Modelling Functional Shifts in Two-Species Hypercycles
Abstract
:1. Introduction
2. General Mathematical Model
- : autonomous self-replication rate (Malthusian growth) of species . implies that species can replicate themselves without the catalytic aid of the other replicator.
- k: the cross-catalytic replication parameter between and . In the case of , the term allows considering a non-symmetric case in which both species are kinetically different. The case involves symmetric catalytic replication.
- : known as the carrying capacity, which limits the population of replicators due to finite resources or (implicit) space. Due to the large number of parameters, we will set .
- : density-independent spontaneous degradation or death of the species. Analogously to the characteristics of , allows considering a non-symmetric decay scenario for and .
- : the density-dependent degradation rate due to the cleavage (or predation, see below) of species by . We notice that the degradative and predatory dynamics are exclusive. That is, we do not consider a case with directed degradation and predation taking place at the same time (these two different cases will be considered with or , respectively).
- : the fraction of the population still receiving cooperation from replication. The term corresponds to the rest of the population that exerts the degradation or the predation of species .
- : the energetic efficiency coefficient from predation. It can be understood as the amount of energy species can gain from predating and investing it for reproduction (due to energetic constraints ). As mentioned, the investigation of the degradative model will be performed by setting , while the predatory system will consider .
3. Results and Discussion
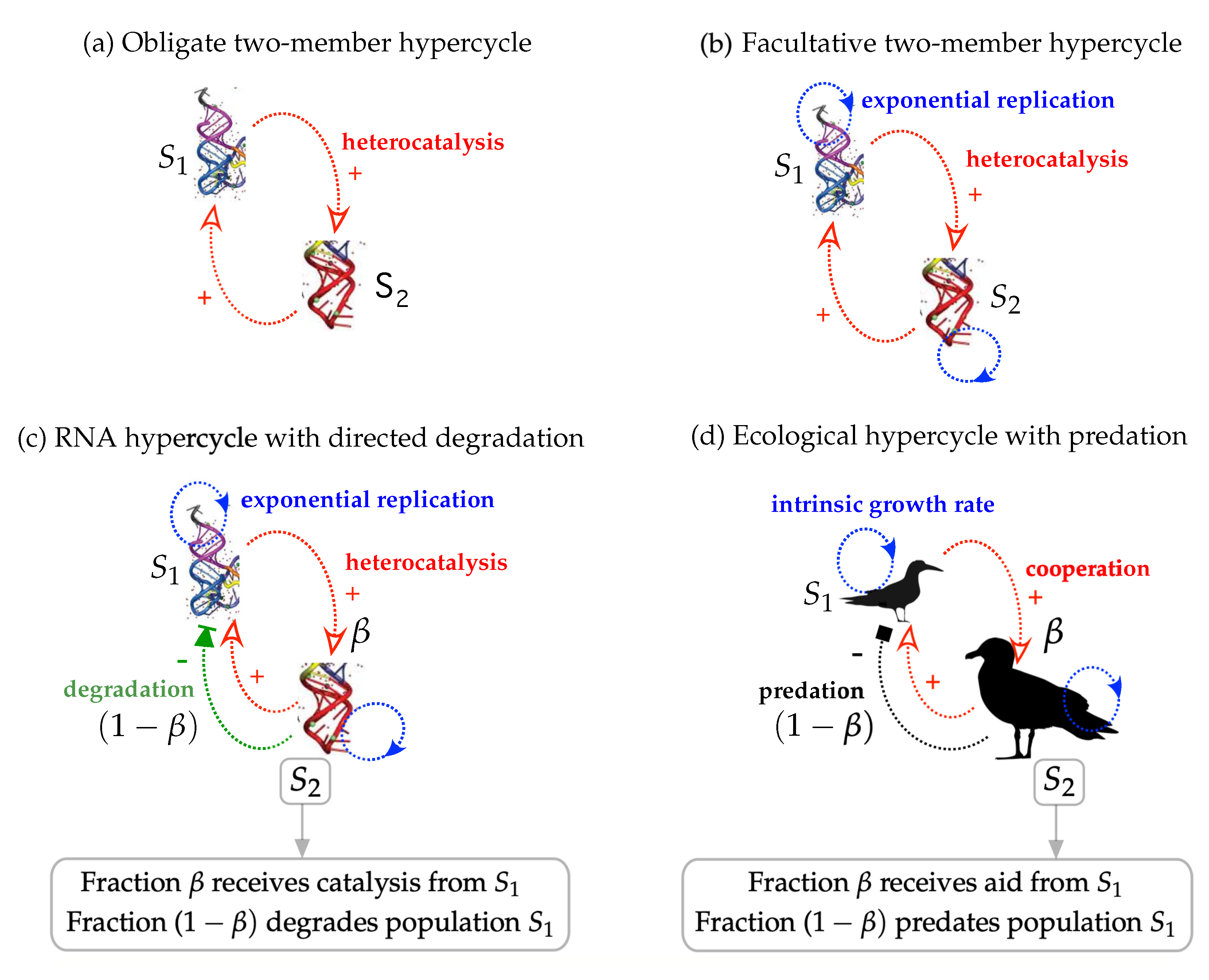
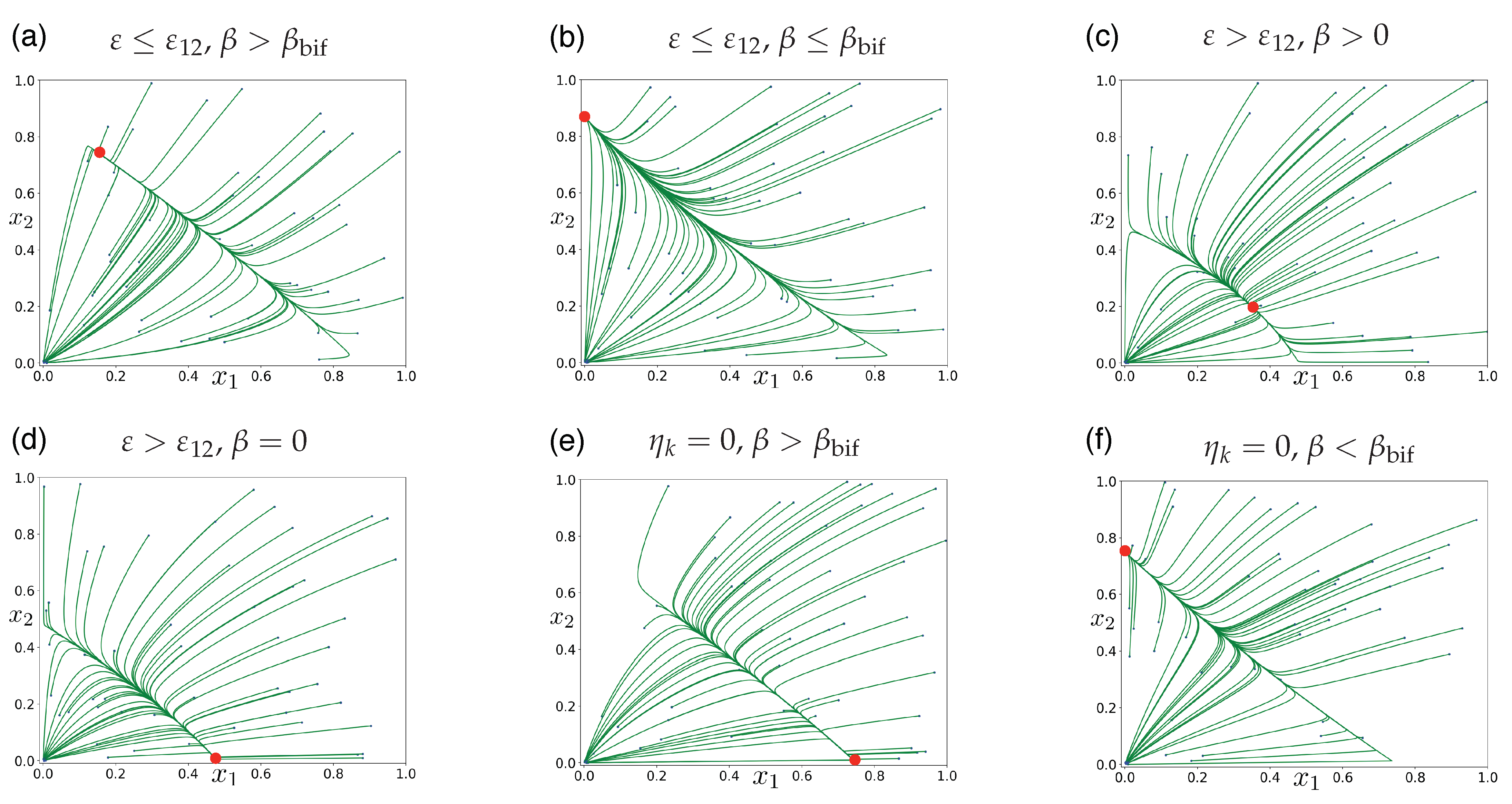
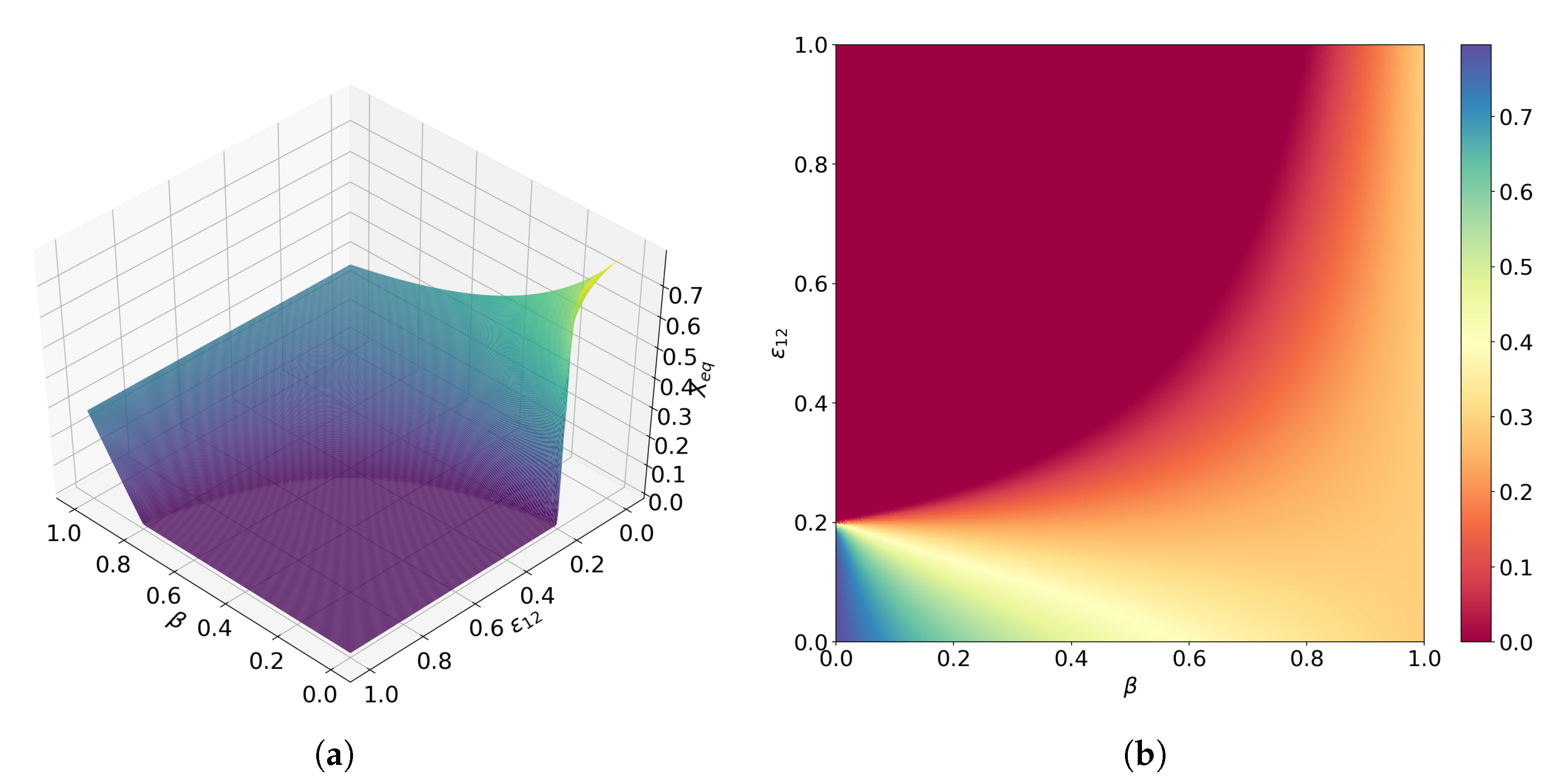
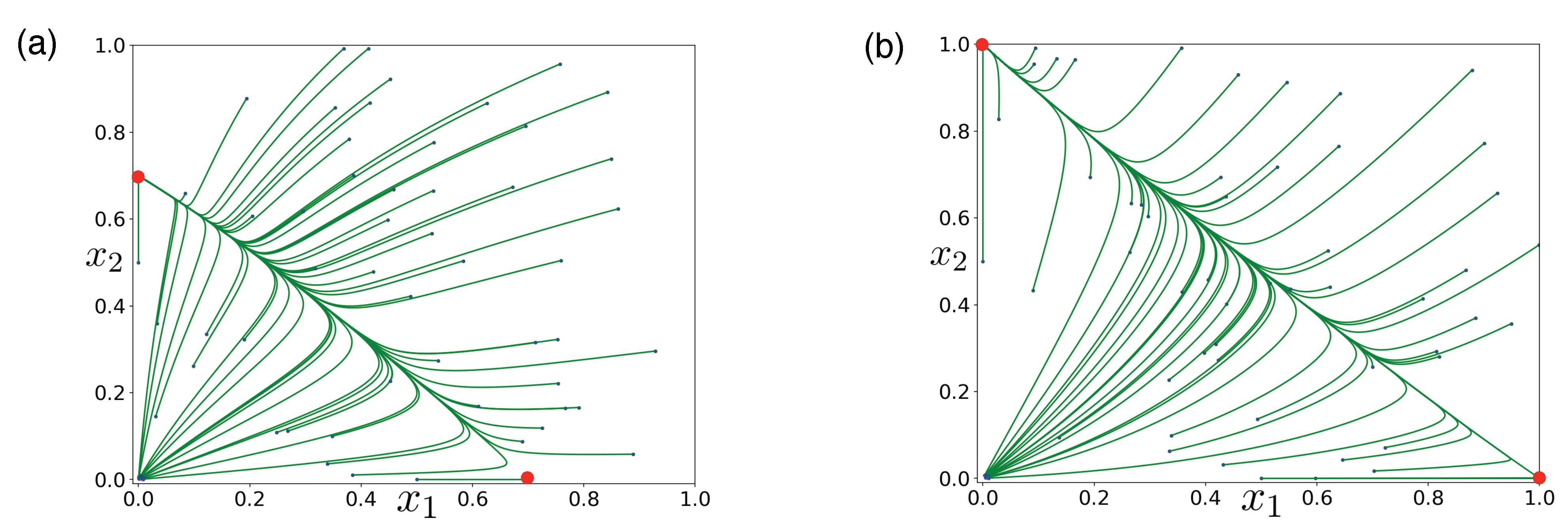
3.1. Obligate Two-Member Hypercycle
3.2. Facultative Two-Member Hypercycle
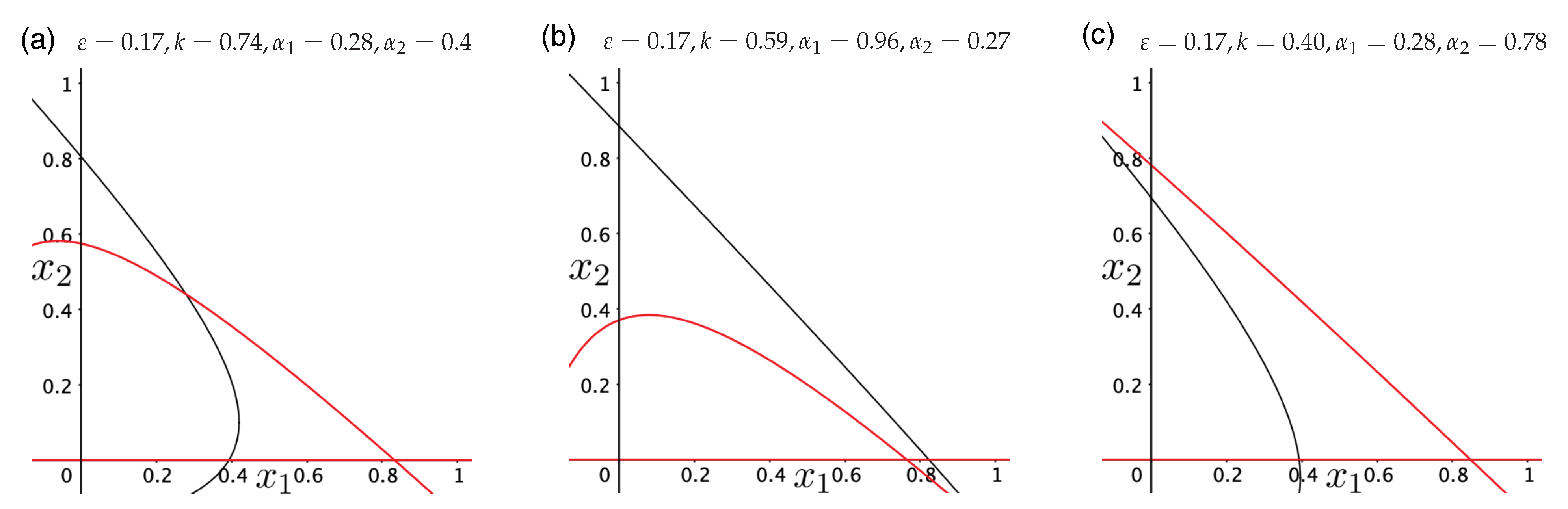
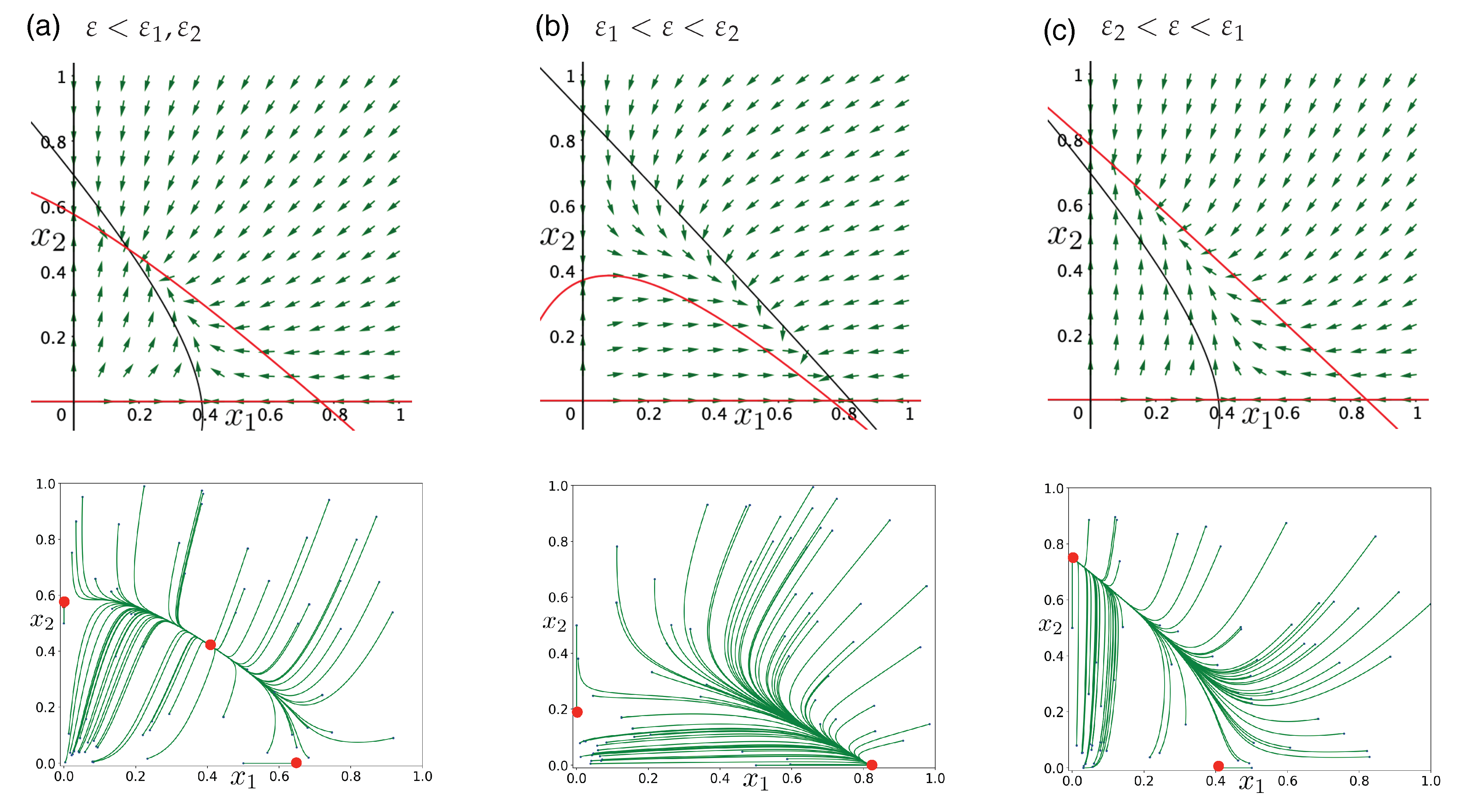
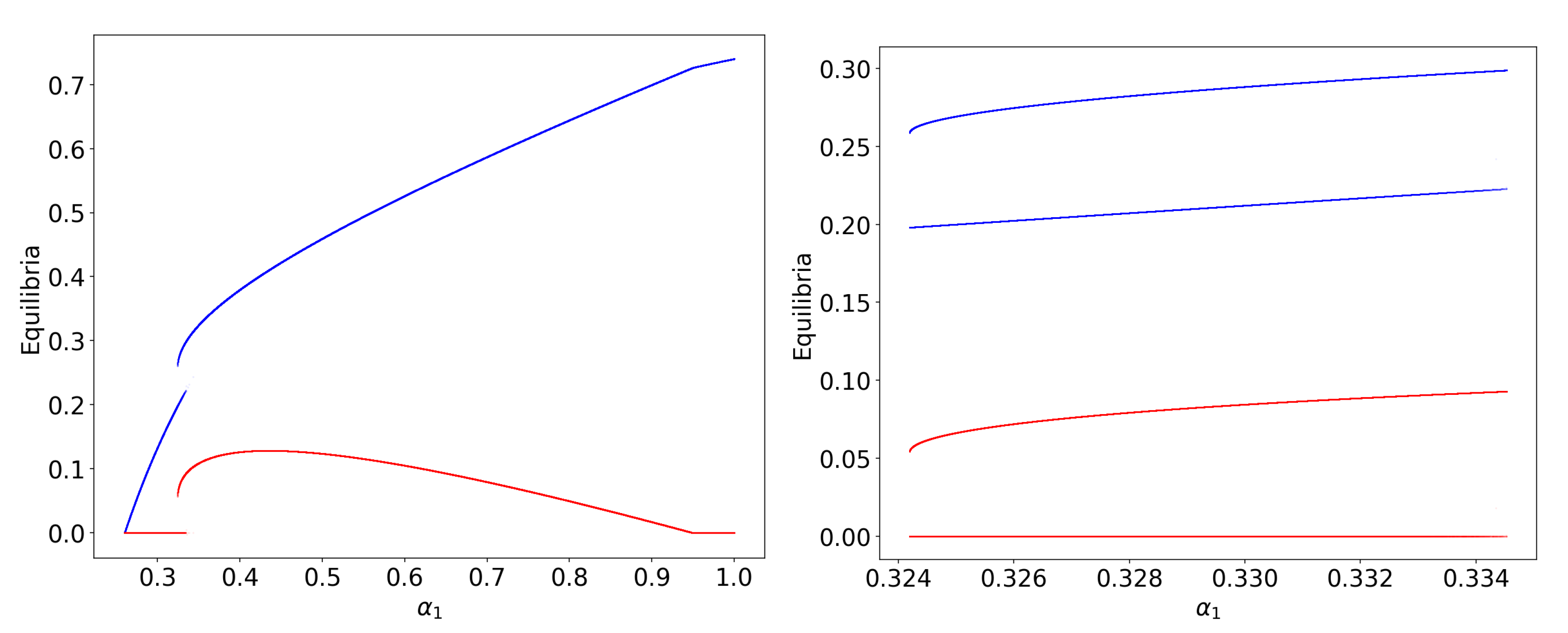
3.3. Directed Degradation and Cooperation in Ribozymes
