Two-Age-Structured COVID-19 Epidemic Model: Estimation of Virulence Parameters through New Data Incorporation
Abstract
:1. Introduction
2. Incorporation of New Data into the Methodology of [23]
2.1. Retrospect
- (a)
- A strict national lockdown rule (scenario a, from 9 March 2020 to 28 April 2020) that removes social contacts in workplaces, schools, markets, and other public areas;
- (b–d)
- A weakened feedback social distancing and contact reduction intervention, which is composed of a weakened lockdown phase (scenario b, from 7 May 2020 to 3 June 2020), a low-distancing phase (scenario c, from 9 June 2020 to 8 September 2020), and a low-distancing + workplace/school-contacts re-activation phase (scenario d, from 15 September 2020 to 27 October 2020), with a progressive release of the population back to their daily routine;
- (e)
- A coordinated intermittent regional action (scenario e, from 7 November 2020 to 29 December 2020), where social distancing measures are put in place or relaxed independently by each region based on the ratio between hospitalized individuals and the specific regional health system capacity;
- (f)
- Direct mRNA-vaccination of subjects—especially the elderly—(scenario f, from 5 January 2021 to 12 May 2021) at highest risk for severe outcomes, along with Vaxzevria vaccination of young subjects within specific occupational categories (to protect subjects at highest risk for severe outcomes indirectly).
2.2. The New Three Subsequent Scenarios
- (G)
- A mitigated coordinated intermittent regional action in conjunction with the II vaccination phase (scenario G, from 19 May 2021 to 14 July 2021): during this phase, only people with a COVID-19 vaccination certificate (Green Pass) can leave their city of residence, and schools that were teaching online until scenario (f) resume normal operation (in presence);
- (H)
- A super-attenuated coordinated intermittent regional action, without mobility restrictions, where even large events (such as sports competitions, congresses, fairs, private parties) can be held in the regions with a sufficiently low number of cases, in conjunction with the II vaccination phase (scenario H, from 21 July 2021 to 22 September 2021);
- (I)
- The last step towards normality (normal reopening of schools, with the vaccination certificate only mandatory for teachers, complete lifting of the obligation to use face masks, reopening of entertainment activities such as discos and ballrooms) and start of the III vaccination phase (scenario I, from 29 September 2021 to 10 November 2021).
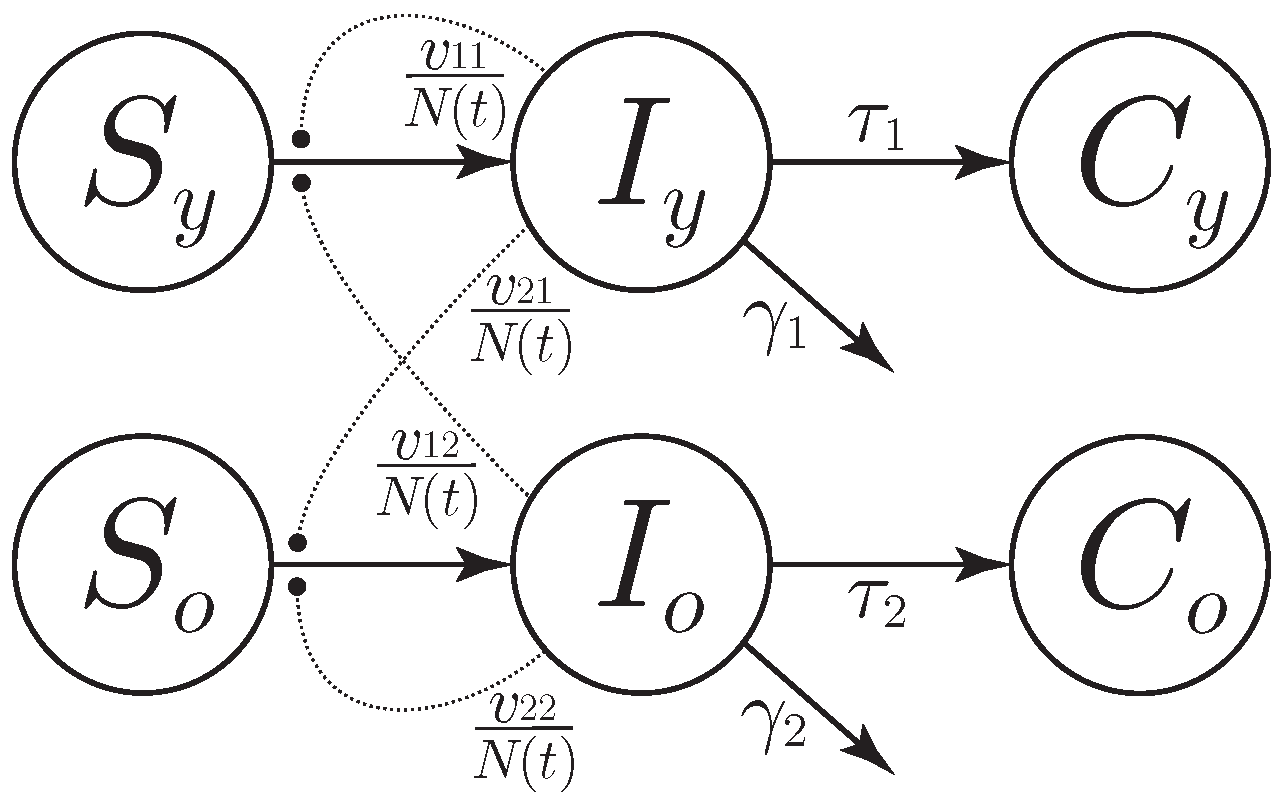
3. Model and Simplifying Assumptions
- Aging, reproduction, and natural death have negligible effects so that the variation in the number of susceptible in the time window only depends on infection.
