadarneh, R.B.; Ouannas, A.; Al-Khasawneh, A.; Momani, S. Fractional-order coronavirus models with vaccination strategies impacted on Saudi Arabia’s infections. AIMS Math. 2022, 7, 12842–12858. [Google Scholar] [CrossRef]
- Debbouche, N.; Ouannas, A.; Batiha, I.M.; Grassi, G. Chaotic dynamics in a novel COVID-19 pandemic model described by commensurate and incommensurate fractional-order derivatives. Nonlinear Dyn. 2022, 109, 1–13. [Google Scholar] [CrossRef]
- Chicchi, L.; Patti, F.D.; Fanelli, D.; Piazza, F.; Ginelli, F. First results with a SEIRD model: Quantifying the population of asymptomatic individuals in Italy, Preprint, Part of the project Analysis and forecast of COVID-19 spreading. 2020. [Google Scholar]
- Samko, S.G.; Kilbas, A.A.; Marichev, O.I. Fractional Integrals and Derivatives: Theory and Applications; Gordon and Breach Science Publishers: Basel, Switzerland, 1993. [Google Scholar]
- Odibat, Z.M.; Shawagfeh, N.T. Generalized Taylor’s formula. Appl. Math. Comput. 2007, 186, 286–293. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Podlubny, I. Stability of fractional-order nonlinear dynamic systems: Lyapunov direct method and generalized Mittag Leffler stability. Comput. Math. Appl. 2010, 59, 1810–1821. [Google Scholar] [CrossRef]
| Variable/Parameter | Description |
|---|---|
| Susceptible class | |
| Exposed class | |
| Infected class | |
| Recovered class | |
| Recruitment rate into susceptible population | |
| Incidence rates | |
| Natural mortality rate | |
| Relapse rate | |
| Progression rate from exposed to infectious class | |
| Treatment rate for infectious individuals | |
| d | COVID-19 death rate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Husban, A.; Djenina, N.; Saadeh, R.; Ouannas, A.; Grassi, G. A New Incommensurate Fractional-Order COVID 19: Modelling and Dynamical Analysis. Mathematics 2023, 11, 555. https://doi.org/10.3390/math11030555
Al-Husban A, Djenina N, Saadeh R, Ouannas A, Grassi G. A New Incommensurate Fractional-Order COVID 19: Modelling and Dynamical Analysis. Mathematics. 2023; 11(3):555. https://doi.org/10.3390/math11030555
Chicago/Turabian StyleAl-Husban, Abdallah, Noureddine Djenina, Rania Saadeh, Adel Ouannas, and Giuseppe Grassi. 2023. "A New Incommensurate Fractional-Order COVID 19: Modelling and Dynamical Analysis" Mathematics 11, no. 3: 555. https://doi.org/10.3390/math11030555







