Dynamics of the Diphtheria Epidemic in Nigeria: Insights from the Kano State Outbreak Data
Abstract
1. Introduction
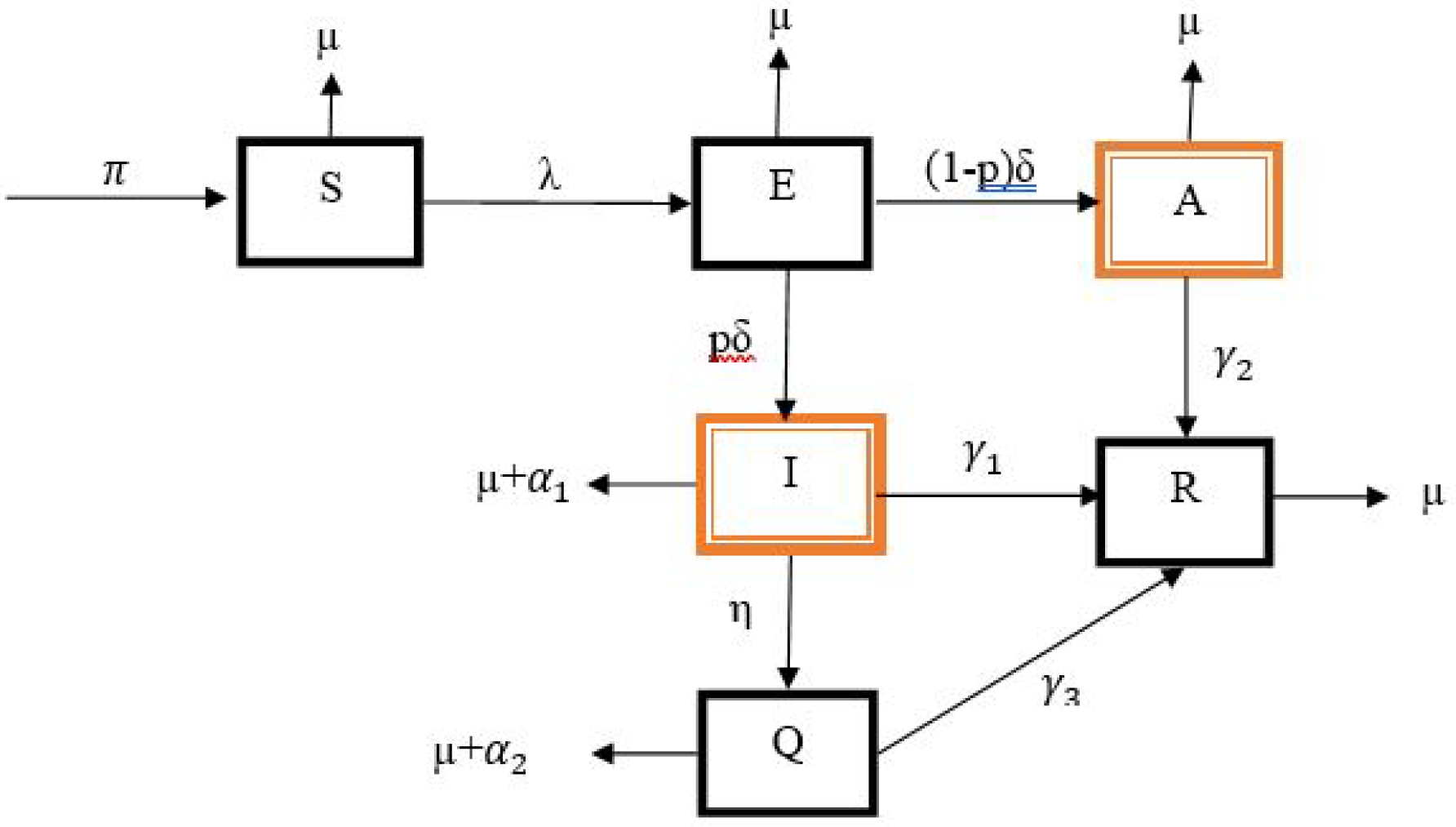
2. Model of Diphtheria Transmission
- (i)
- Susceptible individuals have equal chances of contracting the diphtheria disease (i.e., homogeneous mixing).
- (ii)
- The transmission rate of isolated humans is negligible, so only asymptomatic and infected humans transmit the disease.
- (iii)
- Force of infection is via frequency dependence incidence,where modeled the public enlightenment campaign as in [33].
- (iv)
- Only infected and isolated infected humans die due to the disease.
