Mathematical–Statistical Nonlinear Model of Zincing Process and Strategy for Determining the Optimal Process Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Contextual Setting—Process of Electrochemical Deposition
2.2. Proposed Methodology
2.2.1. Three Stages of the Proposed Methodology
2.2.2. Mathematical Formulation of Optimization Problem
2.3. Experimentation—Data Source
- Anode material zinc with a purity of 99.5%;
- Cathode material: S355J0 (experimental sample);
- The amount of sodium benzoate (C7H5NaO2) in the electrolyte is 5.00 g·L−1;
- Cathode current density J = 2.00 A·dm−2.
3. Results and Discussion
3.1. Results of Statistical Analysis of DOE Data and Model Development
3.2. Results of the Optimization Procedure
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jędrzejczyk, D.; Szatkowska, E. The Impact of Heat Treatment on the Behavior of a Hot-Dip Zinc Coating Applied to Steel During Dry Friction. Materials 2021, 14, 660. [Google Scholar] [CrossRef]
- Klekotka, M.; Zielińska, K.; Stankiewicz, A.; Kuciej, M. Tribological and Anticorrosion Performance of Electroplated Zinc Based Nanocomposite Coatings. Coatings 2020, 10, 594. [Google Scholar] [CrossRef]
- Kavitha, B.; Santhosh, P.; Renukadevi, M.; Kalpana, A.; Shakkthivel, P.; Vasudevan, T. Role of organic additives on zinc plating. Surf. Coat. Technol. 2006, 201, 3438–3442. [Google Scholar] [CrossRef]
- Zhang, X.G.; Valeriote, E.M. Galvanic protection of steel and galvanic corrosion of zinc under thin layer electrolytes. Corros. Sci. 1993, 34, 1957–1972. [Google Scholar] [CrossRef]
- Boshkov, N.; Petrov, K.; Kovacheva, D.; Vitkova, S.; Nemska, S. Influence of the alloying component on the protective ability of some zinc galvanic coatings. Electrochim. Acta 2005, 51, 77–84. [Google Scholar] [CrossRef]
- Yadav, A.P.; Katayama, H.; Noda, K.; Masuda, H.; Nishikata, A.; Tsuru, T. Effect of Al on the galvanic ability of Zn–Al coating under thin layer of electrolyte. Electrochim. Acta 2007, 52, 2411–2422. [Google Scholar] [CrossRef]
