Deterministic Modeling of the Issue of Dental Caries and Oral Bacterial Growth: A Brief Review
Abstract
:1. Introduction
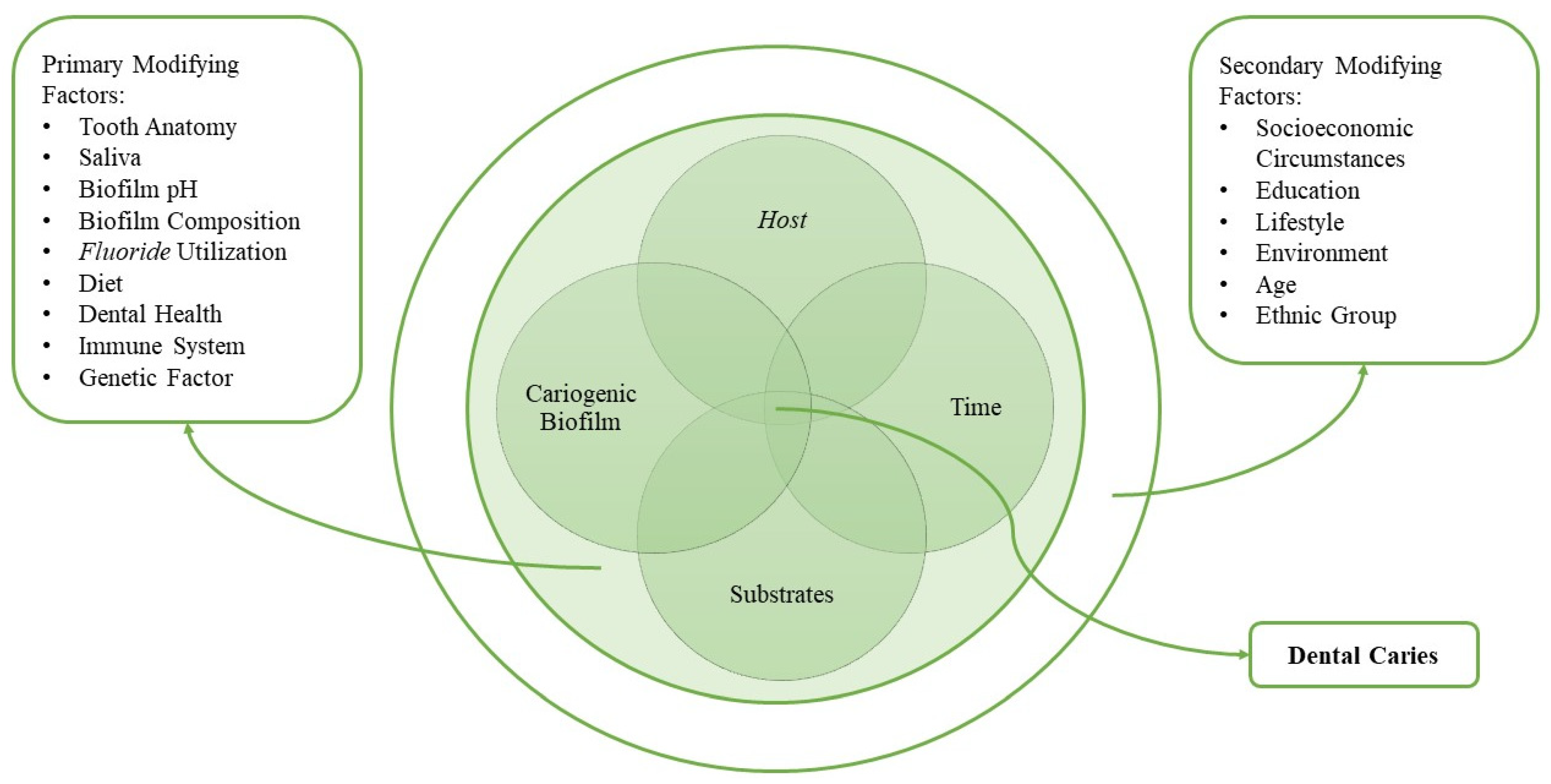
2. Materials and Methods
- Step 1:
- Remove duplicated articles and identify whole papers that meet the review protocol.
- Step 2:
- Screen the papers that pass the first protocol terms. This step allows us to determine the documents that meet the criterion in each step, resulting in the most relevant articles for our review. Generally, it is separated into several stages, as needed.