- Infected people cannot be infected another time in the time window.
- Effect of vaccines against infection is negligible in the time window.
- t is the time, measured in days;
- , are the numbers of susceptible and infected for the two age classes, respectively;
- and are the numbers of reported cases for the two age classes;
- is the number of persons who are not quarantined, hospitalized, or dead at time t.
4. Estimation of Model Parameters
- , characterizing the intra-juvenile virulence;
- , characterizing the juvenile–elder virulence;
- , characterizing the elder–juvenile virulence;
- , characterizing the intra-elder virulence;
- , denoting the average time for disease identification in young subjects;
- , denoting the average time for disease identification in old subjects;
- , representing the young subjects infected at the beginning of the scenario time window;
- , representing the old subjects infected at the beginning of the scenario time window.
- The juvenile–elder virulence—when compared with [23]—will appear to be smaller in scenarios c (namely, low-feedback social distancing and contact reduction intervention) and e (namely, decreased social contacts in schools at a national level and social distancing measures put in place or relaxed independently by each region) to identify successfully, within c and e, a sort of decoupling (that is lost in all the other scenarios) between the two age classes and recognize the benefits, within e, of a reduction in the social contacts in schools at a national level.
- The reasonable punctual reduction—when compared with [23]—of the intra-elder virulence during the summer of scenario c (now of the same magnitude as the intra-juvenile virulence) will preserve the already established trend of such a parameter over the scenarios.
- The increase—when compared with [23]—of the average time for disease identification in old subjects within scenario c better shapes the behavior of such a parameter by consistently moving to scenario d (re-activation of social contacts in workplaces and schools) the moment in which the elderly paid a higher level of attention to symptoms while preserving the remaining, already established trend over the scenarios.
5. Discussion
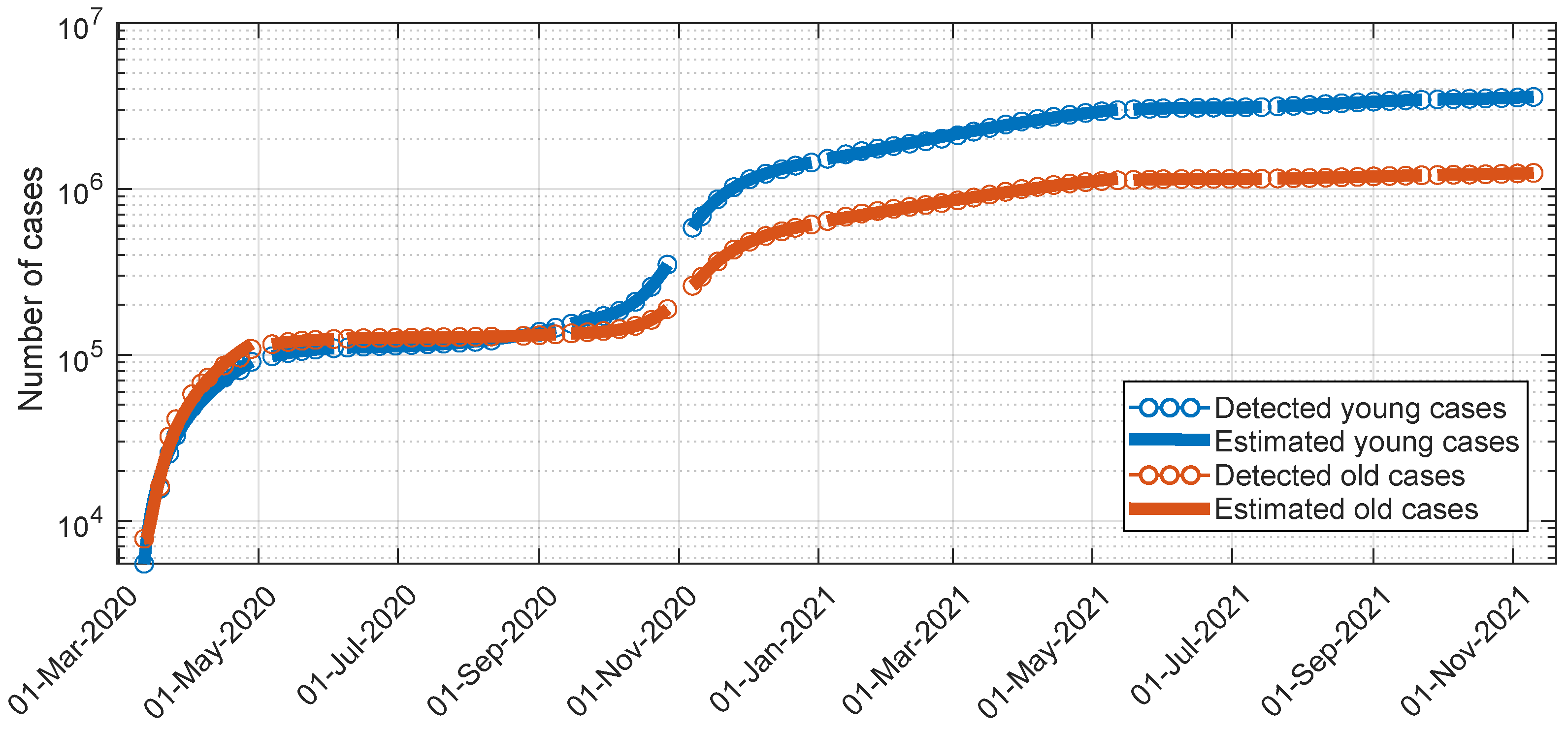
- All the estimates corresponding to the different scenarios, including the estimated , (initial young subjects infected; initial old subjects infected), allow the estimated profile to reproduce the actual one along the different scenarios satisfactorily, as shown in Figure 2.
