Application of Hyperelastic Nodal Force Method to Evaluation of Aortic Valve Cusps Coaptation: Thin Shell vs. Membrane Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Shell Kinematics
2.2. Constitutive Equations
2.3. Weak Formulation
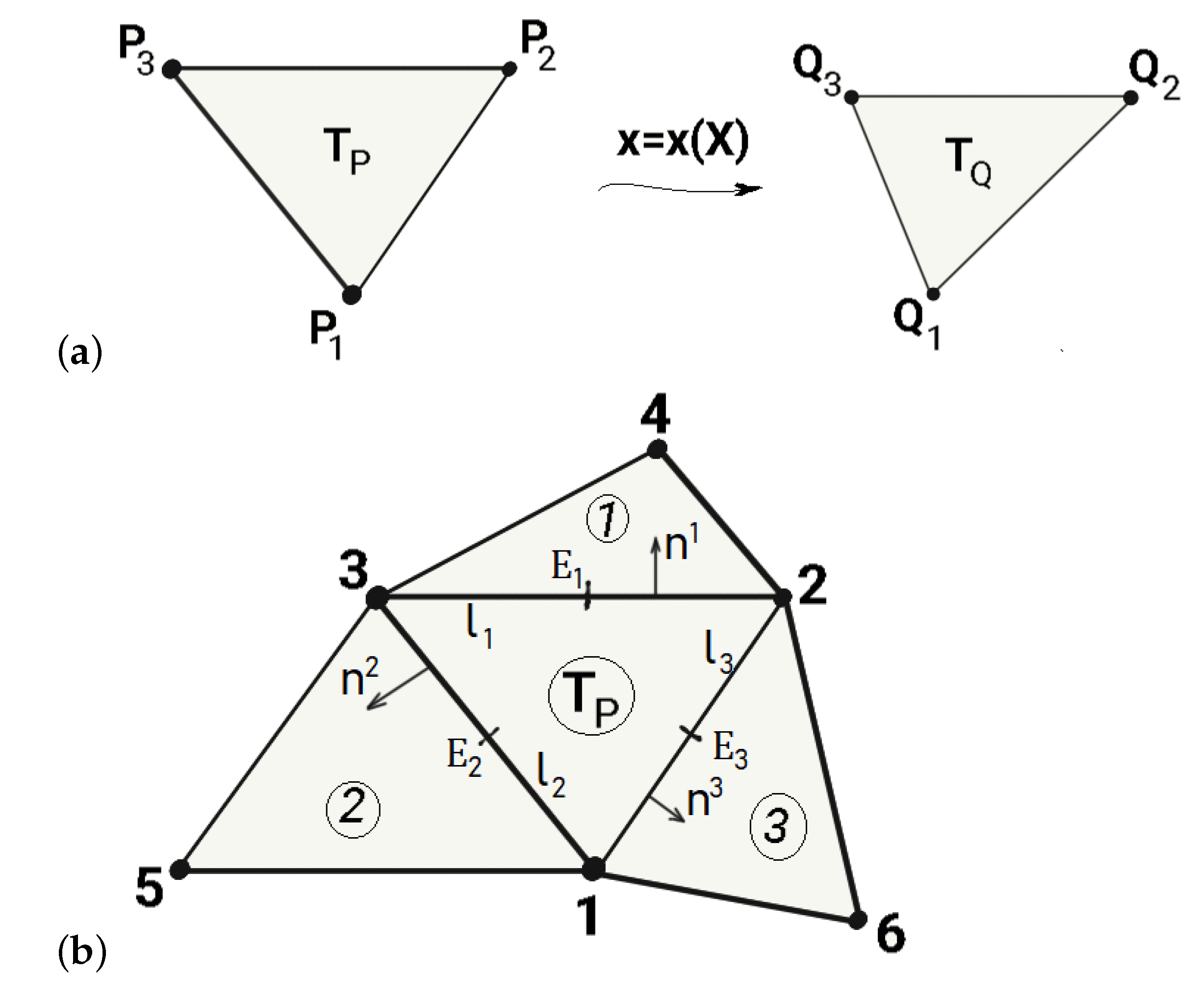
2.4. Discretization
2.4.1. Discretization of the Membrane Part
2.4.2. Bending Part
2.4.3. Discretized Equilibrium Equations
3. Numerical Results
3.1. Benchmarks
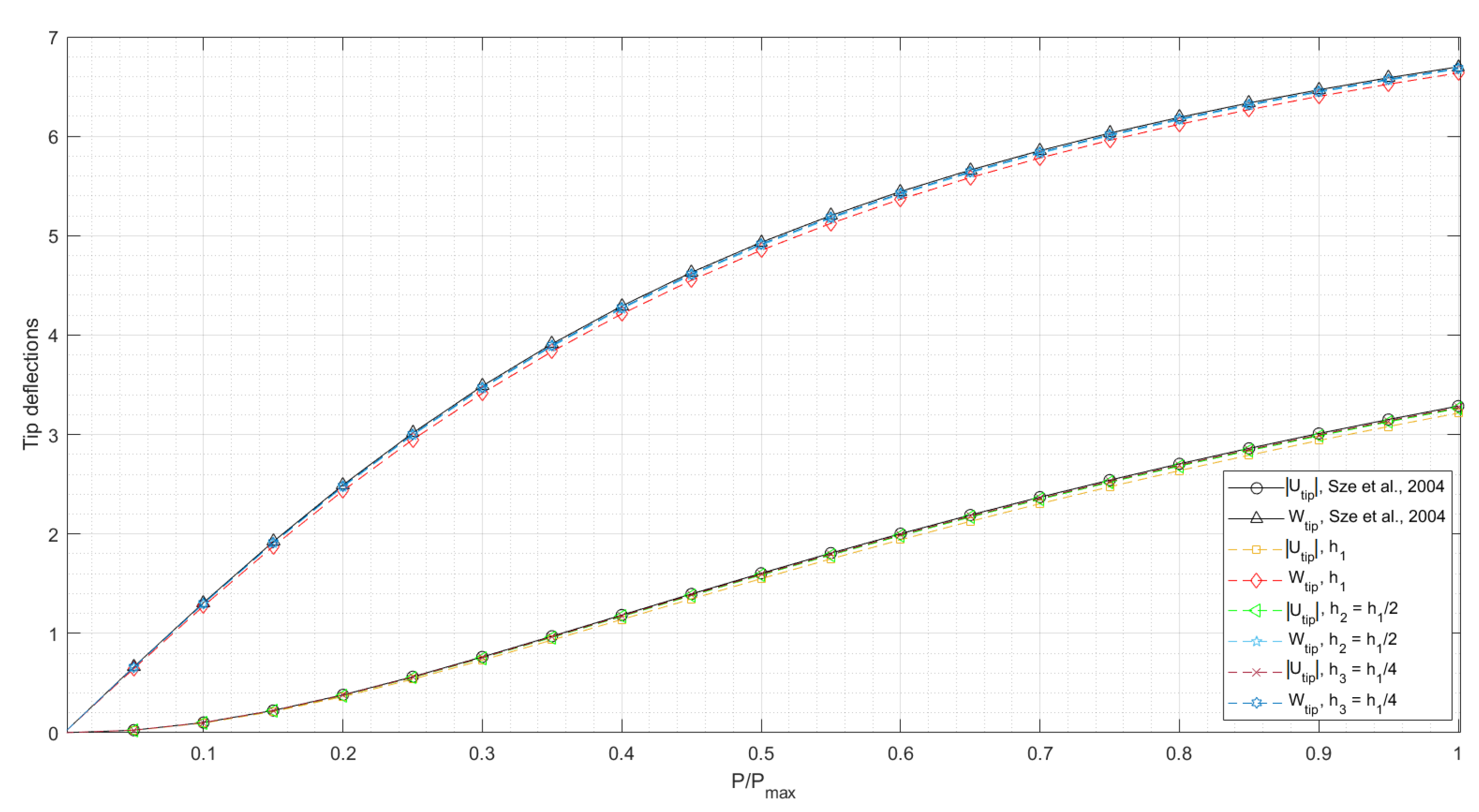
3.1.1. Cantilever Subjected to End Shear Force
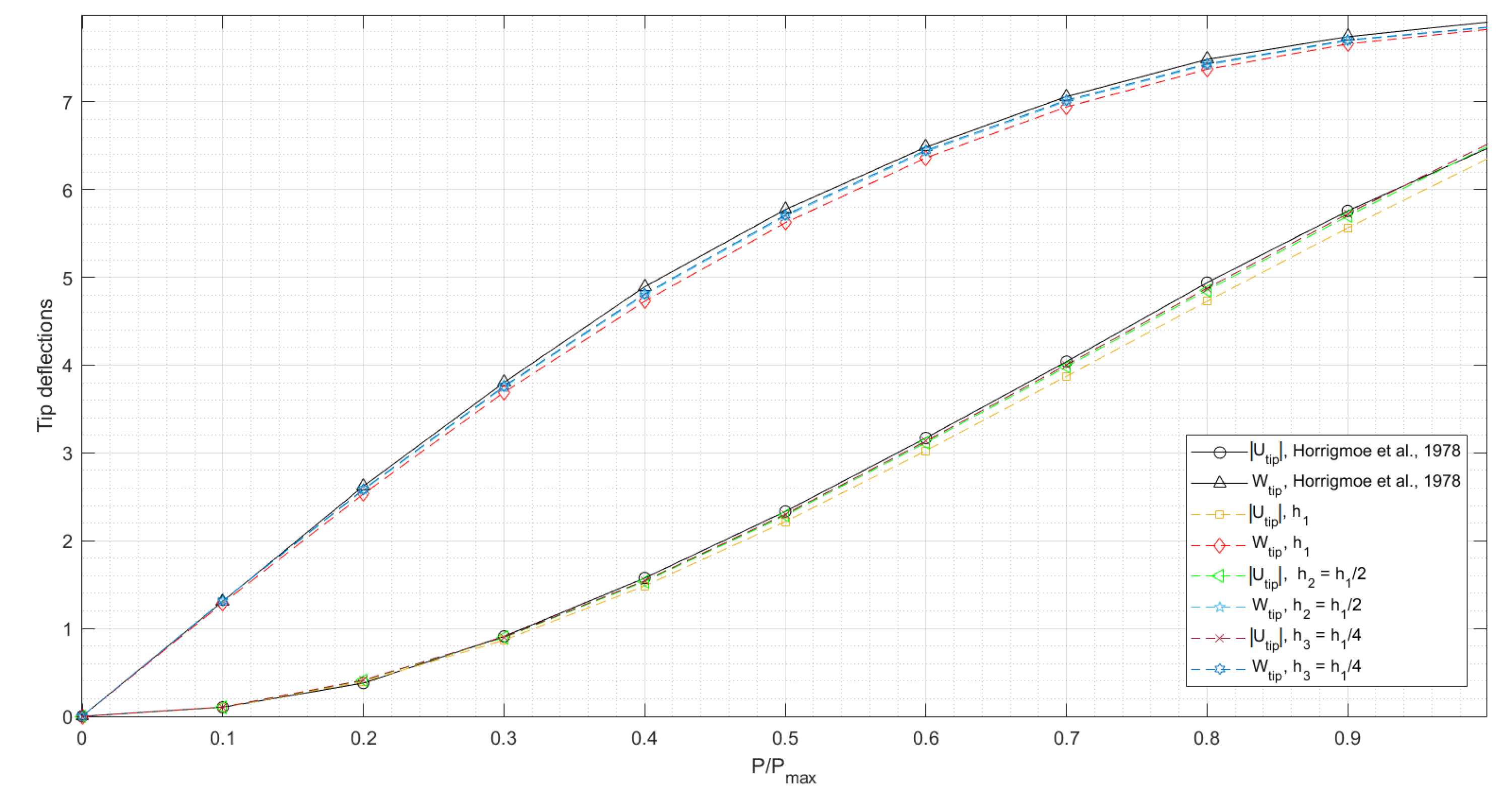
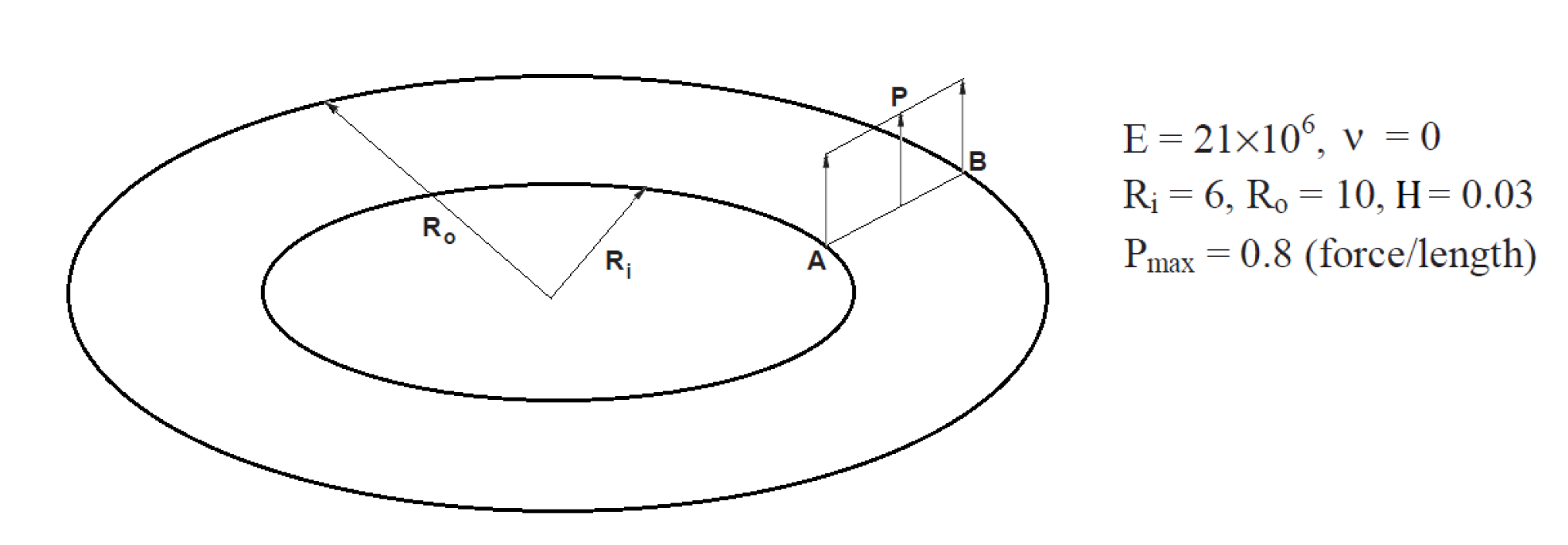
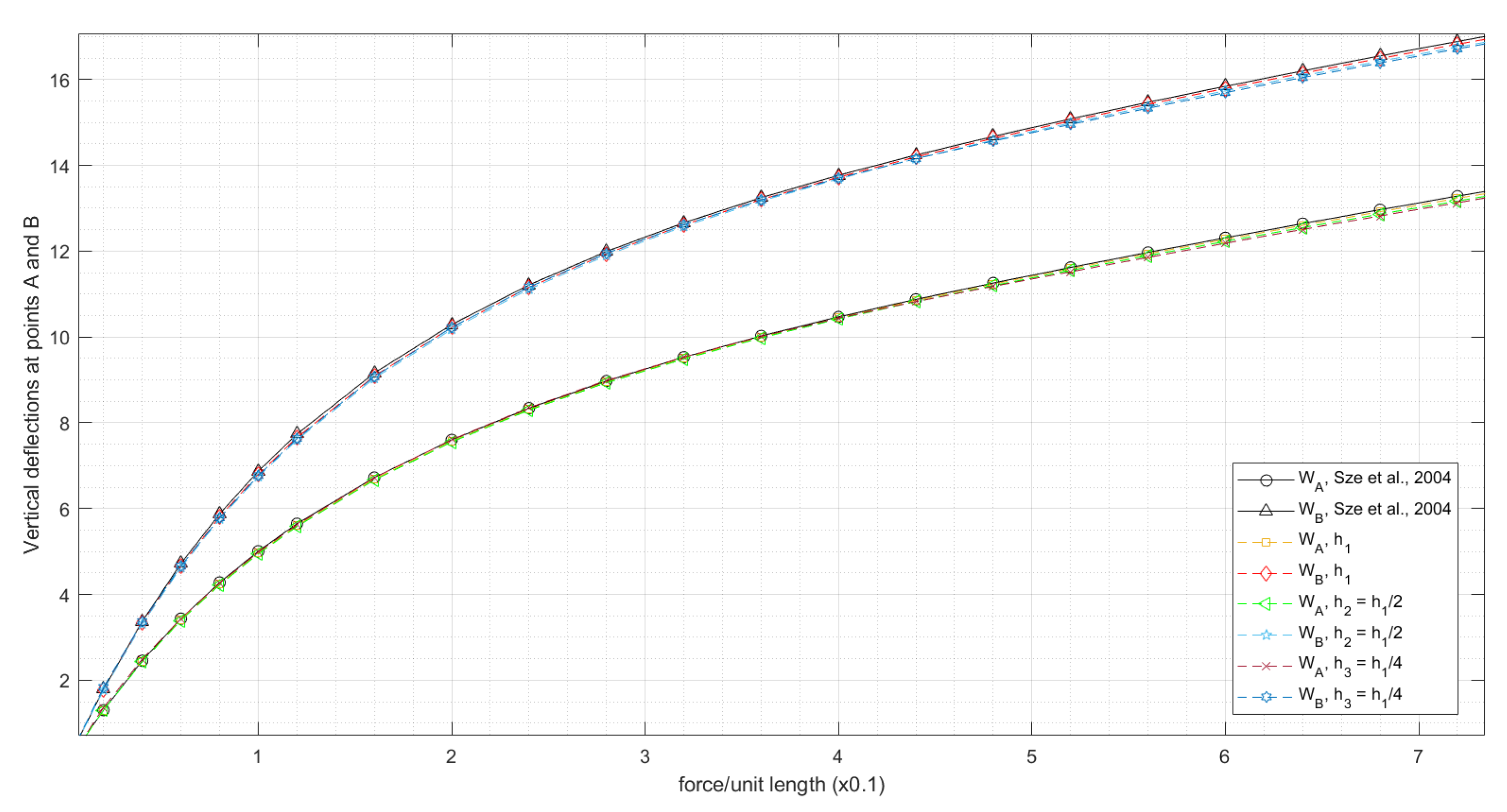
3.1.2. Slit Annular Plate Subjected to Lifting Line Force
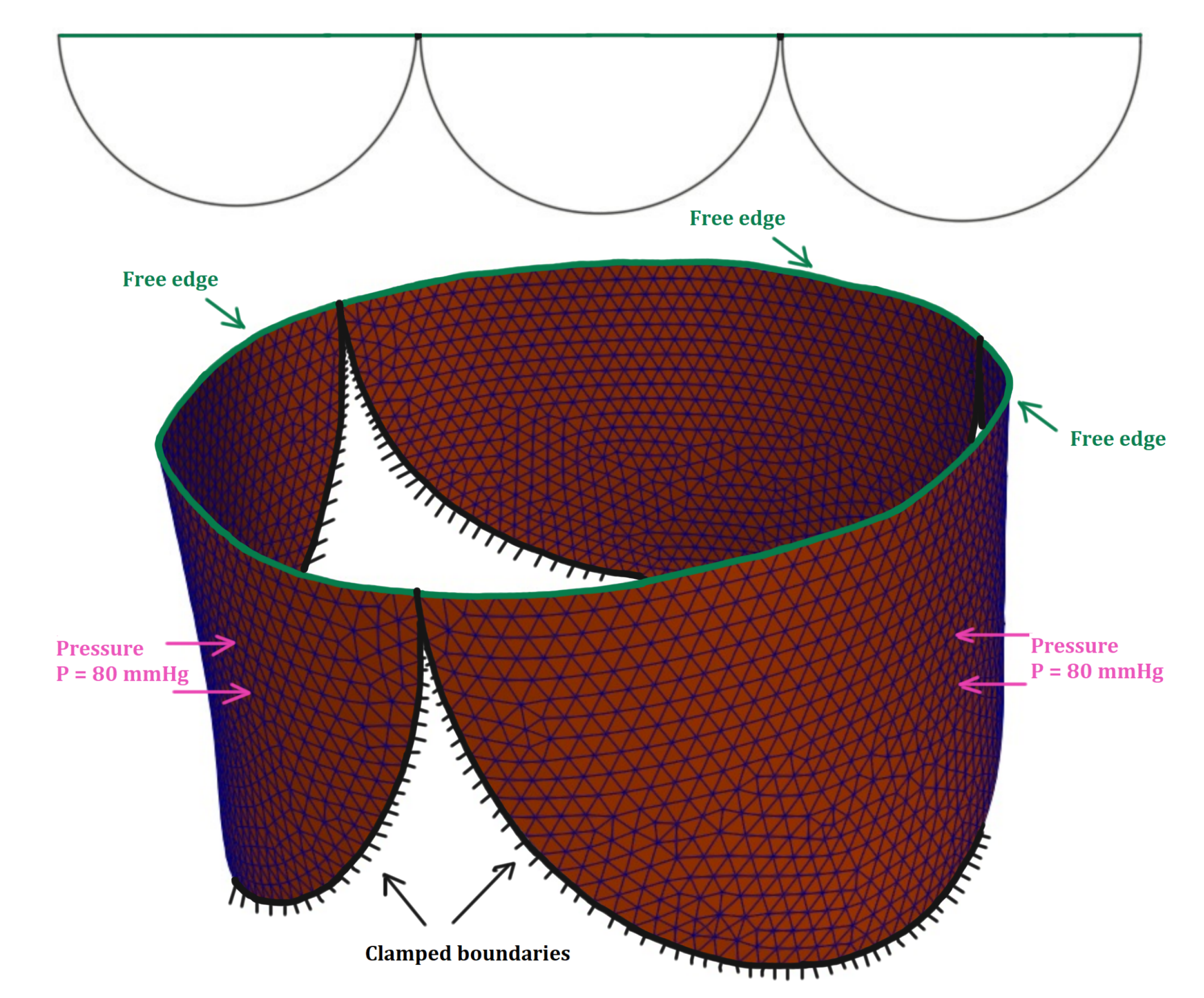
3.2. Aortic Valve in Diastolic State
| Algorithm 1 Solution of the static equilibrium equation and computation of the contact forces |
|