- Step 3:
- Collect the suitable studies for further review and analysis.
- The article studies a compartmental-based model utilizing dynamical system theory.
- The compartmental model is constructed in an ordinary differential equations system (ODEs) or delay differential equations system (DDEs).
- The papers utilize the constructed model to study a population dynamics issue.
- The population dynamics studied in the selected paper represent the dental health issue in epidemiology or bacteria population growth terms.
3. Results and Discussion
3.1. Output of PRISMA Method
3.2. Elaboration of Selected Articles
3.2.1. Elaboration of the First Human-to-Human Transmission Dental Health Issue
| susceptible subpopulation dynamic | (1) |
| exposed subpopulation dynamic | |
| infectious subpopulation dynamic | |
| treated subpopulation dynamic | |
| recovered subpopulation dynamic | |
3.2.2. Elaboration of the Bacterial Population Growth Model Studies in Oral Biofilm
| susceptible bacteria proportion | (3) |
| persister bacteria proportion | |
| dead bacteria proportion | |
| EPS proportion | |
| nutrition concentration | |
| antimicrobial agents | |
| quorum sensing concentration | |
| growth factor concentration | |
| slow-penetration factor | |
| live bacteria proportion | (5) |
| dead bacteria proportion | |
| EPS proportion | |
| antimicrobial agents | |
| quorum sensing molecules | |
| growth factors | |
| slow-penetration factors | |
3.3. Revealing the Gap for Further Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 12 September 2023).
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental Caries. Nat. Rev. Dis. Prim. 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Fejerskov, O.; Nyvad, B.; Kidd, E. Dental Caries: The Disease and Its Clinical Management; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 978-1-118-93582-8. [Google Scholar]
- Santos, A.P.; Soviero, V.M. Caries Prevalence and Risk Factors among Children Aged 0 to 36 Months. Pesqui. Odontol. Bras. 2002, 16, 203–208. [Google Scholar] [CrossRef]
- Thomas, J.G.; Nakaishi, L.A. Managing the Complexity of a Dynamic Biofilm. J. Am. Dent. Assoc. 2006, 137, S10–S15. [Google Scholar] [CrossRef]
- Chen, X.; Daliri, E.; Kim, N.; Kim, J.R.; Yoo, D.; Oh, D.-H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, D.J.; Lynch, R.J.M. Diet and the Microbial Aetiology of Dental Caries: New Paradigms. Int. Dent. J. 2013, 63, 64–72. [Google Scholar] [CrossRef]
- Esra, K.; Nurhan, O.; Yilmaz, A.D.; Berrin, O. Vertical and Horizontal Transmission of Streptococcus Mutans and Effective Factors: An In Vivo Study. J. Adv. Oral Res. 2020, 11, 172–179. [Google Scholar] [CrossRef]
- Kateeb, E.; Lim, S.; Amer, S.; Ismail, A. Behavioral and Social Determinants of Early Childhood Caries among Palestinian Preschoolers in Jerusalem Area: A Cross-Sectional Study. BMC Oral Health 2023, 23, 152. [Google Scholar] [CrossRef]
- Ellakany, P.; Madi, M.; Fouda, S.M.; Ibrahim, M.; Alhumaid, J. The Effect of Parental Education and Socioeconomic Status on Dental Caries among Saudi Children. Int. J. Environ. Res. Public Health 2021, 18, 11862. [Google Scholar] [CrossRef]