3. Analysis of the Model
3.1. Mathematical Well Posedness
3.2. Diphtheria Infection-Free Equilibrium (DIFE)
3.3. Endemic Equilibrium Point
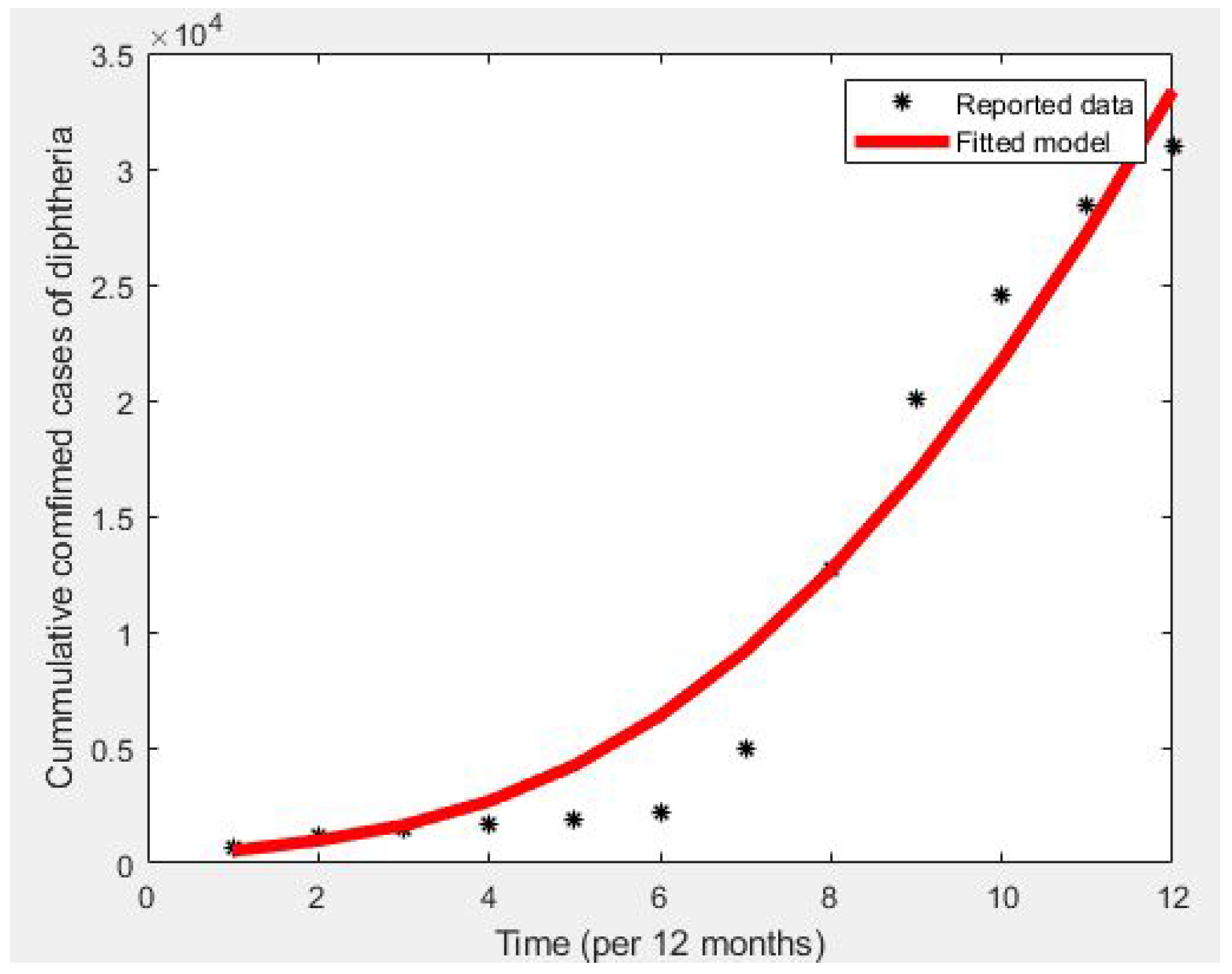
4. Model Fitting and Parameter Estimation
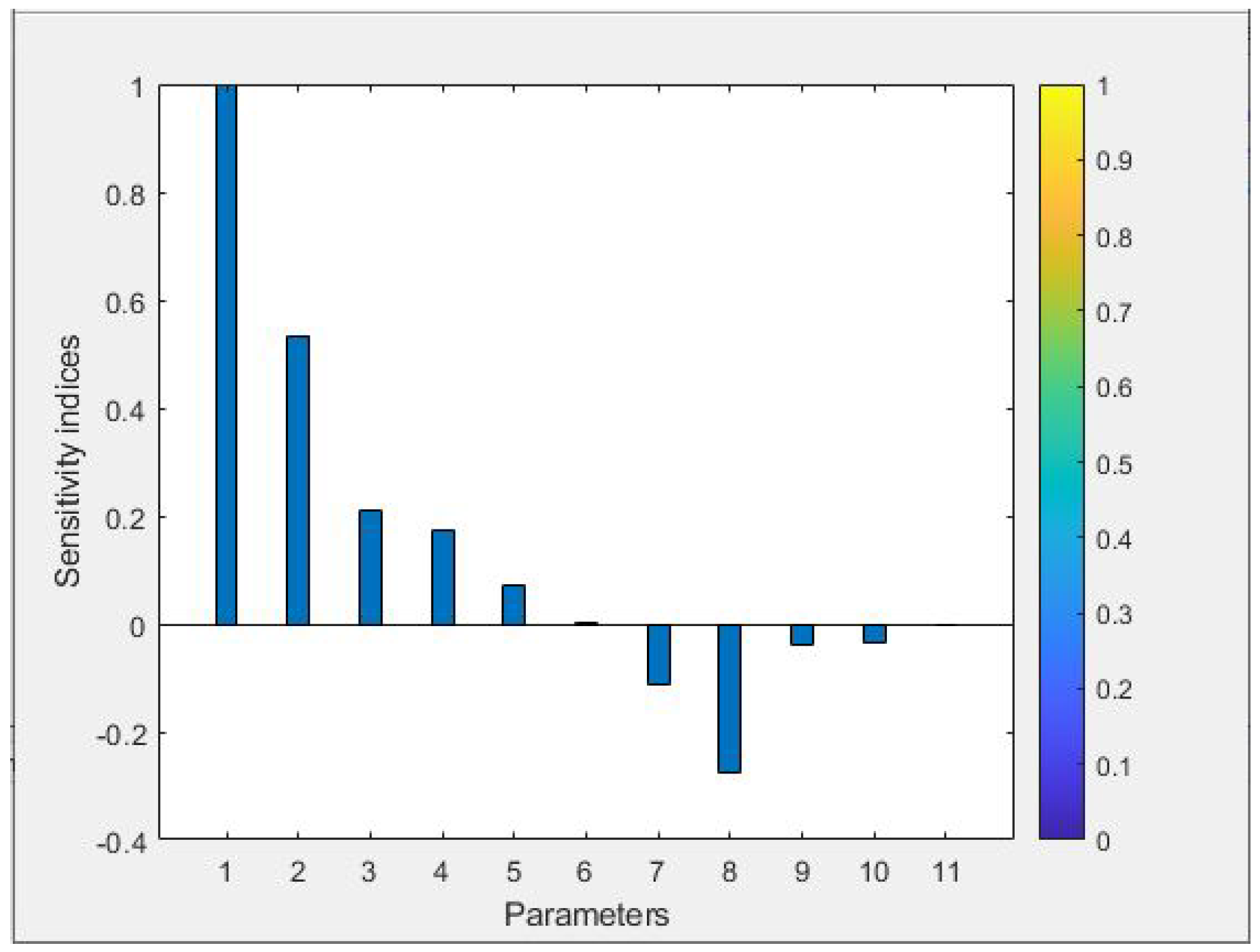
4.1. Most Sensitive Parameters
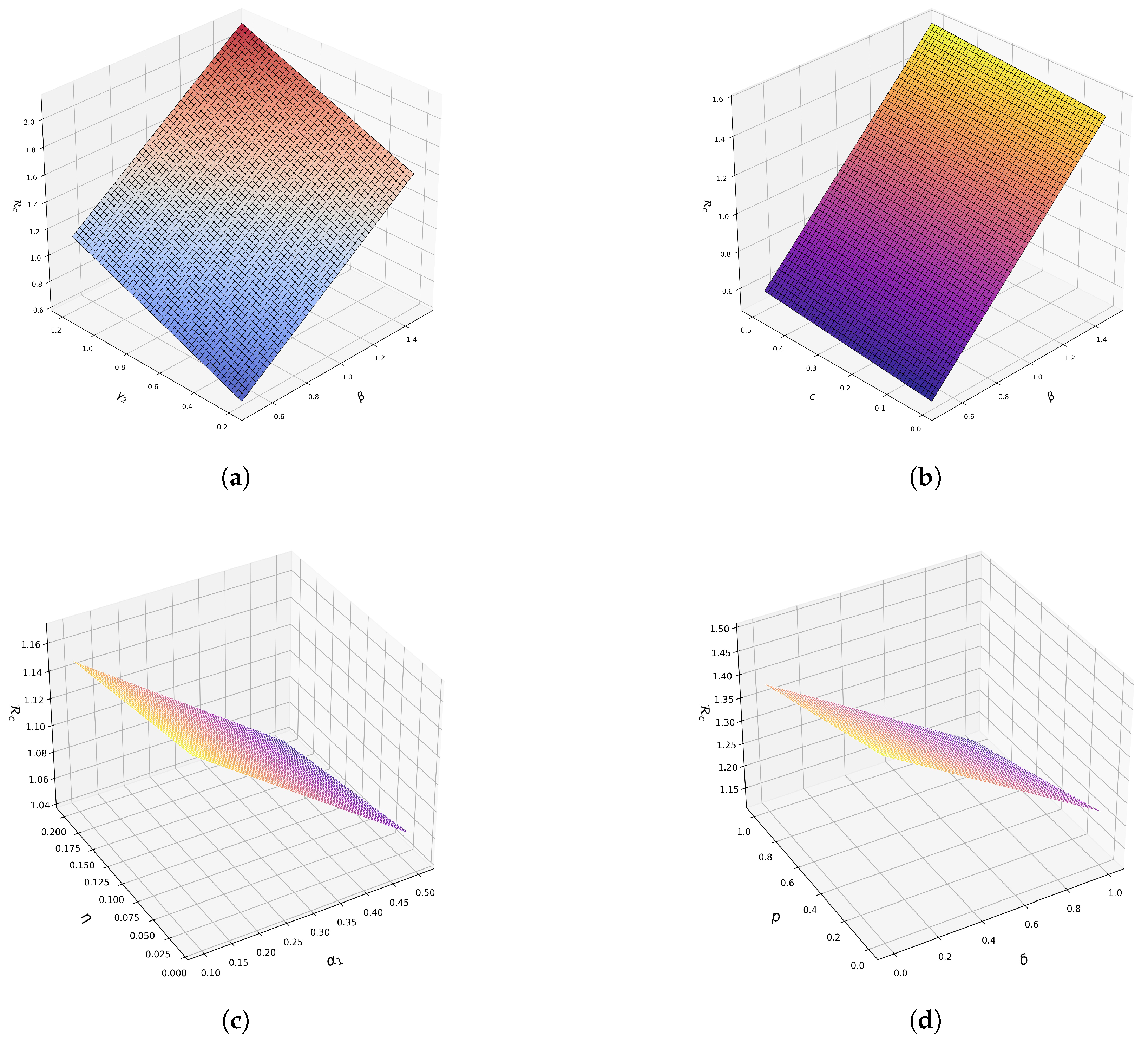
4.2. Surface Plots of Some Model Parameters with
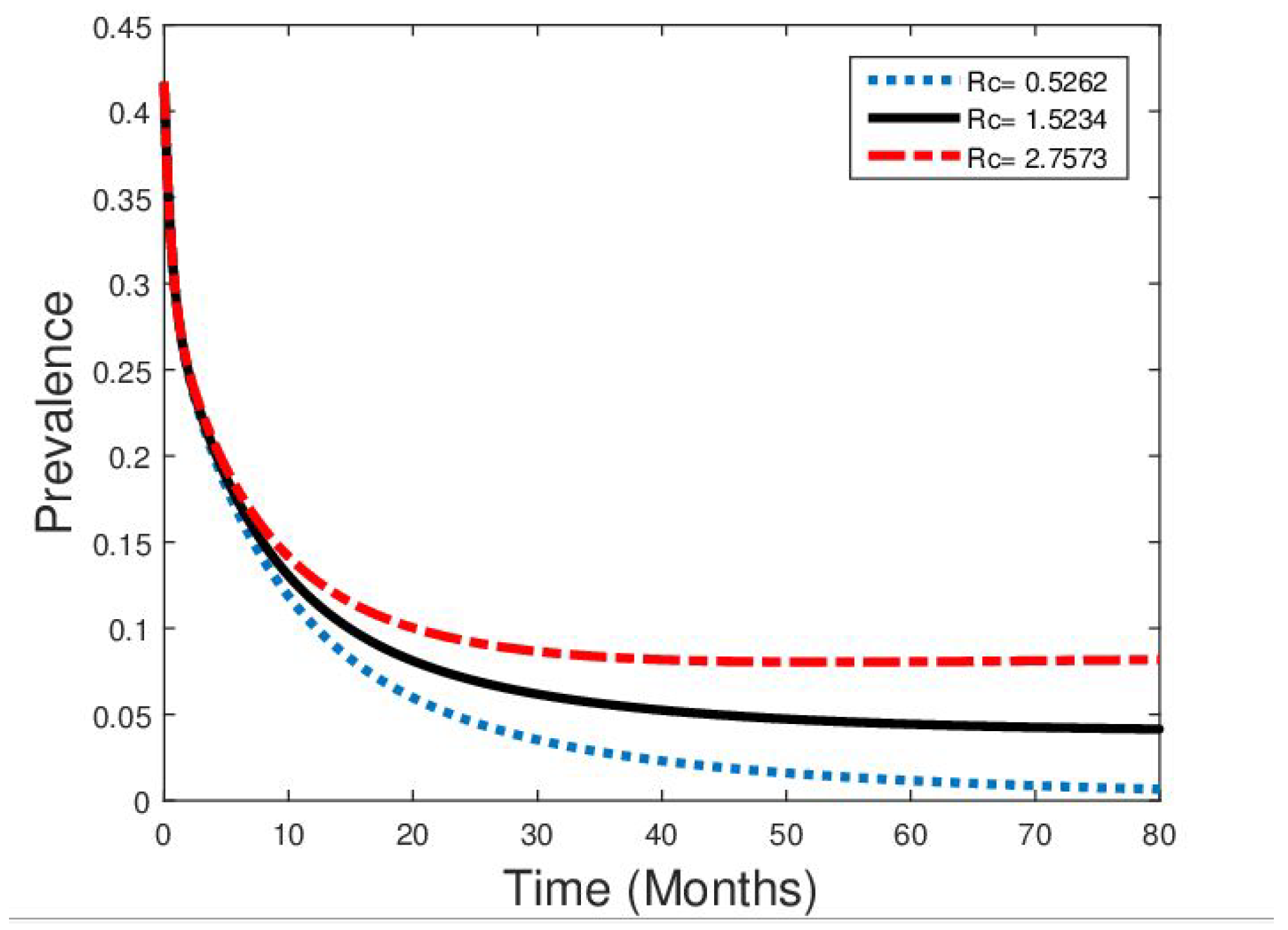
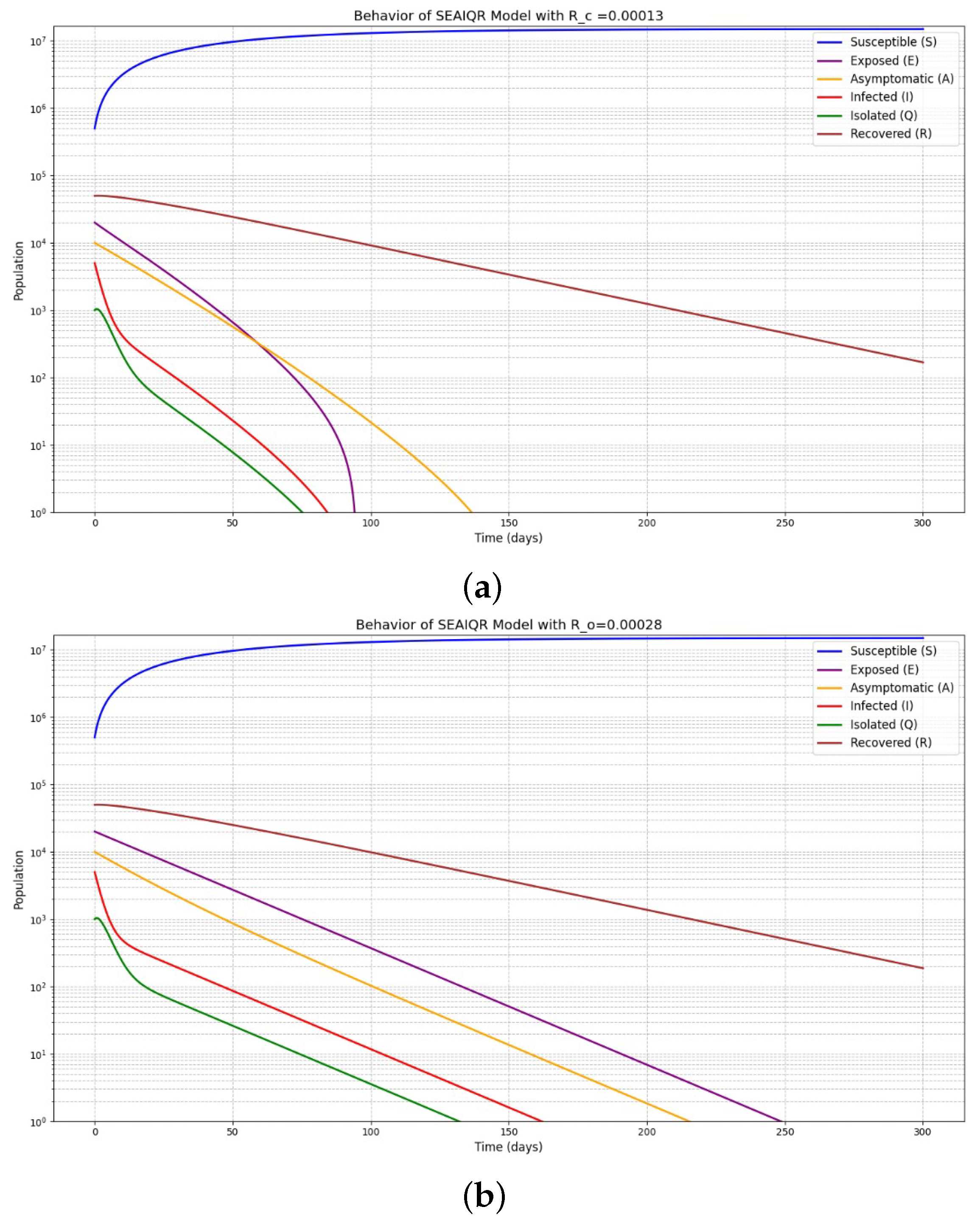
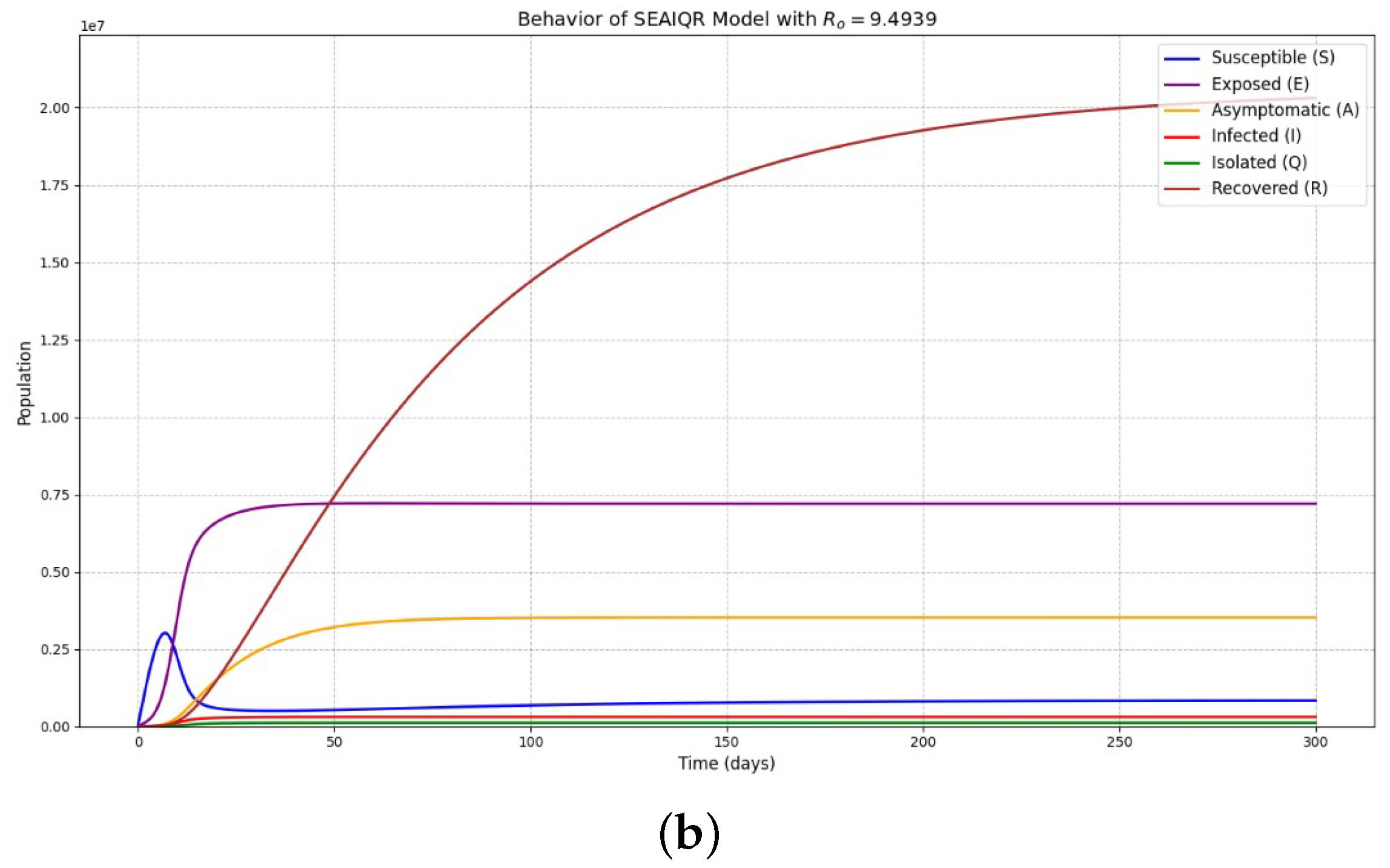
5. Numerical Simulations
6. Conclusions
- i.