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coffey, S.; Cairns, B.J.; Iung, B. The modern epidemiology of heart valve disease. Heart 2016, 102, 75–85. [Google Scholar] [CrossRef]
- Rankin, J.S.; Nöbauer, C.; Crooke, P.S.; Schreiber, C.; Lange, R.; Mazzitelli, D. Techniques of autologous pericardial leaflet replacement for aortic valve reconstruction. Ann. Thorac. Surg. 2014, 98, 743–745. [Google Scholar] [CrossRef]
- Labrosse, M.R.; Beller, C.J.; Robicsek, F.; Thubrikar, M.J. Geometric modeling of functional trileaflet aortic valves: Development and clinical applications. J. Biomech. 2006, 39, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Kawase, I.; Yamashita, H.; Uchida, S.; Nozawa, Y.; Takatoh, M.; Hagiwara, S. A total of 404 cases of aortic valve reconstruction with glutaraldehyde-treated autologous pericardium. J. Thorac. Cardiovasc. Surg. 2014, 147, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakerzadeh, R.; Hsu, M.C.; Sacks, M.S. Computational methods for the aortic heart valve and its replacements. Expert Rev. Med. Devices 2017, 14, 849–866. [Google Scholar] [CrossRef] [PubMed]
- Sturla, F.; Votta, E.; Stevanella, M.; Conti, C.A.; Redaelli, A. Impact of modeling fluid–structure interaction in the computational analysis of aortic root biomechanics. Med. Eng. Phys. 2013, 35, 1721–1730. [Google Scholar] [CrossRef]
- Berra, I.G.; Hammer, P.E.; Berra, S.; Irusta, A.O.; Ryu, S.C.; Perrin, D.P.; Vasilyev, N.V.; Cornelis, C.J.; Delucis, P.G.; Pedro, J. An intraoperative test device for aortic valve repair. J. Thorac. Cardiovasc. Surg. 2019, 157, 126–132. [Google Scholar] [CrossRef]
- Pappalardo, O.A.; Sturla, F.; Onorati, F.; Puppini, G.; Selmi, M.; Luciani, G.B.; Faggian, G.; Redaelli, A.; Votta, E. Mass-spring models for the simulation of mitral valve function: Looking for a trade-off between reliability and time-efficiency. Med. Eng. Phys. 2017, 47, 93–104. [Google Scholar] [CrossRef]