- Iwasaki, M.; Kakuta, S.; Ansai, T. Associations among Internet Addiction, Lifestyle Behaviors, and Dental Caries among High School Students in Southwest Japan. Sci. Rep. 2022, 12, 17342. [Google Scholar] [CrossRef]
- Kaewkamnerdpong, I.; Krisdapong, S. The Associations of School Oral Health-Related Environments with Oral Health Behaviours and Dental Caries in Children. Caries Res. 2018, 52, 166–175. [Google Scholar] [CrossRef]
- Bassa, S.; Workie, S.B.; Kassa, Y.; Tegbaru, D.W. Prevalence of Dental Caries and Relation with Nutritional Status among School-Age Children in Resource Limited Setting of Southern Ethiopia. BMC Oral Health 2023, 23, 84. [Google Scholar] [CrossRef]
- Luo, H.; Moss, M.E.; Wright, W.; Webb, M.; Pardi, V.; Lazorick, S. Racial/Ethnic Disparities in Preventive Dental Services Use and Dental Caries among Children. J. Public Health Dent. 2023, 83, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Do, T.; Devine, D.; Marsh, P.D. Oral Biofilms: Molecular Analysis, Challenges, and Future Prospects in Dental Diagnostics. Clin. Cosmet. Investig. Dent. 2013, 5, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Chen, V.; Xu, X. Novel Approaches to the Control of Oral Microbial Biofilms. Biomed Res. Int. 2018, 2018, 6498932. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Jakubovics, N.S.; Chalmers, N.I.; Palmer, R.J. Human Oral Multispecies Biofilms: Bacterial Communities in Health and Disease. In Biofilm Mode of Life: Mechanisms and Adaptations; Horizon Sciencetific Press: Poole, UK, 2007; pp. 175–193. ISBN 978-1-904933-36-6. [Google Scholar]
- Zhang, Y.; Fang, J.; Yang, J.; Gao, X.; Dong, L.; Zheng, X.; Sun, L.; Xia, B.; Zhao, N.; Ma, Z.; et al. Streptococcus Mutans-Associated Bacteria in Dental Plaque of Severe Early Childhood Caries. J. Oral Microbiol. 2022, 14, 2046309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Zhang, S.; Li, J.; Li, X.; Ying, Y.; Yuan, J.; Chen, K.; Deng, S.; Wang, Q. Association of Polymicrobial Interactions with Dental Caries Development and Prevention. Front. Microbiol. 2023, 14, 1162380. [Google Scholar] [CrossRef]
- Melok, A.L.; Lee, L.H.; Yussof, S.A.M.; Chu, T. Green Tea Polyphenol Epigallocatechin-3-Gallate-Stearate Inhibits the Growth of Streptococcus Mutans: A Promising New Approach in Caries Prevention. Dent. J. 2018, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Mount, G.J.; Hume, W.R.; Ngo, H.C.; Mark, S. Wolff Preservation and Restoration of Tooth Structure: Third Edition, 3rd ed.; Wiley Blackwell: Hoboken, NJ, USA, 2016; pp. 1–328. ISBN 978-1118766590. [Google Scholar]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Mathur, M.R.; Listl, S.; Keller Celeste, R.; Kearns, C.; Benzian, H.; Allison, P. Oral Disease: A Global Public Health Challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, L.; Messer, L.B. Early Childhood Caries: An Update. Singap. Dent. J. 2004, 26, 21–29. [Google Scholar]
- Keyes, P.H. Present and Future Measures for Dental Caries Control. J. Am. Dent. Assoc. 1969, 79, 1395–1404. [Google Scholar] [CrossRef]