- The diphtheria infection-free equilibrium of model (1) exhibits both local and global asymptotic stability, indicating that the disease will be eradicated if .
- ii.
- The existence of an endemic equilibrium in the presence of diphtheria infection has been confirmed. This equilibrium is shown to be globally asymptotically stable when implying that diphtheria will persist as long as asymptomatic or infected individuals continue to generate new cases within the population.
- iii.
- A nonlinear least squares curve-fitting approach, utilizing MATLAB’s version 2023 optimization toolbox, was employed to fit the model to the data of Kano State confirmed cases from January to December 2023.
- iv.
- Sensitivity analysis identified the effective contact rate as the most sensitive model parameter. Additionally, is associated with the public enlightenment campaign c and its efficacy . Thus, prioritizing and is crucial for effectively managing diphtheria outbreaks.
- vi.
- We show via sensitivity analysis that a public enlightenment campaign would have a positive impact on the community if the efficacy of the campaign is high. It also underscores that isolation of infected individuals would have a positive impact when the proportion of exposed individuals becoming infectious is large and their associated progression rate to the infected class is high. This is because the higher the proportion of exposed subpopulation moving to the infectious class, the lower the number of asymptomatic individuals and the higher the isolation rate. On a similar note, the numerical simulation results reveal that combining the public enlightenment campaign and isolation of infected individuals is the best strategy to contain the spread of diphtheria.
- v.
- Numerical simulations of model (1) demonstrated that increasing the level of an effective public enlightenment campaign significantly reduces the prevalence of diphtheria in the population.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Available online: http://www.cdc.gov (accessed on 30 December 2023).
- Nigerian Center for Disease Control and Prevention. Available online: www.ncdc.gov.ng (accessed on 30 December 2023).
- Islam, Z. Developing a Mathematical Model for Optimal Cost-Effective Treatment Strategies Applied to a Diphtheria Outbreak. 2020. Available online: http://lib.buet.ac.bd:8080/xmlui/handle/123456789/5873 (accessed on 30 December 2023).