3.4. Interplay between Predation and Cooperation in Ecology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Obligate Two-Member Hypercycle: Coexistence Equilibrium
References
- Eigen, M. Selforganization of matter and the evolution of biological macromolecules. Die Naturwissenschaften 1971, 58, 465–523. [Google Scholar] [CrossRef] [PubMed]
- Müller-Herold, U. What is a hypercycle? J. Theor. Biol. 1983, 102, 569–584. [Google Scholar] [CrossRef]
- Eigen, M.; Schuster, P. The Hypercycle: A Principle of Natural Selforganization; Springer: Berlin, Germany, 1979. [Google Scholar]
- Kauffman, S. The Origins of Order: Self-Organization and Selection in Evolution; Oxford University: Oxford, UK, 1993. [Google Scholar]
- Eigen, M.; Biebricher, C.K.; Gebinoga, M.; Gardiner, W.C. The hypercycle. Coupling of RNA and protein biosynthesis in the infection cycle of an RNA bacteriophage. Biochemistry 1991, 30, 11005–11018. [Google Scholar] [CrossRef]
- Szathmáry, E. Natural selection and dynamical coexistence of defective and complementing virus segments. J. Theor. Biol. 1992, 157, 383–406. [Google Scholar] [CrossRef]
- Szathmáry, E. Co-operation and defection: Playing the field in virus dynamics. J. Theor. Biol. 1993, 165, 341–356. [Google Scholar] [CrossRef]
- Sardanyés, J.; Elena, S.F. Error threshold in RNA quasispecies models with complementation. J. Theor. Biol. 2010, 265, 278–286. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.A.; Grossberg, S. Absolute stability and global pattern formation and parallel memory storage by competitive neural networks. IEEE Trans. Syst. Man Cybern. 1983, 13, 815–826. [Google Scholar] [CrossRef]
- Cohen, M.A.; Grossberg, S. Pattern Recognition by Self-Organizing Neural Networks; MIT Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Farmer, J.D.; Kauffman, S.A.; Packard, N.H.; Perelson, A.S. Adaptive dynamic networks as models for the immune system and autocatalytic sets. In Perspectives in Biological Dynamics and Theoretical Medicine; Annals of the New York Academy of Sciences: New York, NY, USA, 1987. [Google Scholar]