- Damle, S.G.; Yadav, R.; Garg, S.; Dhindsa, A.; Beniwal, V.; Loomba, A.; Chatterjee, S. Transmission of Mutans Streptococci in Mother-Child Pairs. Indian J. Med. Res. 2016, 144, 264–270. [Google Scholar] [CrossRef]
- Ravikumar, D.; Mahesh, R.; Ningthoujam, S.; Robindro, W.; Gayathri, R.; Priya, V.V. Genotypic Characterization of Streptococcus Mutans in Child-Mother Pair—A PCR Based Study. J. Oral Biol. Craniofacial Res. 2018, 8, 225–230. [Google Scholar] [CrossRef]
- Mattos-Graner, R.O.; Li, Y.; Caufield, P.W.; Duncan, M.; Smith, D.J. Genotypic Diversity of Mutans Streptococci in Brazilian Nursery Children Suggests Horizontal Transmission. J. Clin. Microbiol. 2001, 39, 2313–2316. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, R.J. Mutans Streptococci: Acquisition and Transmission. Pediatr. Dent. 2006, 28, 106–109. [Google Scholar] [CrossRef]
- Tedjosasongko, U.; Kozai, K. Initial Acquisition and Transmission of Mutans Streptococci in Children at Day Nursery. J. Dent. Child. 2002, 69, 284–288. [Google Scholar]
- Baca, P.; Castillo, A.-M.; Liébana, M.-J.; Castillo, F.; Martín-Platero, A.; Liébana, J. Horizontal Transmission of Streptococcus Mutans in Schoolchildren. Med. Oral Patol. Oral Cir. Bucal 2012, 17, 495–500. [Google Scholar] [CrossRef]
- Martini, A.M.; Moricz, B.S.; Woods, L.J.; Jones, B.D. Type IV Pili of Streptococcus Sanguinis Contribute to Pathogenesis in Experimental Infective Endocarditis. Microbiol. Spectr. 2021, 9, e01752-21. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Tao, H. Dental Caries and Systemic Diseases; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783662474501. [Google Scholar]
- Martini, A.M.; Moricz, B.S.; Ripperger, A.K.; Tran, P.M.; Sharp, M.E.; Forsythe, A.N.; Kulhankova, K.; Salgado-Pabón, W.; Jones, B.D. Association of Novel Streptococcus Sanguinis Virulence Factors with Pathogenesis in a Native Valve Infective Endocarditis Model. Front. Microbiol. 2020, 11, 10. [Google Scholar] [CrossRef]
- Aarabi, G.; Thomalla, G.; Heydecke, G.; Seedorf, U. Chronic Oral Infection: An Emerging Risk Factor of Cerebral Small Vessel Disease. Oral Dis. 2019, 25, 710–719. [Google Scholar] [CrossRef]
- Watt, R.G. Social Determinants of Oral Health Inequalities: Implications for Action. Community Dent. Oral Epidemiol. 2012, 40 (Suppl. S2), 44–48. [Google Scholar] [CrossRef]
- Jürgensen, N.; Petersen, P.E. Promoting Oral Health of Children through Schools—Results from a WHO Global Survey 2012. Community Dent. Health 2013, 30, 204–218. [Google Scholar] [CrossRef]
- Lofgren, E.T.; Halloran, M.E.; Rivers, C.M.; Drake, J.M.; Porco, T.C.; Lewis, B.; Yang, W.; Vespignani, A.; Shaman, J.; Eisenberg, J.N.S.; et al. Opinion: Mathematical Models: A Key Tool for Outbreak Response. Proc. Natl. Acad. Sci. USA 2014, 111, 18095–18096. [Google Scholar] [CrossRef]
- Brauer, F. Mathematical Epidemiology: Past, Present, and Future. Infect. Dis. Model. 2017, 2, 113–127. [Google Scholar] [CrossRef]
- Kermack, W.O.; McKendrick, A.G. A Contribution to the Mathematical Theory of Epidemics. Proc. Roy Soc. Lond. 1927, 45, 700–721. [Google Scholar] [CrossRef]