- Momani, S.; Batiha, I.M.; Bendib, I.; Al-Nana, A.; Ouannas, A.; Dalah, M. On finite-time stability of some COVID-19 models using fractional discrete calculus. Comput. Methods Programs Biomed. Update 2025, 7, 100–188. [Google Scholar]
- Momani, S.; Batiha, I.M.; Hijazi, M.S.; Bendib, I.; Ouannas, A.; Anakira, N. Fractional-Order SEIR Model for COVID-19: Finite-Time Stability Analysis and Numerical Validation. Int. J. Neutrosophic Sci. (IJNS) 2025, 26, 266–282. [Google Scholar]
- Oyeyemi, B.O.; Suleiman, A.O.; Suleiman, B.M.; Ajetomobi, B.M.; Ibrahim, A. A report of two clusters of diphtheria outbreak involving three contiguous Local government areas in Katsina State. Abstract 013 presented at Annual General and Scientific conference of the Paediatric Association of Nigeria. Niger. J. Paediatr. 2011, 38, 10–64. [Google Scholar]
- Sadoh, A.E.; Okhakhu, A.; Omuemu, V.; Lofor PV, O.; Oviawe, O. Diphtheria in Nigeria: Is there resurgence? Ann. Biomed. Sci. 2009, 8. [Google Scholar] [CrossRef]
- Blythe, S.P. Towards a Unified Theory of Mixing and Pair Formation; Department of Rural Sociology [New York State College of Agriculture and Life Sciences]: New York, NY, USA, 1990; Volume 2. [Google Scholar]
- Blythe, S.; Busenberg, S.; Castillo-Chavez, C. Affinity in paired event probability. Math. Biosci. 1995, 128, 265–284. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Busenberg, S.; Castillo-Chavez, C. Interaction, pair formation and force of infection terms in sexually transmitted diseases. In Mathematical and Statistical Approaches to AIDS Epidemiology; Springer: Berlin/Heidelberg, Germany, 1989; pp. 289–300. [Google Scholar]
- Brauer, F. Epidemic models with treatment and heterogeneous mixing. Bull. Math. Biol. 2008, 70, 1869–1885. [Google Scholar] [CrossRef]
- Brauer, F. Age-of-infection and the final size relation. Math. Biosci. Eng. 2008, 5, 681–690. [Google Scholar] [PubMed]
- van den Driessche, P.; Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartments models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef]
- Diekmann, O.; Heesterbeek JA, P.; Roberts, M.G. The construction of next-generation matrices for compartmental epidemic models. J. R. Soc. Interface 2010, 7, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Hethcote, H.W. The mathematics of infectious diseases. SIAM Rev. 2000, 42, 599–653. [Google Scholar] [CrossRef]
- Hethcote, H.W. An age-structured model for pertussis transmission. Math. Biosci. 1997, 145, 89–136. [Google Scholar] [CrossRef]
- Hethcote, H.W. Three basic epidemiological models. In Applied Mathematical Ecology; Springer: Berlin/Heidelberg, Germany, 1989; pp. 119–144. [Google Scholar]
- Hethcote, H.W.; Stech, H.W.; van den Driessche, P. Periodicity and stability in epidemic models: A survey. In Differential Equations and Applications in Ecology, Epidemics, and Population Problems; Academic Press: Cambridge, MA, USA, 1981; pp. 65–82. [Google Scholar]
- Hethcote, H.W. An immunization model for a heterogeneous population. Theor. Popul. Biol. 1978, 14, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Adewale, S.O.; Olopade, I.A.; Adeniran, G.A.; Ajao, S.O. Mathematical Analysis of Quarantine on the Dynamical Transmission of Diphtheria Disease. Int. J. Sci. Eng. Investig. 2017, 6, 8–17. [Google Scholar]
- Akhi, A.A.; Tasnim, F.; Akter, S.; Kamrujjaman, M. A Mathematical Model of a Diphtheria outbreak in Rohingya Settlement in Bangladesh. J. Mahani Math. Res. Cent. 2023, 12, 547–563. [Google Scholar]
- Djaafara, B.; Adrian, V.; Eriawati, E.; Elyazar, I.R.; Hamers, R.L.; Baird, J.K.; Thwaites, G.E.; Clapham, H.E. Transmission Dynamics and Control Strategies During the 2017 Diphtheria Outbreak in Jakarta, Indonesia: A Modelling Study. Preprint 2023. [Google Scholar] [CrossRef]
- Husain, H.S. An SIR mathematical model for Dipterid disease. J. Phys. Conf. Ser. 2019, 1280, 022051. [Google Scholar] [CrossRef]
- Ilahi, F.; Widiana, A. The effectiveness of vaccine in the outbreak of diphtheria: Mathematical model and simulation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 434, 012006. [Google Scholar] [CrossRef]
- Fauzi, I.S.; Nuraini, N.; Sari, A.M.; Wardani, I.B.; Taurustiati, D.; Simanullang, P.M.; Lestari, B.W. Assessing the impact of booster vaccination on diphtheria transmission: Mathematical modeling and risk zone mapping. Infect. Dis. Model. 2024, 9, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Loyinmi, A.C.; Ijaola, A.L. Investigating the effects of some controls measures on the dynamics of diphtheria infection using fractional order model. Math. Comput. Sci. 2024, 5, 26–47. [Google Scholar]
- Ahmed, I.; Kiataramkul, C.; Muhammad, M.; Tariboon, J. Existence and sensitivity analysis of a Caputo fractional-order diphtheria epidemic model. Mathematics 2024, 12, 2033. [Google Scholar] [CrossRef]