- Nowak, M.A.; Krakauer, D.C. The evolution of language. Proc. Natl. Acad. Sci. USA 1999, 96, 8028–8033. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Severin, K.; Ghadiri, M.R. Autocatalytic networks: The transition from molecular self-replication to molecular ecosystems. Curr. Opin. Chem. Biol. 1997, 1, 491–496. [Google Scholar] [CrossRef]
- Shou, W.; Ram, S.; Vilar, J.M.G. Synthetic cooperation in engineered yeast populations. Proc. Natl. Acad. Sci. USA 2007, 104, 1877–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amor, D.R.; Montañez, R.; Duran-Nebreda, S.; Solé, R. Spatial dynamics of synthetic microbial mutualists and their parasites. PLoS Comput. Biol. 2017, 13, e15689. [Google Scholar] [CrossRef] [Green Version]
- Stadler, B.; Stadler, P. Molecular replicator dynamics. Adv. Complex Syst. 2003, 6, 47–77. [Google Scholar] [CrossRef] [Green Version]
- Niesert, U.; Harnasch, D.; Bresch, C. Origin of life between Scylla and Charybdis. J. Mol. Evol. 1981, 17, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Usher, D.A.; McHale, A.H. Hydrolyitc stability of helical RNA: A selective advantage for the natural 3’, 5’-bond. Proc. Natl. Acad. Sci. USA 1976, 73, 1149–1153. [Google Scholar] [CrossRef] [Green Version]
- Lohrmann, R.; Orgel, L.E. Self-condensation of activated dinucleotides on polynucleotide templates with alternating sequences. J. Mol. Evol. 1979, 14, 243–250. [Google Scholar] [CrossRef]
- Cech, T.R. The chemistry of self-replicating RNA and RNA enzymes. Science 1987, 236, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Kruger, K.; Grabowski, P.J.; Zaug, A.J.; Sands, J.; Gottschling, D.E.; Cech, T.R. Self-splicing RNA: Autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell 1982, 31, 147–157. [Google Scholar] [CrossRef]
- Daròs, J.A. Eggplant latent viroid: A friendly experimental system in the family Asunviroidae. Mol. Plant Pathol. 2016, 17, 1170–1177. [Google Scholar] [CrossRef] [Green Version]
- Vlassov, A.V. Mini-ribozymes and freezing environment: A new scenario for the early RNA world. Biogeosci. Discuss. 2005, 2, 1719–1737. [Google Scholar]
- Robertson, M.P.; Joyce, G.F. The origins of the RNA world. Cold Spring Harb. Perspect. Biol. 2012, 4, a003608. [Google Scholar] [CrossRef]