- Hammer, P.E.; Pedro, J.; Howe, R.D. Anisotropic mass-spring method accurately simulates mitral valve closure from image-based models. In Functional Imaging and Modeling of the Heart. FIMH 2011. Lecture Notes in Computer Science; Metaxas, D.N., Axel, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 6666, pp. 233–240. [Google Scholar]
- Hammer, P.E.; Sacks, M.S.; Pedro, J.; Howe, R.D. Mass-spring model for simulation of heart valve tissue mechanical behavior. Ann. Biomed. Eng. 2011, 39, 1668–1679. [Google Scholar] [CrossRef] [Green Version]
- Salamatova, V.Y.; Liogky, A.A.; Karavaikin, P.A.; Danilov, A.A.; Kopylov, P.Y.; Kopytov, G.V.; Kosukhin, O.N.; Pryamonosov, R.A.; Shipilov, A.A.; Yurova, A.S.; et al. Numerical assessment of coaptation for auto-pericardium based aortic valve cusps. Russ. J. Numer. Anal. Math. Model. 2019, 34, 277–287. [Google Scholar] [CrossRef]
- Salamatova, V.Y.; Liogky, A.A. Method of Hyperelastic Nodal Forces for Deformation of Nonlinear Membranes. Differ. Equ. 2020, 56, 950–958. [Google Scholar] [CrossRef]
- Murdock, K.; Martin, C.; Sun, W. Characterization of mechanical properties of pericardium tissue using planar biaxial tension and flexural deformation. J. Mech. Behav. Biomed. Mater. 2018, 77, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Gärdsback, M.; Tibert, G. A comparison of rotation-free triangular shell elements for unstructured meshes. Comput. Methods Appl. Mech. Eng. 2007, 196, 5001–5015. [Google Scholar] [CrossRef]
- Oñate, E.; Flores, F.G. Advances in the formulation of the rotation-free basic shell triangle. Comput. Methods Appl. Mech. Eng. 2005, 194, 2406–2443. [Google Scholar] [CrossRef]