- Badida, M.; Sobotova, L.; Gombar, M.; Kmec, J.; Kucerka, D.; Hrmo, R. The contribution to coating quality evaluation by statistical methods. Metalurgija 2016, 55, 445–448. [Google Scholar]
- Yadav, A.P.; Katayama, H.; Noda, K.; Masuda, H.; Nishikata, A.; Tsuru, T. Surface potential distribution over a zinc/steel galvanic couple corroding under thin layer of electrolyte. Electrochim. Acta 2007, 52, 3121–3129. [Google Scholar] [CrossRef]
- Jong-Min, L. Numerical analysis of galvanic corrosion of Zn/Fe interface beneath a thin electrolyte. Electrochim. Acta 2006, 51, 3256–3260. [Google Scholar] [CrossRef]
- Dubent, S.; Mertens, M.L.A.D.; Saurat, M. Electrodeposition, characterization and corrosion behaviour of tin–20wt.% zinc coatings electroplated from a non-cyanide alkaline bath. Mater. Chem. Phys. 2010, 120, 371–380. [Google Scholar] [CrossRef]
- Maniam, K.K.; Paul, S. Progress in Electrodeposition of Zinc and Zinc Nickel Alloys Using Ionic Liquids. Appl. Sci. 2020, 10, 5321. [Google Scholar] [CrossRef]
- Wang, Y. Study on Influence Factors of zinc layer thickness via Response Surface Method, Taguchi Method and Genetic Algorithm. Ind. Eng. Manag. 2018, 07, 1000245. [Google Scholar] [CrossRef]
- Luis Pérez, C.J. A Proposal of an Adaptive Neuro-Fuzzy Inference System for Modeling Experimental Data in Manufacturing Engineering. Mathematics 2020, 8, 1390. [Google Scholar] [CrossRef]
- Dobránsky, J.; Gombár, M.; Stejskal, T. The Influence of the Use of Technological Waste and the Simulation of Material Lifetime on the Unnotched Impact Strength of Two Different Polymer Composites. Materials 2022, 15, 8516. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk-Świercz, D.; Świercz, R.; Michna, Š. Evaluation of Prediction Models of the Microwire EDM Process of Inconel 718 Using ANN and RSM Methods. Materials 2022, 15, 8317. [Google Scholar] [CrossRef]