- Kamadjeu, R.; Okunromade, O.; Lawal, B.B.; Gadanya, M.; Suwaid, S.A.; Blanco, E.C.; Kelvin, E.A. Diphtheria transmission dynamics-unveiling generation time and reproduction numbers from the 2022–2023 outbreak in Kano State, Nigeria. Infect. Dis. Model. 2025, 10, 680–690. [Google Scholar] [CrossRef]
- Islam, Z.; Ahmed, S.; Rahman, M.M.; Karim, M.F.; Amin, M.R. Global stability analysis and parameter estimation for a diphtheria model: A case study of an epidemic in Rohingya refugee camp in Bangladesh. Comput. Math. Methods Med. 2022, 2022, 6545179. [Google Scholar] [CrossRef] [PubMed]
- Izzati, N.; Andriani, A. Dynamical analysis of diphtheria epidemic model with natural immunity rate on exposed individuals. J. Phys. Conf. Ser. 2021, 1869, 012117. [Google Scholar] [CrossRef]
- Kanchanarat, S.; Chinviriyasit, S.; Chinviriyasit, W. Mathematical assessment of the impact of the imperfect vaccination on diphtheria transmission dynamics. Symmetry 2022, 14, 2000. [Google Scholar] [CrossRef]
- Matsuyama, R.; Akhmetzhanov, A.R.; Endo, A.; Lee, H.; Yamaguchi, T.; Tsuzuki, S.; Nishiura, H. Uncertainty and sensitivity analysis of the basic reproduction number of diphtheria: A case study of a Rohingya refugee camp in Bangladesh, November–December 2017. PeerJ 2018, 6, e4583. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Akinwande, N.I.; Ashezua, T.T.; Gweryina, R.; Somma, S.A.; Oguntolu, F.A.; Kadunad, F.S.; Adajimee, T.P.; Kuta, F.A.; Shehu, M.D.; et al. Mathematical model of COVID-19 transmission dynamics incorporating booster vaccine program and environmental contamination. Heliyon 2022, 8, e11513. [Google Scholar]
- Castillo-Chavez, C. On the computation of r. And its role on global stability carlos castillo-chavez, zhilan feng, and wenzhang huang. Math. Approaches Emerg. Reemerging Infect. Dis. Introd. 2002, 1, 229. [Google Scholar]
- La Salle, J.P. The Stability of Dynamical Systems; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1976. [Google Scholar] [CrossRef]
- Truelove, S.A.; Keegan, L.T.; Moss, W.J.; Chaisson, L.H.; Macher, E.; Azman, A.S.; Lessler, J. Clinical and epidemiological aspects of diphtheria: A systematic review and pooled analysis. Clin. Infect. Dis. 2020, 71, 89–97. [Google Scholar]
- Chitnis, N.; Hyman, J.M.; Cushing, J.M. Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull. Math. Biol. 2008, 70, 1272–1296. [Google Scholar]

| Variable | Description |
|---|---|
| N | Total human population |
| S | Susceptible individuals |
| E | Exposed individuals |
| A | Asymptotic infected individuals |
| Q | Isolated individuals |
| R | Recovered individuals |
| Parameter | |
| Recruitment rate | |
| Natural mortality rate | |
| Mortality rate due to disease in infected class | |
| Mortality rate due to disease in isolated class | |
| Progression rate from infected to recovered class | |
| Progression rate from asymptomatic to recovered class | |
| Progression rate from isolated to recovered class | |
| p | Proportion of exposed population becoming infectious |
| Modification parameter | |
| Transmission rate | |
| c | Public enlightenment campaign rate |
| Efficacy (people compliance) rate of public enlightenment campaign | |
| Progression rate from exposed to either asymptomatic | |
| class or infected class | |
| Progression rate from infected class to isolated class |
| Parameter | Estimated Value | Sources |
|---|---|---|
| fitted | ||
| 6,808,999 | fitted | |
| [1] | ||
| Assumed | ||
| fitted | ||
| c | [33] | |
| [33] | ||
| fitted | ||
| fitted | ||
| fitted | ||
| fitted | ||
| fitted | ||
| fitted | ||
| p | [36] |
| Parameter | Index | Index Value |
|---|---|---|
| 1.0000 | ||
| 0.5349 | ||
| −0.2760 | ||
| 0.2089 | ||
| c | 0.1725 | |
| −0.1111 | ||
| 0.06951 | ||
| −0.0381 | ||
| −0.0357 | ||
| 0.0020 | ||
| p | −0.00001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, S.; Usaini, S.; Ahmed, I.; Kiataramkul, C.; Tariboon, J. Dynamics of the Diphtheria Epidemic in Nigeria: Insights from the Kano State Outbreak Data. Mathematics 2025, 13, 1189. https://doi.org/10.3390/math13071189
Musa S, Usaini S, Ahmed I, Kiataramkul C, Tariboon J. Dynamics of the Diphtheria Epidemic in Nigeria: Insights from the Kano State Outbreak Data. Mathematics. 2025; 13(7):1189. https://doi.org/10.3390/math13071189
Chicago/Turabian StyleMusa, Sani, Salisu Usaini, Idris Ahmed, Chanakarn Kiataramkul, and Jessada Tariboon. 2025. "Dynamics of the Diphtheria Epidemic in Nigeria: Insights from the Kano State Outbreak Data" Mathematics 13, no. 7: 1189. https://doi.org/10.3390/math13071189
APA StyleMusa, S., Usaini, S., Ahmed, I., Kiataramkul, C., & Tariboon, J. (2025). Dynamics of the Diphtheria Epidemic in Nigeria: Insights from the Kano State Outbreak Data. Mathematics, 13(7), 1189. https://doi.org/10.3390/math13071189