- Tepole, A.B.; Kabaria, H.; Bletzinger, K.U.; Kuhl, E. Isogeometric Kirchhoff–Love shell formulations for biological membranes. Comput. Methods Appl. Mech. Eng. 2015, 293, 328–347. [Google Scholar] [CrossRef] [Green Version]
- Holzapfel, G. Nonlinear Solid Mechanics: A Continuum Approach for Engineering Science; John Wiley & Sons Ltd.: Chichester, UK, 2000; p. 470. [Google Scholar]
- Lu, J.; Zhou, X.; Raghavan, M.L. Inverse method of stress analysis for cerebral aneurysms. Biomech. Model. Mechanobiol. 2008, 7, 477–486. [Google Scholar] [CrossRef]
- Ciarlet, P.G. Mathematical Elasticity. Volume I: Three-Dimensional Elasticity; North-Holland: Amsterdam, The Netherlands, 1988; p. 451. [Google Scholar]
- Sze, K.Y.; Liu, X.H.; Lo, S.H. Popular benchmark problems for geometric nonlinear analysis of shells. Finite Elem. Anal. Des. 2004, 40, 1551–1569. [Google Scholar] [CrossRef]
- Horrigmoe, G.; Bergan, P.G. Nonlinear analysis of free-form shells by flat finite elements. Comput. Methods Appl. Mech. Eng. 1978, 16, 11–35. [Google Scholar] [CrossRef]
- Aguiari, P.; Fiorese, M.; Iop, L.; Gerosa, G.; Bagno, A. Mechanical testing of pericardium for manufacturing prosthetic heart valves. Interact. Cardiovasc. Thorac. Surg. 2015, 22, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Zigras, T.C. Biomechanics of Human Pericardium: A Comparative Study of Fresh and Fixed Tissue. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 2007. [Google Scholar]
- Raghupathi, L.; Grisoni, L.; Faure, F.; Marchal, D.; Cani, M.P.; Chaillou, C. An intestinal surgery simulator: Real-time collision processing and visualization. IEEE Trans. Vis. Comput. Graph. 2004, 10, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Method btSoftBody::PSolve_SContacts. Available online: https://github.com/bulletphysics/bullet3/blob/master/src/BulletSoftBody/btSoftBody.cpp (accessed on 20 June 2021).