- Valíček, J.; Czán, A.; Harničárová, M.; Šajgalík, M.; Kušnerová, M.; Czánová, T.; Kopal, I.; Gombár, M.; Kmec, J.; Šafář, M. A new way of identifying, predicting and regulating residual stress after chip-forming machining. Int. J. Mech. Sci. 2019, 155, 343–359. [Google Scholar] [CrossRef]
- Świercz, R.; Oniszczuk-Świercz, D.; Chmielewski, T. Multi-Response Optimization of Electrical Discharge Machining Using the Desirability Function. Micromachines 2019, 10, 72. [Google Scholar] [CrossRef]
- Hrehová, S. Possibilities of Data Analysis Using Data Model. In Proceedings of the 4th EAI International Conference on Management of Manufacturing System (MMS 2019), Krynica Zdroj, Poland, 8–10 October 2019; EAI/Springer Innovations in Communication and Computing. Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Hoskova-Mayerova, S.; Kalvoda, J.; Bauer, M.; Rackova, P. Development of a Methodology for Assessing Workload within the Air Traffic Control Environment in the Czech Republic. Sustainability 2022, 14, 7858. [Google Scholar] [CrossRef]
- Bekesiene, S.; Samoilenko, I.; Nikitin, A.; Meidute-Kavaliauskiene, I. The Complex Systems for Conflict Interaction Modelling to Describe a Non-Trivial Epidemiological Situation. Mathematics 2022, 10, 537. [Google Scholar] [CrossRef]
- Panda, A.; Zaloga, V.; Dyadyura, K.; Rybalka, I.; Pandova, I. Modelling Business Process of Manufacturing for Air Compressors. TEM J. 2019, 8, 430–436. [Google Scholar] [CrossRef]
- Sánchez, L.; Leiva, V.; Saulo, H.; Marchant, C.; Sarabia, J.M. A New Quantile Regression Model and Its Diagnostic Analytics for a Weibull Distributed Response with Applications. Mathematics 2021, 9, 2768. [Google Scholar] [CrossRef]
- Anand, V.; Srivastava, V.C. Zinc oxide nanoparticles synthesis by electrochemical method: Optimization of parameters for maximization of productivity and characterization. J. Alloy. Compd. 2015, 636, 288–292. [Google Scholar] [CrossRef]
