1. Introduction
Cancer remains a large global health issue and is a major cause of deaths annually, despite there being several successful drugs that have been used for the treatment of tumour diseases, for example, cisplatin (CIS), carboplatin (CAR), and doxorubicin (DOX). CIS is utilized in the treatment of solid tumours, and it is deemed as the most effective anticancer drug. It has been utilized for the treatment of number of types of human cancers, including lung, head, ovarian, neck, testicular, and bladder cancers [
1]. The first synthesis of cisplatin was carried out in 1844 by Michele Peyrone, and in 1893, Alfred Werner offered the first explanation of the chemical structure of the drug [
1]. CAR, derived from cisplatin, is also an anticancer drug, and it has the same mechanism of action; the only differences between these two drugs are their toxicity and structure [
2]. In addition, DOX is an anti-cancer that is also utilized as a chemotherapy drug, and it was isolated from bacteria in the early 1960s [
3]. It shows potential in the treatment of breast cancer, soft tissue sarcomas, childhood tumours, and many other cancers [
4,
5]. These drugs are proven to be effective in the treatment of many of the cancers, and the damage that they may incur to healthy cells is a known lacuna. Moreover, anticancer drugs are toxic to deoxyribonucleic acid (DNA), and they target nuclear DNA. These drugs may cause oxidative DNA death, and they considerably damage cells by inhibiting topoisomerase and generating free radicals [
6].
Over time, several nanocarriers have been presented as drug delivery carriers, such as nanotubes, inorganic nanoparticles, liposomes, and lipid particles [
7,
8,
9]. For example, the use of graphene and graphene-based materials may resensitise cells and tumour tissues to reduce the side effects of cytotoxic chemotherapy [
10]. In addition, gold nanostructures can be used for light-mediated cancer theranostics, as many gold nanostructures have shown tremendous potential in the field of experimental medicine for plasmonic photo-thermal therapy [
11]. The encapsulation of these drugs by using nanocarriers not only allows for the drugs to be carried, but they are also safely and effectively delivered to target cells. Moreover, this can help to avoid the problem of non-specific toxicity, and to increase the efficacy of these drugs on target cells, they should be delivered directly to the nucleus to initiate a pharmacological response. Nanoparticles and nanotubes can be designed to deliver multiple drugs to target cells while avoiding the known toxic effect of anticancer drugs on healthy cells by using nanotechnology, which depends on surface functionality, the surrounding environment, and the shape and size of materials. These characteristics can be engineered via incorporation either within nanocarriers’ interiors or on their surface so as to enable them to carry one or more functional groups. For example, nanocarriers can act as disparity agents (magnetic, optical, or other) to enable a means of tracking treatment, and as near-infrared agents, they can provide recognition signals for targeting or the capacity to cause cell death via heat. In particular, nanotubes with one open end can be used, as then they can be filled and subsequently capped to provide an appropriate filling. Assuming the nanotube is a vehicle for the delivery of a drug, the process may consist of the following: (i) the addition of a chemical receptor to the nanotube’s surface; (ii) capping of the open end when the drug is filled inside the nanotube; (iii) insertion of the nanocapsule inside the body either by intravenous injection or orally; (iv) target cells internalizing the nanocapsule by receptor mediation; (v) biodegradation of the cap into the cell (otherwise it will be removed); and, finally, (vi) release of drug molecules inside the cell.
There are several advantages of using nanoparticles and nanotubes as drug carriers; for example, drugs might be protected from interacting with the biological environment and from premature degradation; they are able to reach the cell nucleus; and they possess the ability to be readily taken up by cells. However, their main advantage is their multifunctionality, which means that they may be incorporated in multiple therapeutic, diagnostic, targeting, and barrier-avoiding agents. Nanotubes (NTs), such as carbon nanotubes (CNTs), silicon nanotubes (SiNTs), and boron nitride nanotubes (BNNTs), have several important properties, such as chemical stability and mechanical properties; therefore, they have a large number of potential applications, especially in the medical area, where they can act as drug carrier systems. NTs may be configured by rolling up a sheet in a cylindrical shape, where they can be described in terms of two integers
; this is called a chiral [
12,
13]. The tubes with
are referred to as zigzag nanotubes, and those with
are called armchair nanotubes.