- Marom, G.; Haj-Ali, R.; Raanani, E.; Schäfers, H.J.; Rosenfeld, M. A fluid-structure interaction model of the aortic valve with coaptation and compliant aortic root. Med. Biol. Eng. Comput. 2012, 50, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Danilov, A.; Ivanov, Y.; Pryamonosov, R.; Vassilevski, Y. Methods of graph network reconstruction in personalized medicine. Int. J. Numer. Methods Biomed. Eng. 2016, 32, e02754. [Google Scholar] [CrossRef] [PubMed]



| Model | Max. Coaptation | Free Edge | |||
|---|---|---|---|---|---|
| Height, mm | Length, mm | ||||
| SVK | MPa, (membrane) | ||||
| MPa, (shell) | |||||
| Neo-Hookean | MPa, (membrane) | ||||
| MPa, (shell) | |||||
| Gent | MPa, , (membrane) | ||||
| MPa, , (shell) | |||||
| SVK | MPa, (membrane) | ||||
| MPa, (shell) | * | * | |||
| Neo-Hookean | MPa, (membrane) | ||||
| MPa, (shell) | * | * | |||
| Gent | MPa, , (membrane) | ||||
| MPa, , (shell) | * | * | |||
| Model | CPU Time, s | |
|---|---|---|
| SVK | MPa, (membrane) | 220 |
| MPa, (shell) | 3761 | |
| Neo-Hookean | MPa, (membrane) | 157 |
| MPa, (shell) | 3940 | |
| Gent | MPa, , (membrane) | 170 |
| MPa, , (shell) | 5047 | |
| SVK | MPa, (membrane) | 831 |
| MPa, (shell) | 5736 | |
| Neo-Hookean | MPa, (membrane) | 810 |
| MPa, (shell) | 5561 | |
| Gent | MPa, , (membrane) | 832 |
| MPa, , (shell) | 4496 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassilevski, Y.; Liogky, A.; Salamatova, V. Application of Hyperelastic Nodal Force Method to Evaluation of Aortic Valve Cusps Coaptation: Thin Shell vs. Membrane Formulations. Mathematics 2021, 9, 1450. https://doi.org/10.3390/math9121450
Vassilevski Y, Liogky A, Salamatova V. Application of Hyperelastic Nodal Force Method to Evaluation of Aortic Valve Cusps Coaptation: Thin Shell vs. Membrane Formulations. Mathematics. 2021; 9(12):1450. https://doi.org/10.3390/math9121450
Chicago/Turabian StyleVassilevski, Yuri, Alexey Liogky, and Victoria Salamatova. 2021. "Application of Hyperelastic Nodal Force Method to Evaluation of Aortic Valve Cusps Coaptation: Thin Shell vs. Membrane Formulations" Mathematics 9, no. 12: 1450. https://doi.org/10.3390/math9121450
APA StyleVassilevski, Y., Liogky, A., & Salamatova, V. (2021). Application of Hyperelastic Nodal Force Method to Evaluation of Aortic Valve Cusps Coaptation: Thin Shell vs. Membrane Formulations. Mathematics, 9(12), 1450. https://doi.org/10.3390/math9121450