- Neveu, M.; Kim, H.J.; Benner, S.A. The strong RNA world hypothesis: Fifty years old. Astrobiology 2013, 13, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Doudna, J.A.; Cech, T.R. The chemical repertoire of natural ribozymes. Nature 2002, 418, 222–228. [Google Scholar] [CrossRef]
- Zhang, B.; Cech, T.R. Peptidyl-transferase ribozymes: Trans reactions, structural characterization and ribosomal RNA-like features. Chem. Biol. 1998, 5, 539–553. [Google Scholar] [CrossRef] [Green Version]
- Been, M.D.; Barford, E.T.; Burke, J.M.; Price, J.V.; Tanner, N.K.; Zaug, A.J.; Cech, T.R. Structures involved in Tetrahymena rRNA self-splicing and RNA enzyme activity. Cold Spring Harb. Symp. Quant. Biol. 1987, 52, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Zielinksi, W.S.; Orgel, L.E. Oligomerization of activated derivatives of 3’-amino-3’-deoxyguanosine on poly(C) and poly(dC) template. Nucl. Acid Res. 1985, 13, 8999–9009. [Google Scholar]
- Von Kiedrowski, G. A self-replicating hexadeoxynucleotide. Angew. Chem. Int. Ed. 1986, 119, 932–935. [Google Scholar] [CrossRef]
- Jimenez, R.M.; Polanco, J.A.; Lupták, A. Chemistry and biology of self-cleaving ribozymes. Trends Biochem. Sci. 2015, 40, 648–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbonell, A.; Flores, R.; Gago, S. Trans-cleaving hammerhead ribozymes with tertiary stabilizing motifs: In vitro and in vivo activity against a structured viroid RNA. Nucl. Acid Res. 2011, 39, 2432–2444. [Google Scholar] [CrossRef]
- Weinberg, C.E.; Weinberg, Z.; Hammann, C. Noverl ribozymes: Discovery, catalytic mechanisms, and the quest to understand biological functions. Nucl. Acid Res. 2019, 47, 9480–9494. [Google Scholar] [CrossRef]
- Weinberg, C.E.; Weinberg, Z.; Hammann, C. In-ice evolution of RNA polymerase ribozyme activity. Nature Chem. 2013, 5, 1011–1018. [Google Scholar]
- Vaidya, N.; Manapat, M.L.; Chen, I.A.; Xulvi-Brunet, R.; Hayden, E.J.; Lehman, N. Spontaneous network formation among cooperative RNA replicators. Nature 2002, 491, 72–77. [Google Scholar] [CrossRef]