- The number of susceptible individuals in both age groups continuously decreases in all the scenarios starting from scenario G (including additional scenario J), as an effect of the vaccination campaign starting within scenario f and continuing within G. However, a non-drastic reduction in such a number seems to confirm that the vaccination action principally protects against severe symptomatology rather than giving total immunity [24].
- Starting from scenario G, again as an effect of the vaccination campaign starting within scenario f and continuing within G, the juvenile–elder and the intra-elder virulences exhibited a large reduction. The major strength of the vaccination action for the elderly, however, allows for relatively large elder–juvenile virulence values.
- The average time for disease identification in young subjects in all the scenarios, a–f and G–I, ranges from 1 to 9 days across the scenarios. Notably, scenarios b–c have an average time of approximately 5–7 days, whereas new scenario G has a slightly longer time of about 8 days. This variation can be attributed to the fact that, after the lockdown period and related concerns, young individuals tended to pay less attention to their symptoms, particularly in scenarios b and c (covering the summer period, from 7 May 2020 to 8 September 2020). The same phenomenon was observed in new scenario G, which occurred from 19 May 2021 to 14 July 2021. This period coincided with weakened, intermittent regional actions and the second vaccination stage (booster), along with no festivities, like Christmas and Easter. In contrast, scenarios H–I exhibited a substantial reduction in the average time for disease identification among young subjects, possibly due to more immediate reliance on testing in the presence of: (i) typical entertainment habits during the summer that saw a large increase in travel (compared with previous analogous scenario c); (ii) normal reopening of schools and entertainment activities such as discos and ballrooms. These scenarios also witnessed an increase in intra-juvenile virulence (, ) compared with G ().
- Even the average time for disease identification in old subjects in all the scenarios, a–f and G–I, varies from 1 to 9 days, with less than 4 days occurring in scenarios d–f (in which the elderly paid a higher level of attention to symptoms) and H–I (corresponding to an increase of the intra-juvenile and elder–juvenile virulences).