- Yip, K.C.M.; Huang, K.W.H.; Ho, E.W.Y.; Chan, W.K.; Lee, I.L.Y. Optimized Staff Allocation for Inpatient Phlebotomy and Electrocardiography Services via Mathematical Modelling in an Acute Regional and Teaching Hospital. Health Syst. 2017, 6, 102–111. [Google Scholar] [CrossRef]
- Panovska-Griffiths, J.; Kerr, C.C.; Waites, W.; Stuart, R.M. Mathematical Modeling as a Tool for Policy Decision Making: Applications to the COVID-19 Pandemic. In Handbook of Statistics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 291–326. [Google Scholar] [CrossRef]
- Cartocci, A.; Cevenini, G.; Barbini, P. A Compartment Modeling Approach to Reconstruct and Analyze Gender and Age-Grouped COVID-19 Italian Data for Decision-Making Strategies. J. Biomed. Inform. 2021, 118, 103793. [Google Scholar] [CrossRef]
- Zhang, P.; Feng, K.; Gong, Y.; Lee, J.; Lomonaco, S.; Zhao, L. Usage of Compartmental Models in Predicting COVID-19 Outbreaks. AAPS J. 2022, 24, 98. [Google Scholar] [CrossRef]
- Okuonghae, D.; Omame, A. Analysis of a Mathematical Model for COVID-19 Population Dynamics in Lagos, Nigeria. Chaos Solitons Fractals 2020, 139, 110032. [Google Scholar] [CrossRef] [PubMed]
- Sardar, T.; Rana, S.; Chattopadhyay, J. A Mathematical Model of Dengue Transmission with Memory. Commun. Nonlinear Sci. Numer. Simul. 2015, 22, 511–525. [Google Scholar] [CrossRef]
- Tresna, S.T.; Subiyanto; Supian, S. Mathematical Models for Typhoid Disease Transmission: A Systematic Literature Review. Mathematics 2022, 10, 2506. [Google Scholar] [CrossRef]
- Luo, J.; Wang, W.; Chen, H.; Fu, R. Bifurcations of a Mathematical Model for HIV Dynamics. J. Math. Anal. Appl. 2016, 434, 837–857. [Google Scholar] [CrossRef]
- Hasibuan, A.; Supriatna, A.K.; Rusyaman, E.; Biswas, H.A. Predator—Prey Model Considering Implicit Marine Reserved Area and Linear Function of Critical Biomass Level. Mathematics 2023, 11, 4015. [Google Scholar] [CrossRef]
- Cogan, N.G. Effects of Persister Formation on Bacterial Response to Dosing. J. Theor. Biol. 2006, 238, 694–703. [Google Scholar] [CrossRef]
- Tresna, S.T.; Anggriani, N.; Supriatna, A.K. Mathematical Model of Hcv Transmission with Treatment and Educational Effort. Commun. Math. Biol. Neurosci. 2022, 2022, 46. [Google Scholar] [CrossRef]
- Jing, X.; Huang, X.; Haapasalo, M.; Shen, Y.; Wang, Q. Modeling Oral Multispecies Biofilm Recovery after Antibacterial Treatment. Sci. Rep. 2019, 9, 804. [Google Scholar] [CrossRef]
- Kumar, P.; Govindaraj, V.; Erturk, V.S. A Novel Mathematical Model to Describe the Transmission Dynamics of Tooth Cavity in the Human Population. Chaos Solitons Fractals 2022, 161, 112370. [Google Scholar] [CrossRef]
- Stovold, E.; Beecher, D.; Foxlee, R.; Noel-Storr, A. Study Flow Diagrams in Cochrane Systematic Review Updates: An Adapted PRISMA Flow Diagram. Syst. Rev. 2014, 3, 54. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Patil, S.; Kulkarni, V.; Bhise, A. Algorithmic Analysis for Dental Caries Detection Using an Adaptive Neural Network Architecture. Heliyon 2019, 5, e01579. [Google Scholar] [CrossRef]
- Askar, H.; Krois, J.; Rohrer, C.; Mertens, S.; Elhennawy, K.; Ottolenghi, L.; Mazur, M.; Paris, S.; Schwendicke, F. Detecting White Spot Lesions on Dental Photography Using Deep Learning: A Pilot Study. J. Dent. 2021, 107, 103615. [Google Scholar] [CrossRef]