- Smith, J.M.; Szathmáry, E. The Major Transitions in Evolution; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Sardanyés, J. The hypercycle: From molecular to ecosystems dynamics. In Landscape Ecology Research Trend; Nova Publishers: Hauppauge, NY, USA, 2009. [Google Scholar]
- Dugatkin, L.A. Cooperation Among Animals. An Evolutionary Perspective; Dugatkin, L.A., Ed.; Oxford Series in Ecology and Evolution; Oxford University Press: New York, NY, USA, 1997; pp. 90–115. [Google Scholar]
- Packer, C.; Ruttan, L. The evolution of cooperative hunting. Am. Nat. 1988, 132, 159–198. [Google Scholar] [CrossRef]
- Cordes, E.E.; Arthur, M.A.; Shea, K.; Arvidson, R.S.; Fisher, C.R. Modeling the Mutualistic Interactions between Tubeworms and Microbial Consortia. PLoS Biol. 2005, 3, e77. [Google Scholar] [CrossRef] [Green Version]
- van Schaik, C.P.; Kappeler, P.M. Cooperation in Primates and Humans. Mechanisms of Evolution; Kappeler, P.M., van Schaik, C.P., Eds.; Springer: New York, NY, USA, 2006; pp. 4–21. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic: New York, NY, USA, 1996. [Google Scholar]
- McNab, B.K. Energetics and the distribution of vampires. J. Mammol. 1973, 54, 131–144. [Google Scholar] [CrossRef]
- Wilkinson, G. Reciprocal food sharing in vampire bats. Nature 1984, 308, 181–184. [Google Scholar] [CrossRef]
- Wilkinson, G. The social organization of the common vampire bat. II. Mating systems, genetic structure and relatedness. Behav. Ecol. Sociobiol. 1985, 17, 123–134. [Google Scholar]
- Stander, P.E. Cooperative hunting in lions: The role of the individual. Behav. Ecol. Sociobiol. 1992, 29, 445–454. [Google Scholar] [CrossRef]
- Mills, M.G.L. Foraging behaviour of the brown hyaena (Hyaena brunnea Thunberg, 1820) in the southern Kalahari. Z. Tierpsychol. 1978, 48, 113–141. [Google Scholar] [CrossRef]
- Boesch, C.; Boesch, H.; Vigilant, L. Cooperative hunting in chimpanzees: Kinship or mutualism? In Cooperation in Primates and Humans: Mechanisms and Evolution; Springer: Berlin/Heidelberg, Germany, 2006; pp. 139–150. [Google Scholar]
- Major, P.F. Predator-Prey Interactions in Two Schooling Fishes, Caranx ignobilis and Stolephorus purpureus. Anim. Behav. 1978, 26, 760–777. [Google Scholar] [CrossRef]