- The elder–juvenile virulences exhibit a specific increasing trend starting from scenario e (namely, coordinated intermittent regional action), except for scenario G (namely, weakened intermittent regional actions, second vaccination stage (booster), and, mainly, no festivities, like Christmas and Easter), in which all the virulences show a relatively large reduction. The subsequent increase of intra-juvenile and elder–juvenile virulences seems to suggest a sort of greater decoupling of social habits between young subjects and old ones, and allows us to pose a question while recognizing scenario G as the most favourable one in terms of virulence values: what would have happened if, after the first vaccination campaign, the super-attenuated coordinated intermittent regional actions and the last step towards normality had been delayed until after the summer vacations, and actions like the ones in a–b, e had been performed to reduce the intra-juvenile virulence?
6. One and Two Years Later
- (J)
- from 1 March 2023 to 3 May 2023
| Time Window J from 1 March 2023 to 3 May 2023 | |||||
|---|---|---|---|---|---|
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, M.; Jiang, J. COVID-19 and social distancing. J. Public Health 2020, 30, 259–261. [Google Scholar] [CrossRef]
- Della Rossa, F.; Salzano, D.; Di Meglio, A.; De Lellis, F.; Coraggio, M.; Calabrese, C.; Guarino, A.; Cardona-Rivera, R.; De Lellis, P.; Liuzza, D.; et al. A network model of Italy shows that intermittent regional strategies can alleviate the COVID-19 epidemic. Nat. Commun. 2020, 11, 5106. [Google Scholar] [CrossRef]
- Kretzschmar, M.E.; Rozhnova, G.; Bootsma, M.C.; van Boven, M.; van de Wijgert, J.H.; Bonten, M.J. Impact of delays on effectiveness of contact tracing strategies for COVID-19: A modelling study. Lancet Public Health 2020, 5, e452–e459. [Google Scholar] [CrossRef] [PubMed]
- Keeling, M.J.; Hollingsworth, T.D.; Read, J.M. Efficacy of contact tracing for the containment of the 2019 novel coronavirus (COVID-19). J. Epidemiol. Community Health 2020, 74, 861–866. [Google Scholar] [CrossRef]
- Tupper, P.; Otto, S.P.; Colijn, C. Fundamental limitations of contact tracing for COVID-19. Facets 2021, 6, 1993–2001. [Google Scholar] [CrossRef]
- Italia, M.; Della Rossa, F.; Dercole, F. Model-informed health and socio-economic benefits of enhancing global equity and access to COVID-19 vaccines. Sci. Rep. 2023, 13, 21707. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, A.J.; Klepac, P.; Conlan, A.J.; Kissler, S.M.; Tang, M.L.; Fry, H.; Gog, J.R.; Edmunds, W.J.; Emery, J.C.; Medley, G.; et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: A mathematical modelling study. Lancet Infect. Dis. 2020, 20, 1151–1160. [Google Scholar] [CrossRef]
- Musa, S.S.; Qureshi, S.; Zhao, S.; Yusuf, A.; Mustapha, U.T.; He, D. Mathematical modeling of COVID-19 epidemic with effect of awareness programs. Infect. Dis. Model. 2021, 6, 448–460. [Google Scholar] [CrossRef]
- Ancona, C.; Lo Iudice, F.; Garofalo, F.; De Lellis, P. A model-based opinion dynamics approach to tackle vaccine hesitancy. Sci. Rep. 2022, 12, 11835. [Google Scholar] [CrossRef] [PubMed]
- Maji, C.; Al Basir, F.; Mukherjee, D.; Ravichandran, C.; Nisar, K. COVID-19 propagation and the usefulness of awareness-based control measures: A mathematical model with delay. AIMs Math 2022, 7, 12091–12105. [Google Scholar] [CrossRef]
- Thunström, L.; Newbold, S.C.; Finnoff, D.; Ashworth, M.; Shogren, J.F. The benefits and costs of using social distancing to flatten the curve for COVID-19. J. Benefit-Cost Anal. 2020, 11, 179–195. [Google Scholar] [CrossRef]
- Moosa, I.A. The effectiveness of social distancing in containing COVID-19. Appl. Econ. 2020, 52, 6292–6305. [Google Scholar] [CrossRef]