- Peres, M.A.; Ju, X.; Mittinty, M.; Spencer, A.J.; Do, L.G. Modifiable Factors Explain Socioeconomic Inequalities in Children’s Dental Caries. J. Dent. Res. 2019, 98, 1211–1218. [Google Scholar] [CrossRef]
- Head, D.; Marsh, P.D.; Devine, D.A.; Tenuta, L.M.A. In Silico Modeling of Hyposalivation and Biofilm Dysbiosis in Root Caries. J. Dent. Res. 2021, 100, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Tamanai-Shacoori, Z.; Bronsard, J.; Ginguené, F.; Meuric, V.; Mahé, F.; Bonnaure-Mallet, M. A New Mathematical Model of Bacterial Interactions in Two-Species Oral Biofilms. PLoS ONE 2017, 12, e0173153. [Google Scholar] [CrossRef]
- Rene, L.; Fabregas, I.; Rubinstein, J. A Mathematical Model for the Progression of Dental Caries. Math. Med. Biol. 2014, 31, 319–337. [Google Scholar] [CrossRef]
- Scott, D.C.; Coggan, J.W.; Cruze, C.A.; He, T.; Johnson, R.D. Topical Oral Cavity Pharmacokinetic Modeling of a Stannous Fluoride Dentifrice: An Unusual Two Compartment Model. J. Pharm. Sci. 2012, 101, 2271–2280. [Google Scholar] [CrossRef]
- Martínez, E.A.; Giulietti, M.; de Almeida e Silva, J.B.; Derenzo, S. Kinetics of the Xylitol Crystallization in Hydro-Alcoholic Solution. Chem. Eng. Process. Process Intensif. 2008, 47, 2157–2162. [Google Scholar] [CrossRef]
- Chen, C.W.; Ou-Yang, C.C.; Yeh, C.W. Synthesis of Galactooligosaccharides and Transgalactosylation Modeling in Reverse Micelles. Enzyme Microb. Technol. 2003, 33, 497–507. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, J.; De La Fuente-Núñez, C.; Wang, Z.; Hancock, R.E.W.; Roberts, C.R.; Ma, J.; Li, J.; Haapasalo, M.; Wang, Q. Experimental and Theoretical Investigation of Multispecies Oral Biofilm Resistance to Chlorhexidine Treatment. Sci. Rep. 2016, 6, 27537. [Google Scholar] [CrossRef]
- Brejning, J.; Jespersen, L. Protein Expression during Lag Phase and Growth Initiation in Saccharomyces Cerevisiae. Int. J. Food Microbiol. 2002, 75, 27–38. [Google Scholar] [CrossRef]
- Larsen, N.; Boye, M.; Siegumfeldt, H.; Jakobsen, M. Differential Expression of Proteins and Genes in the Lag Phase of Lactococcus Lactis Subsp. Lactis Grown in Synthetic Medium and Reconstituted Skim Milk. Appl. Environ. Microbiol. 2006, 72, 1173–1179. [Google Scholar] [CrossRef]
- Gefen, O.; Fridman, O.; Ronin, I.; Balaban, N.Q. Direct Observation of Single Stationary-Phase Bacteria Reveals a Surprisingly Long Period of Constant Protein Production Activity. Proc. Natl. Acad. Sci. USA 2014, 111, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Chenicheri, S.; Usha, R.; Ramachandran, R.; Thomas, V.; Wood, A. Insight into Oral Biofilm: Primary, Secondary and Residual Caries and Phyto-Challenged Solutions. Open Dent. J. 2017, 11, 312–333. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Tay, F.R.; Niu, L.N.; Chen, J.H. Advancing Antimicrobial Strategies for Managing Oral Biofilm Infections. Int. J. Oral Sci. 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Ruff, R.R.; Saxena, D.; Niederman, R. School-Based Caries Prevention and Longitudinal Trends in Untreated Decay: An Updated Analysis with Markov Chains. BMC Res. Notes 2020, 13, 25. [Google Scholar] [CrossRef]