- Dhak, D.; Mahon, M.; Asselin, E.; Alfantazi, A. Characterizing industrially electrowon sticky zinc deposits. Hydrometallurgy 2012, 111–112, 136–140. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Alamdari, E.K.; Zamanzade, M.; Salasi, M.; Behrouzghaemi, S.; Heydari, J.; Haghshenas, D.F.; Zolala, V. Empirical equations for electrical conductivity and density of Zn, Cd and Mn sulphate solutions in the range of electrowinning and electrorefining electrolytes. J. Mater. Sci. 2007, 42, 9622–9631. [Google Scholar] [CrossRef]
- Yu, J.; Wang, L.; Su, L.; Ai, X.; Yang, H. Temperature Effects on the Electrodeposition of Zinc. J. Electrochem. Soc. 2002, 150, C19–C23. [Google Scholar] [CrossRef]
- Jedrzejczyk, D. Effect of High Temperature Oxidation on Structure and Corrosion Resistance of the Zinc Coating Deposited on Cast Iron. Arch. Met. Mater. 2012, 57, 145–154. [Google Scholar] [CrossRef]
- Xia, X.; Zhitomirsky, I.; McDermid, J.R. Electrodeposition of zinc and composite zinc–yttria stabilized zirconia coatings. J. Mater. Process. Technol. 2009, 209, 2632–2640. [Google Scholar] [CrossRef]
- Alfantazi, A.M.; Dreisinger, D. The role of zinc and sulfuric acid concentrations on zinc electrowinning from industrial sulfate based electrolyte. J. Appl. Electrochem. 2001, 31, 641–646. [Google Scholar] [CrossRef]
- Kania, H.; Mendala, J.; Kozuba, J.; Saternus, M. Development of Bath Chemical Composition for Batch Hot-Dip Galvanizing—A Review. Materials 2020, 13, 4168. [Google Scholar] [CrossRef]
- Mackinnon, D.J.; Brannen, J.M.; Fenn, P.L. Characterization of impurity effects in zinc electrowinning from industrial acid sulphate electrolyte. J. Appl. Electrochem. 1987, 17, 1129–1143. [Google Scholar] [CrossRef]