- Rypstra, A.L. Aggregations of Nephila clavipes (L.) (Araneae, Araneidae) in Relation to Prey Availability. J. Arachnol. 1985, 13, 71–78. [Google Scholar]
- Ward, P.I.; Enders, M.M. Conflict and cooperation in the group feeding of the social spider Stegodyphus mimosarum. Behaviour 1985, 94, 167–182. [Google Scholar]
- Nakasuji, F.; Dyck, V.A. Evaluation of the role of Microvelia douglasi atrolineata (Bergroth) (Hemiptera: Veliidae) as predator of the brown planthopper, Nilaparvata lugens (Stal) (Homoptera: Delphacidae). Res. Pop. Ecol. 1984, 26, 148–163. [Google Scholar] [CrossRef]
- Sardanyés, J.; Solé, R.V. Bifurcations and phase transitions in spatially extended two-member hypercycles. J. Theor. Biol. 2006, 243, 468–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardanyés, J. Error threshold ghosts in a simple hypercycle with error prone self-replication. Chaos Solitons Fractals 2008, 35, 313–319. [Google Scholar] [CrossRef]
- Silvestre, D.A.M.M.; Fontanari, J.F. The information capacity of hypercycles. J. Theor. Biol. 2008, 254, 804–806. [Google Scholar] [CrossRef] [Green Version]
- Guillamon, A.; Fontich, E.; Sardanyés, J. Bifurcations analysis of oscillating hypercycles. J. Theor. Biol. 2015, 387, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boerlijst, M.C.; Hogeweg, P. Spiral wave structure in prebiotic evolution: Hypercycles stable against parasites. Phys. D 1991, 48, 17–28. [Google Scholar] [CrossRef]
- Boerlijst, M.C.; Hogeweg, P. Spatial gradients enhance persistence of hypercycles. Phys. D 1995, 88, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Cronhjort, M.B. Cluster compartmentalization may provide resistance to parasites for catalytic networks. Phys. D 1997, 101, 289–298. [Google Scholar] [CrossRef]
- Sardanyés, J.; Solé, R.V. Spatio-temporal dynamics in simple asymmetric hypercycles under weak parasitic coupling. Phys. D 2007, 231, 116–129. [Google Scholar] [CrossRef]
- Kim, P.J.; Jeong, H. Spatio-temporal dynamics in the origin of genetic information. Phys. D 2005, 203, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Sardanyés, J.; Lázaro, J.T.; Guillamon, A.; Fontich, E. Full analysis of small hypercycles with short-circuits in prebiotic evolution. Phys. D 2017, 34, 90–108. [Google Scholar] [CrossRef]