- Shen, C.; Rao, P.V.; Batich, C.D.; Moorhead, J.; Yan, J. Stochastic Modeling of Controlled Release from Poly-Styrene-Co-4-Vinylpyridine Microspheres. J. Control. Release 1994, 32, 139–146. [Google Scholar] [CrossRef]
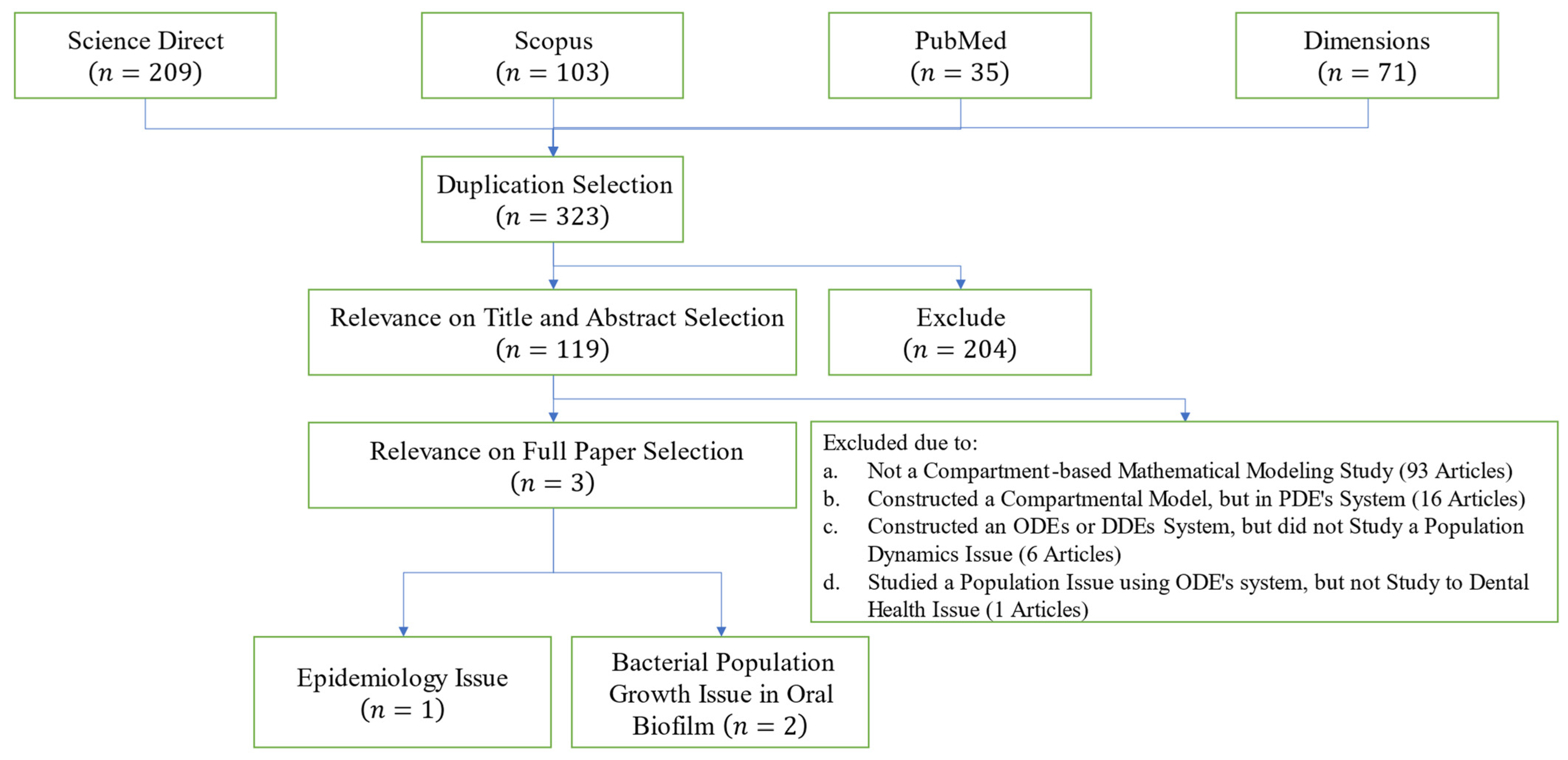
| Parameter | Definition |
|---|---|
| The transition rate from susceptible to persister portion. | |
| The transition rate from persister to susceptible portion. | |
| The natural death rate of susceptible bacteria. | |
| The death rate of susceptible bacteria is due to the application of antimicrobial agents. | |
| The persister bacteria death rate is due to the application of antimicrobial agents. | |
| The carrying capacity for susceptible bacteria. | |
| The carrying capacity for persister bacteria. | |
| The rate of half-saturation for Hill-type reactive kinetics. | |
| The rate of maximum breakdown for dead bacteria. | |
| The rate of half-saturation for Hill-type switch function. | |
| The rate of maximum EPS production. | |
| The rate of half-saturation for Hill functions. | |
| The rate of maximum consumption for nutrient and antimicrobial agents. | |
| The rate of maximum production for quorum sensing. | |
| The rate of maximum production for growth factors. | |
| The rate of half saturation. | |
| The rate of antimicrobial agent natural decay. | |
| The carrying capacity for quorum sensing. | |
| The carrying capacity for growth factor. |
| Parameter | Definition |
|---|---|
| The live cells proliferation rate. | |
| The constant of Hill-function. | |
| The live cell natural death rate. | |
| The live cell death rate is due to the application of an antibacterial agent. | |
| The degradation rate of dead bacteria. | |
| The rate of half-saturation for Hill-type switch function. | |
| The EPS production rate is due to live bacteria. | |
| The carrying capacity for the EPS portion. | |
| The killing rate of the bacteria by antibacterial agents. | |
| The degradation rate of antibacterial agent. | |
| The growth rate of quorum sensing molecules. | |
| The growth rate of growth factors. | |
| The carrying capacity for quorum sensing molecules. | |
| The carrying capacity of growth factors. | |
| The relative diffusivity of EPS. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tresna, S.T.; Anggriani, N.; Napitupulu, H.; Ahmad, W.M.A.W. Deterministic Modeling of the Issue of Dental Caries and Oral Bacterial Growth: A Brief Review. Mathematics 2024, 12, 2218. https://doi.org/10.3390/math12142218
Tresna ST, Anggriani N, Napitupulu H, Ahmad WMAW. Deterministic Modeling of the Issue of Dental Caries and Oral Bacterial Growth: A Brief Review. Mathematics. 2024; 12(14):2218. https://doi.org/10.3390/math12142218
Chicago/Turabian StyleTresna, Sanubari Tansah, Nursanti Anggriani, Herlina Napitupulu, and Wan Muhamad Amir W. Ahmad. 2024. "Deterministic Modeling of the Issue of Dental Caries and Oral Bacterial Growth: A Brief Review" Mathematics 12, no. 14: 2218. https://doi.org/10.3390/math12142218