- Silva, P.C.; Batista, P.V.; Lima, H.S.; Alves, M.A.; Guimarães, F.G.; Silva, R.C. COVID-ABS: An agent-based model of COVID-19 epidemic to simulate health and economic effects of social distancing interventions. Chaos Solitons Fractals 2020, 139, 110088. [Google Scholar] [CrossRef]
- Childs, M.L.; Kain, M.P.; Kirk, D.; Harris, M.; Couper, L.; Nova, N.; Delwel, I.; Ritchie, J.; Mordecai, E.A. The impact of long-term non-pharmaceutical interventions on COVID-19 epidemic dynamics and control. MedRxiv 2020. [Google Scholar] [CrossRef]
- Goldsztejn, U.; Schwartzman, D.; Nehorai, A. Public policy and economic dynamics of COVID-19 spread: A mathematical modeling study. PLoS ONE 2020, 15, e0244174. [Google Scholar] [CrossRef] [PubMed]
- Childs, M.L.; Kain, M.P.; Harris, M.J.; Kirk, D.; Couper, L.; Nova, N.; Delwel, I.; Ritchie, J.; Becker, A.D.; Mordecai, E.A. The impact of long-term non-pharmaceutical interventions on COVID-19 epidemic dynamics and control: The value and limitations of early models. Proc. R. Soc. B 2021, 288, 20210811. [Google Scholar] [CrossRef] [PubMed]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. Jama 2020, 323, 1775–1776. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yang, W.; Lai, J.; Hou, J.; Lin, W. Vaccination and quarantine effect on COVID-19 transmission dynamics incorporating Chinese-spring-festival travel rush: Modeling and simulations. Bull. Math. Biol. 2022, 84, 30. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, X.; Deng, M.; Liu, P.; Li, F.; Xie, N.; Pang, Y.; Zhang, X.; Luo, W.; Peng, Y.; et al. Epidemiology of COVID-19 in older persons, Wuhan, China. Age Ageing 2020, 49, 706–712. [Google Scholar] [CrossRef]
- Balabdaoui, F.; Mohr, D. Age-stratified discrete compartment model of the COVID-19 epidemic with application to Switzerland. Sci. Rep. 2020, 10, 21306. [Google Scholar] [CrossRef] [PubMed]
- Bentout, S.; Tridane, A.; Djilali, S.; Touaoula, T.M. Age-structured modeling of COVID-19 epidemic in the USA, UAE and Algeria. Alex. Eng. J. 2021, 60, 401–411. [Google Scholar] [CrossRef]
- Verrelli, C.M.; Della Rossa, F. Two-age-structured COVID-19 epidemic model: Estimation of virulence parameters to interpret effects of national and regional feedback interventions and vaccination. Mathematics 2021, 9, 2414. [Google Scholar] [CrossRef]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccines Immunother. 2022, 18, 2027160. [Google Scholar] [CrossRef]

| Scenario a | |||||
| 0.7532 | 0.0000 | 1.3718 | 0.0001 | 0.349 | 0.2372 |
| Scenario b | |||||
| 0.13 | 0.1928 | ||||
| Scenario c | |||||
| 0.0018 | 0.4953 | 0.1906 | 0.2327 | ||
| Scenario d | |||||
| 0.24 | 0.32 | ||||
| Scenario e | |||||
| 0.0256 | 0.3023 | 0.5258 | |||
| Scenario f | |||||
| 0.4465 | 0.5754 | ||||
| Scenario G | |||||
| 0.1203 | 0.1134 | ||||
| Scenario H | |||||
| 0.8976 | 0.5847 | ||||
| Scenario I | |||||
| Scenario a | |||
| Scenario b | |||
| Scenario c | |||
| Scenario d | |||
| Scenario e | |||
| Scenario f | |||
| Scenario G | |||
| Scenario H | |||
| Scenario I | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verrelli, C.M.; Della Rossa, F. Two-Age-Structured COVID-19 Epidemic Model: Estimation of Virulence Parameters through New Data Incorporation. Mathematics 2024, 12, 825. https://doi.org/10.3390/math12060825
Verrelli CM, Della Rossa F. Two-Age-Structured COVID-19 Epidemic Model: Estimation of Virulence Parameters through New Data Incorporation. Mathematics. 2024; 12(6):825. https://doi.org/10.3390/math12060825
Chicago/Turabian StyleVerrelli, Cristiano Maria, and Fabio Della Rossa. 2024. "Two-Age-Structured COVID-19 Epidemic Model: Estimation of Virulence Parameters through New Data Incorporation" Mathematics 12, no. 6: 825. https://doi.org/10.3390/math12060825
APA StyleVerrelli, C. M., & Della Rossa, F. (2024). Two-Age-Structured COVID-19 Epidemic Model: Estimation of Virulence Parameters through New Data Incorporation. Mathematics, 12(6), 825. https://doi.org/10.3390/math12060825