- Perona, J.; Fontich, E.; Sardanyés, J. Dynamical effects of loss of cooperation in discrete-time hypercycles. Phys. D 2020, 406, 132425. [Google Scholar] [CrossRef]
- Scott, M.D.; Chivers, S.J.; Olson, R.J.; Holland, K. Pelagic predator associations: Tuna and dolphins in the eastern tropical Pacific Ocean. Mar. Ecol. Prog. Ser. 2012, 458, 283–302. [Google Scholar] [CrossRef]
- Maldini, D. Evidence of predation by a tiger shark (Galeocerdo cuvier) on a spotted dolphin (Stenella attenuata) off Oahu, Hawaii. Aquat. Mamm. 2003, 29, 84–87. [Google Scholar] [CrossRef]
- Melillo-Sweeting, K.; Turnbull, S.D.; Guttridge, T.L. Evidence of shark attacks on Atlantic spotted dolphins (Stenella frontalis) off Bimini, The Bahamas. Mar. Mammal Sci. 2014, 30, 1158–1164. [Google Scholar] [CrossRef]
- Votier, S.C.; Furness, R.W.; Bearhop, S.; Crane, J.E.; Caldow, R.W.G.; Catry, P.; Ensor, K.; Hamer, K.C.; Hudson, A.V.; Kalmbach, E.; et al. Changes in fisheries discard rates and seabird communities. Nature 2004, 427, 727–730. [Google Scholar] [CrossRef]
- Almaraz, P.; Oro, D. Size-mediated non-trophic interactions and stochastic predation drive assembly and dynamics in a seabird community. Ecology 2011, 92, 1948–1958. [Google Scholar] [CrossRef] [PubMed]
- Hatch, J.J. Predation and piracy by gulls at a ternery in Maine. Auk 1970, 87, 244–254. [Google Scholar] [CrossRef]
- Andersson, M. Predation and kleptoparasitism by skuas in a Shetland seabird colony. Ibis 1976, 118, 208–217. [Google Scholar] [CrossRef]
- Götmark, F.; Andersson, M. Breeding Association between Common Gull Larus canus and Arctic Skua Stercorarius parasiticus. Ornis Scand. 1980, 11, 121–124. [Google Scholar] [CrossRef]
- Oro, D. Interspecific kleptoparasitism in Audouin’s Gull Larus audouinii at the Ebro Delta, northeast Spain: A behavioural response to low food availability. Ibis 1996, 138, 218–221. [Google Scholar] [CrossRef]
- Oro, D. Breeding Biology and Population Dynamics of Slender-billed Gulls at the Ebro Delta (Northwestern Mediterranean). Waterbirds 2002, 25, 67–77. [Google Scholar] [CrossRef]