- Verma, N.; Sharma, V.; Badar, M.A. Improving sigma level of galvanization process by zinc over-coating reduction using an integrated Six Sigma and design-of-experiments approach. Arab. J. Sci. Eng. 2022, 47, 8535–8549. [Google Scholar] [CrossRef]
- Bennasr, J.; Snoussi, A.; Bradai, C.; Halouani, F. Optimization of hot-dip galvanizing process of reactive steels: Minimizing zinc consumption without alloy additions. Mater. Lett. 2008, 62, 3328–3330. [Google Scholar] [CrossRef]
- Lorza, R.L.; Calvo, M.Á.M.; Labari, C.B.; Fuente, P.J.R. Using the Multi-Response Method with Desirability Functions to Optimize the Zinc Electroplating of Steel Screws. Metals 2018, 8, 711. [Google Scholar] [CrossRef]
- Vagaská, A.; Gombár, M.; Kmec, J.; Michal, P. Statistical Analysis of the Factors Effect on the Zinc Coating Thickness. Appl. Mech. Mater. 2013, 378, 184–189. [Google Scholar] [CrossRef]
- Michal, P.; Gombár, M.; Vagaská, A.; Piteľ, J.; Kmec, J. Experimental Study and Modeling of the Zinc Coating Thickness. Adv. Mater. Res. 2013, 712–715, 382–386. [Google Scholar] [CrossRef]
- Michal, P.; Piteľ, J.; Vagaská, A.; Bukovský, I. Application of neural networks to evaluate experimental data of galvanic zincing. In Proceedings of the 2014 International Joint Conference on Neural Networks (IJCNN), Beijing, China, 6–11 July 2014; IEEE: New York, NY, USA, 2014; pp. 2997–3001. [Google Scholar]
- Bartl, D.O.; Mudroch, O. Technologie Chemických a Elektrochemických Povrchových Úprav I, 1st ed.; SNTL: Prague, Czech Republic, 1957; p. 448. [Google Scholar]
- Hayter, A. Probability and Statistics for Engineers and Scientists, 4th ed.; Thomson Brooks/Cole, Cengage Learning: Toronto, ON, Canada, 2013; p. 826. [Google Scholar]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters. Design, Innovation, and Discovery, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; p. 639. [Google Scholar]
- Vagaská, A.; Gombár, M.; Straka, Ľ. Selected Mathematical Optimization Methods for Solving Problems of Engineering Practice. Energies 2022, 15, 2205. [Google Scholar] [CrossRef]