- Sadoul, N.; Johnson, A.R.; Walmsley, J.; Levéque, R. Changes in the numbers and the distribution of colonial Charadriifromes breeding in the Camargue, Southern France. Col. Waterbirds 1996, 19, 46–58. [Google Scholar] [CrossRef]
- Martínez-Abraín, A.; Genovart, M.; Oro, D. Kleptoparasitism, disturbance and predation of yellow-legged gulls on Audouin’s gulls in three colonies of the Western Mediterranean. Sci. Mar. 2003, 67, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Guinet, C.; Bouvier, J. Development of intentional stranding hunting techniques in killer whale (Orcinus orca) calves at Crozet Archipelago. Can. J. Zool. 1995, 73, 27–33. [Google Scholar] [CrossRef]
- Sardanyés, J.; Solé, R. Ghosts in the origins of life? Int. J. Bifurc. Chaos 2006, 16, 2761–2765. [Google Scholar] [CrossRef]
- García-Tejedor, A.; Morán, F.; Montero, F. Influence of the Hypercyclic Organization on the Error Threshold. J. Theor. Biol. 1987, 127, 393–402. [Google Scholar] [CrossRef]
- García-Tejedor, A.; Sanz-Nuño, J.C.; Olarrea, J.; de la Rubia, F.J.; Montero, F. Influence of the Hypercycle on the Error Threshold: A Stochastic Approach. J. Theor. Biol. 1988, 134, 431–443. [Google Scholar] [CrossRef]
- Nuño, J.C.; Montero, F.; de la Rubia, F.J. Influence of external fluctuations on a hypercycle formed by two kinetically indistinguishable species. J. Theor. Biol. 1993, 165, 553–575. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassols, B.; Fontich, E.; Oro, D.; Alonso, D.; Sardanyés, J. Modelling Functional Shifts in Two-Species Hypercycles. Mathematics 2021, 9, 1809. https://doi.org/10.3390/math9151809
Bassols B, Fontich E, Oro D, Alonso D, Sardanyés J. Modelling Functional Shifts in Two-Species Hypercycles. Mathematics. 2021; 9(15):1809. https://doi.org/10.3390/math9151809
Chicago/Turabian StyleBassols, Bernat, Ernest Fontich, Daniel Oro, David Alonso, and Josep Sardanyés. 2021. "Modelling Functional Shifts in Two-Species Hypercycles" Mathematics 9, no. 15: 1809. https://doi.org/10.3390/math9151809
APA StyleBassols, B., Fontich, E., Oro, D., Alonso, D., & Sardanyés, J. (2021). Modelling Functional Shifts in Two-Species Hypercycles. Mathematics, 9(15), 1809. https://doi.org/10.3390/math9151809