- Hatefi, E.; Hatefi, A. Estimation of Critical Collapse Solutions to Black Holes with Nonlinear Statistical Models. Mathematics 2022, 10, 4537. [Google Scholar] [CrossRef]
- Yang, W.Y.; Cao, W.; Chung, T.S.; Morris, J. Applied Numerical Methods Using MATLAB; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; p. 509. [Google Scholar]
- Camacho, E.F.; Alba, C.B. Model Predictive Control, 2nd ed.; Springer Science & Business Media: New York, NY, USA, 2013; pp. 249–287+427. [Google Scholar]
- Hamala, M.; Trnovská, M. Nelineárne Programovanie/Nonlinear Programming; Epos: Bratislava, Slovak Republic, 2012; p. 339. [Google Scholar]
- Behún, M.; Knežo, D.; Cehlár, M.; Knapčíková, L.; Behúnová, A. Recent Application of Dijkstra’s Algorithm in the Process of Production Planning. Appl. Sci. 2022, 12, 7088. [Google Scholar] [CrossRef]
- Ruml, V. Abeceda Povrchových Úprav Kovov, 1st ed.; PRÁCE: Prague, Czech Republic, 1956; p. 175. [Google Scholar]
- Ruml, V.; Soukup, M. Galvanické Pokovování, 1st ed.; SNTL: Prague, Czech Republic, 1981; p. 324. [Google Scholar]
- Rao, S. Engineering Optimization. Theory and Practice, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; p. 830. [Google Scholar]
- Antoniou, A.; Lu, W.S. Practical Optimization. Algorithms and Engineering Applications; Springer Science & Business Media LCC: New York, NY, USA, 2007; pp. 547–558+675. [Google Scholar]
- Boyd, S.; Vandenberghe, L. Convex Optimization; Cambridge University Press: Cambridge, UK, 2009; 701p. [Google Scholar]
- Belegundu, A.D.; Chandrupatla, T.R. Optimization Concepts and Applications in Engineering; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Bakare, E.A.; Hoskova-Mayerova, S. Optimal Control Analysis of Cholera Dynamics in the Presence of Asymptotic Transmission. Axioms 2021, 10, 60. [Google Scholar] [CrossRef]
- Coronado de Koster, O.A.; Domínguez-Navarro, J.A. Multi-Objective Tabu Search for the Location and Sizing of Multiple Types of FACTS and DG in Electrical Networks. Energies 2020, 13, 2722. [Google Scholar] [CrossRef]

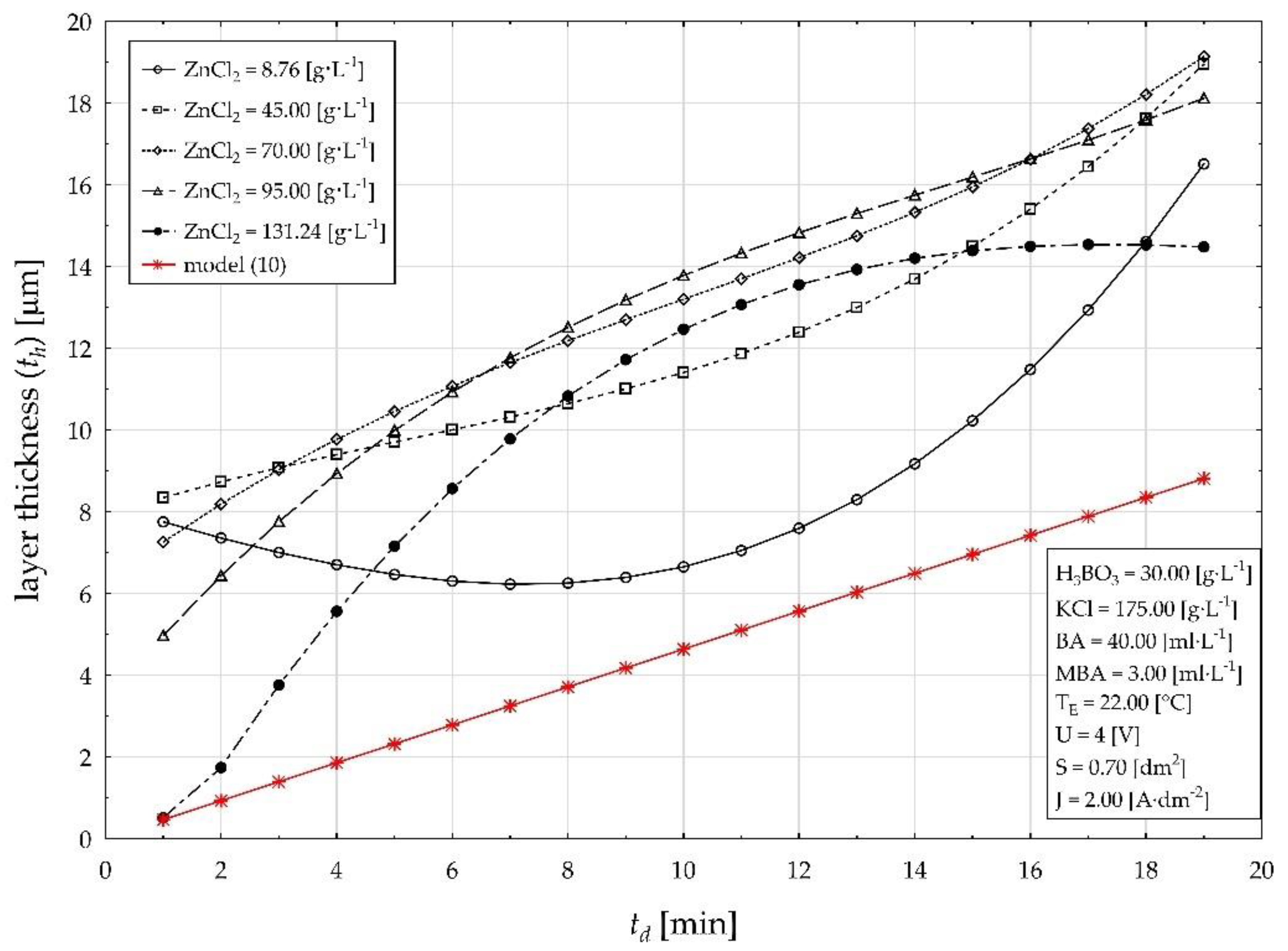
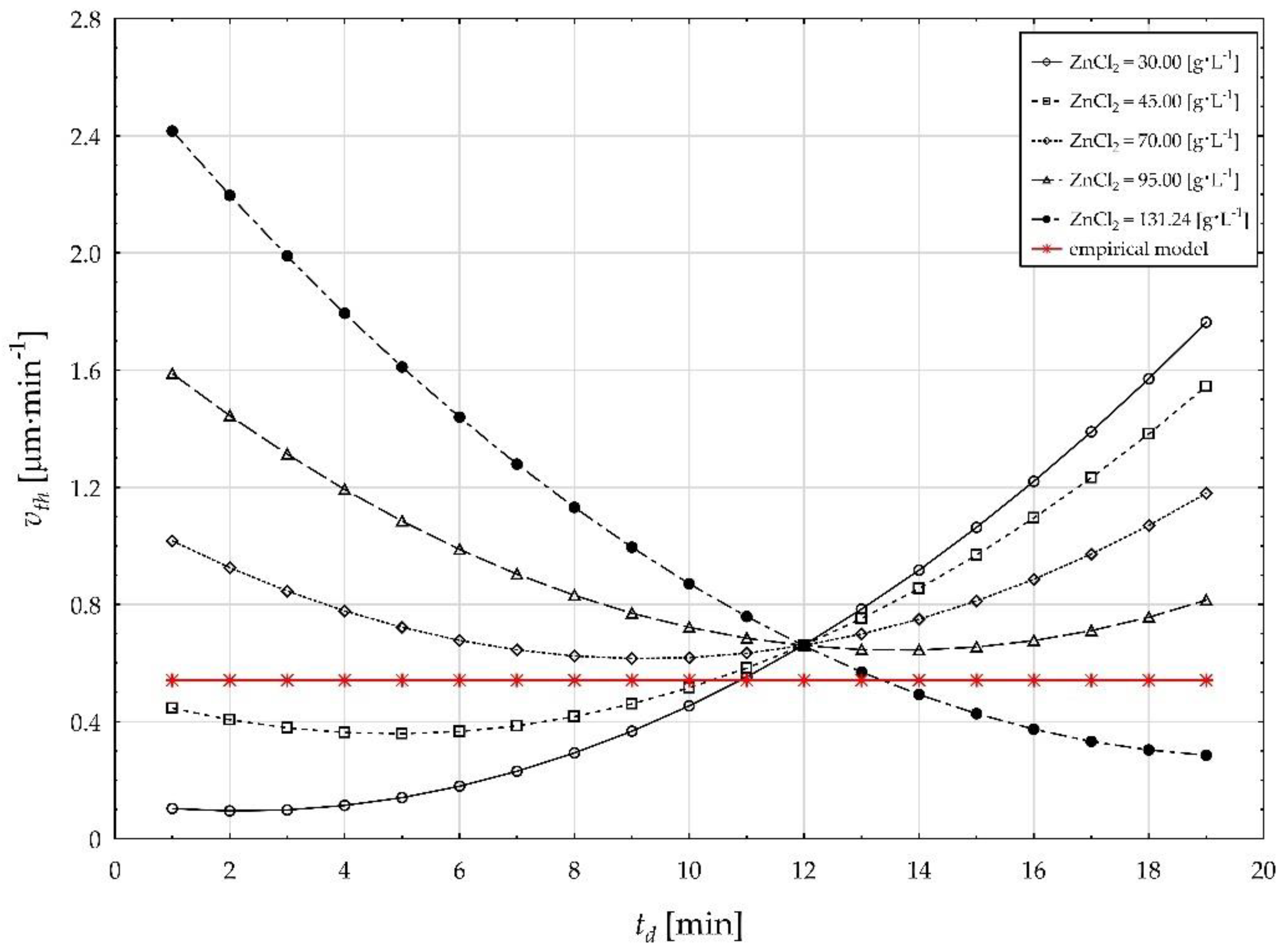
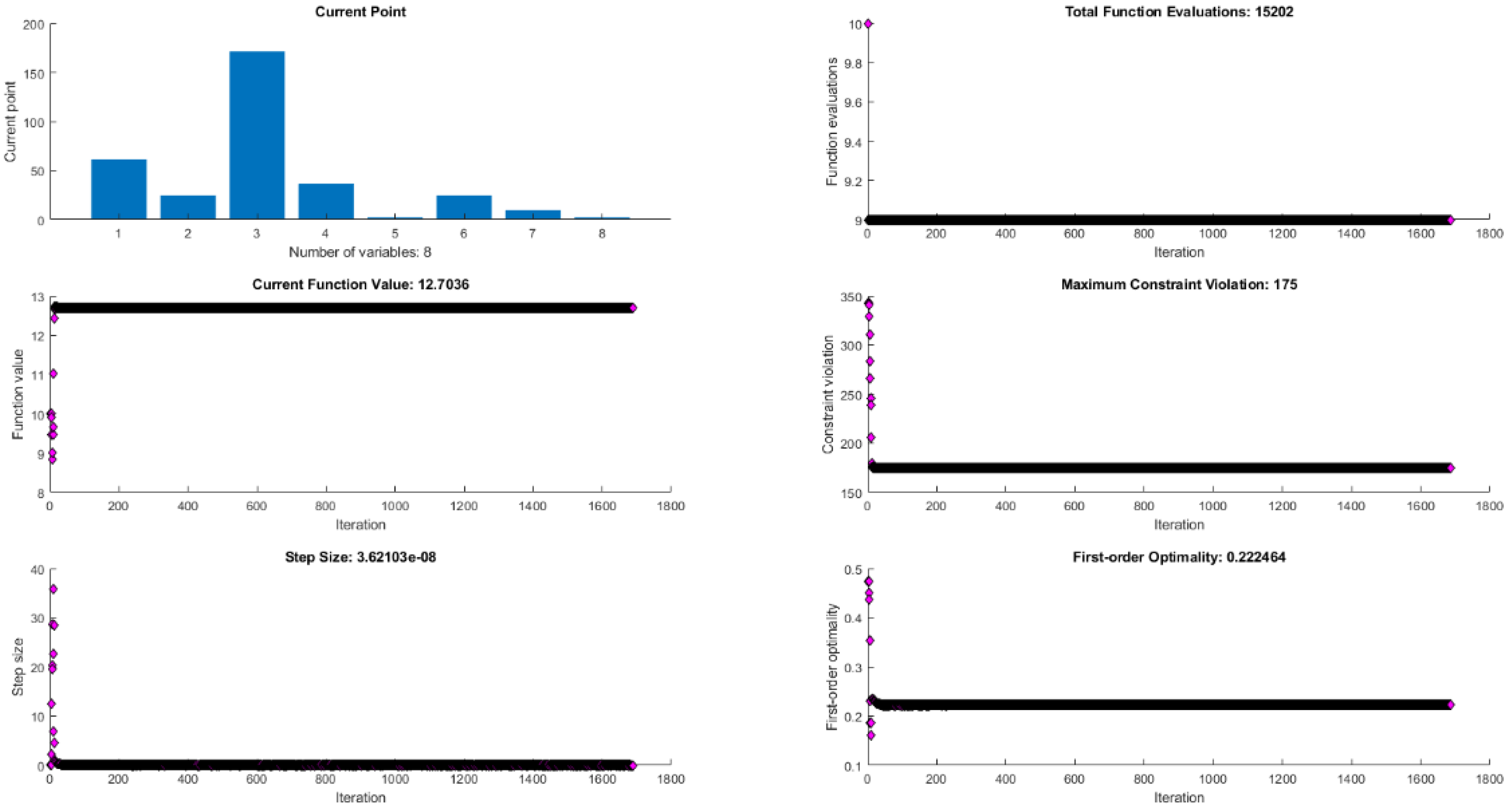
| Factor (Code) | Factor (Natural) | Factor Unit | Factor Level | ||||
|---|---|---|---|---|---|---|---|
| −2.449 | −1 | 0 | +1 | +2.449 | |||
| x1 | ZnCl2 | g·L−1 | 8.76 | 45.00 | 70.00 | 95.00 | 131.24 |
| x2 | H3BO3 | g·L−1 | 5.51 | 20.00 | 30.00 | 40.00 | 54.49 |
| x3 | KCl | g·L−1 | 3.54 | 105.00 | 175.00 | 245.00 | 346.46 |
| x4 | BA | ml·L−1 | 3.26 | 25.00 | 40.00 | 60.00 | 76.74 |
| x5 | MBA | ml·L−1 | 0.55 | 2.00 | 3.00 | 4.00 | 5.45 |
| x6 | TE | °C | −2.49 | 12.00 | 22.00 | 32.00 | 46.49 |
| x7 | td | min | 0.20 | 6.00 | 10.00 | 14.00 | 19.80 |
| x8 | U | V | 1.55 | 3.00 | 4.00 | 5.00 | 6.45 |
| Parameter | Value |
|---|---|
| RSquare | 0.959403 |
| RSquare Adj | 0.944829 |
| Root Mean Square Error | 0.609012 |
| Mean of Response | 12.66074 |
| Observations (or Sum Wgts) | 54 |
| Source | df | Sum of Squares | Mean Square | F Ratio | p |
|---|---|---|---|---|---|
| Model | 14 | 341.8378 | 24.417 | 65.8324 | <0.0001 * |
| Error | 39 | 14.46495 | 0.3709 | ||
| C. Total | 53 | 356.3028 |
| Source | df | Sum of Squares | Mean Square | F Ratio | p |
|---|---|---|---|---|---|
| Lack Of Fit | 36 | 13.69565 | 0.380435 | 1.4836 | 0.4264 |
| Pure Error | 3 | 0.7693 | 0.256433 | ||
| Total Error | 39 | 14.46495 |
| Term | Estimate | Std Error | t Ratio | Prob>|t| | −95% CI | +95% CI | VIF |
|---|---|---|---|---|---|---|---|
| Intercept | 13.20027 | 0.106555 | 123.88 | <0.0001 * | 12.98474 | 13.4158 | . |
| x7 | 1.999702 | 0.132512 | 15.09 | <0.0001 * | 1.731672 | 2.267732 | 2.272463 |
| x1 | 1.185961 | 0.175807 | 6.75 | <0.0001 * | 0.830359 | 1.541564 | 1.999999 |
| x6 | 0.536572 | 0.097868 | 5.48 | <0.0001 * | 0.338615 | 0.734528 | 1.239563 |
| x3 | 0.411504 | 0.093711 | 4.39 | <0.0001 * | 0.221954 | 0.601053 | 1.136512 |
| x7·x1 | 0.282726 | 0.106549 | 2.65 | 0.0115 * | 0.06721 | 0.498243 | 1.101924 |
| x1·x1 | −0.60697 | 0.075346 | −8.06 | <0.0001 * | −0.75937 | −0.45457 | 1.000000 |
| x7·x6 | 0.438066 | 0.104858 | 4.18 | 0.0002 * | 0.225971 | 0.650162 | 1.067221 |
| x3·x8 | 0.275512 | 0.125774 | 2.19 | 0.0345 * | 0.021109 | 0.529915 | 1.535445 |
| x7·x5 | 0.40297 | 0.114282 | 3.53 | 0.0011 * | 0.171812 | 0.634128 | 1.267674 |
| x3·x5 | −0.29171 | 0.107228 | −2.72 | 0.0097 * | −0.50859 | −0.07482 | 1.115997 |
| x1·x4 | −0.46195 | 0.116077 | −3.98 | 0.0003 * | −0.69674 | −0.22716 | 1.307807 |
| x6·x4 | 0.324827 | 0.128789 | 2.52 | 0.0159 * | 0.064326 | 0.585328 | 1.609937 |
| x7·x7·x7 | 0.126336 | 0.041106 | 3.07 | 0.0039 * | 0.04319 | 0.209481 | 1.132125 |
| x7·x7·x1 | −0.44179 | 0.212534 | −2.08 | 0.0443 * | −0.87168 | −0.0119 | 1.384377 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagaská, A. Mathematical–Statistical Nonlinear Model of Zincing Process and Strategy for Determining the Optimal Process Conditions. Mathematics 2023, 11, 771. https://doi.org/10.3390/math11030771
Vagaská A. Mathematical–Statistical Nonlinear Model of Zincing Process and Strategy for Determining the Optimal Process Conditions. Mathematics. 2023; 11(3):771. https://doi.org/10.3390/math11030771
Chicago/Turabian StyleVagaská, Alena. 2023. "Mathematical–Statistical Nonlinear Model of Zincing Process and Strategy for Determining the Optimal Process Conditions" Mathematics 11, no. 3: 771. https://doi.org/10.3390/math11030771
APA StyleVagaská, A. (2023). Mathematical–Statistical Nonlinear Model of Zincing Process and Strategy for Determining the Optimal Process Conditions. Mathematics, 11(3), 771. https://doi.org/10.3390/math11030771