A number of researchers have studied the possibility of reducing the side effects of the chemotherapy treatment by directly transporting anticancer drugs to target cells using a drug carrier. Tripisciano et al. showed that CIS can be encapsulated inside single-wall carbon nanotubes (SWCNTs) with a radius of 0.60 to 0.8 nm [
14]. Roosta et al. studied encapsulation behaviour of CIS drug into (10, 0) CNTs and BNNTs, and they showed that, after computing the complexation free energies of CIS inside NTs, the encapsulation is stable, and BNNTs appear to provide optimal drug delivery compared with carbon nanotubes [
15]. Hosni et al. examined the behaviour of the encapsulation of CIS molecules into different sizes of CNTs a with diameter range from 6.26 Å to 12.04 Å. Using ab initio calculations, they observed that weak stabilization energy of the drug into (11, 0) CNT equals −70 kcal/mol, which corresponds to a CNT with diameter of 8.5, and the drug could not be inserted inside CNTs whose diameters were less than 7.6 Å [
16]. Ali-Boucetta et al. studied the insertion of the complexes of an antitumour drug into CNTs, and they showed that the DOX molecule can enter the CNT with a diameter larger than 12.5 Å [
17]. Hilder and Hill used the hybrid discrete–continuum approximation and the continuum approach together with the Lennard–Jones potential function to determine the interaction energies of the encapsulation of CIS and DOX drugs into different types of nanotubes, including CNTs, BNNTs, and SiNTs [
18,
19,
20,
21]. Their results show the size of tubes that gives rise to the optimum encapsulation mechanics for drug molecules, and they showed that, for CIS and DOX acceptance, nanotubes must have a radius of at least 4.785 Å and 8.855 Å, respectively. Mejri et al. used MD simulations to investigate the interaction between anticancer CIS molecules and different types of CNTs in order to study their encapsulation behaviour. Their results show the most favourable CNT radius is about 6.9 Å [
22]. Mahdavifar and Moridzadeh used density functional theory to investigate the encapsulation of CIS molecule inside (7, 7) CNTs and BNNTs [
23]. Nejad et al. investigated the adsorption of DOX molecules into BNNTs of varying radii, and they found that adsorption of DOX is strong for small nanotubes (radius of 9 Å) and that the adsorption energy decreases when the radius of the nanotube is larger (radii: 12–15 Å) [
24].
Moreover, Meng et al. found that SWCNTs are highly efficient in carrying DOX to cancer tissue [
25]. Their results indicate that the overall stabilities of the complexes are dependent on the van der Waals forces, electrostatic interactions, and hydrogen bonding, and they suggest that BNNTs can act as better drug delivery vehicles than CNTs due to the predicted electronic and structural properties of BNNTs. Khatti and Hashemianzadeh investigated the insertion of CAR drugs inside boron nitride nanotubes (14 Å diameter) using traditional molecular dynamics (MD) simulation. Their results show that the van der Waals interaction between CAR and BNNTs has an influence on encapsulating the drug into BNNTs [
26]. El Khalifi et al. investigated the interactions and loading of CAR anticancer drugs inside single-walled (10, 10) CNTs using density functional theory (DFT) calculations, and they found that the stability of the carboplatin molecule inside the (10, 10) CNTs is strong and that the deep energy well is around −0.55 (eV) [
27] Moreover, El Khalifi et al. studied the stability and mechanism of CAR molecules inside (10, 10) BNNTs using MD simulations, and their results indicate that there is a large storage capacity for CAR molecules in BNNTs. In addition, the interaction energy between the CAR molecule and BNNTs is about −40 (kcal/mol) [
28]. Bentin et al. studied the confinement of CAR molecule inside BNNTs using functional theory and MD simulations, and their results show that the insertion of the drug molecule inside BNNTs depends on the tube radius, and the (12, 12) BNNT is the most favourable tube to encapsulate the drug molecule [
29].
The purpose of this work is to computationally examine the energetics of three different types of the anticancer drugs encapsulated into carbon, silicon, and boron nitride nanotubes in order to determine the appropriate size and type of nanotubes to encapsulate anticancer drugs, where the nanotubes are used as drug vehicles to carry anticancer drugs to cancer tissue. The system of the interactions between drug molecules and nanotubes comprising a large amount of atoms is considered here; thus, the use atomistic modelling or molecular dynamics may be computationally expensive. In terms of a mathematical model, the continuum approach with the Lennard–Jones function is utilized to calculate the interaction energies of drug molecules in the nanotubes.
2. Modelling Approach
Here, I determine the biophysical model that explains the encapsulation of anticancer drug molecules into nanotubes as a mathematical model by using van der Waals forces between non-bonded atoms. The proposed model is designed to examine the mechanism of encapsulation of three different anticancer compounds, namely, cisplatin, carboplatin, and doxorubicin, inside carbon, silicon, and boron nitride nanotubes. I adopt the continuum approach together with the 6–12 Lennard–Jones potential to determine the van der Waals interaction energy. The 6–12 Lennard–Jones potential with the distance
between two atoms is expressed as
where
A and
B indicate the two parameters (attractive and repulsive) of the Lennard–Jones potential. These are related to the well-depth
and the van der Waals distance
, which are called the empirical combining rules, where
and
, and they are listed in
Table 1. The values of the well-depths
and the van der Waals diameters
for C, B, N, Si, Pt, H, Cl, and O atoms are taken from Rappi et al. [
30].
With reference to
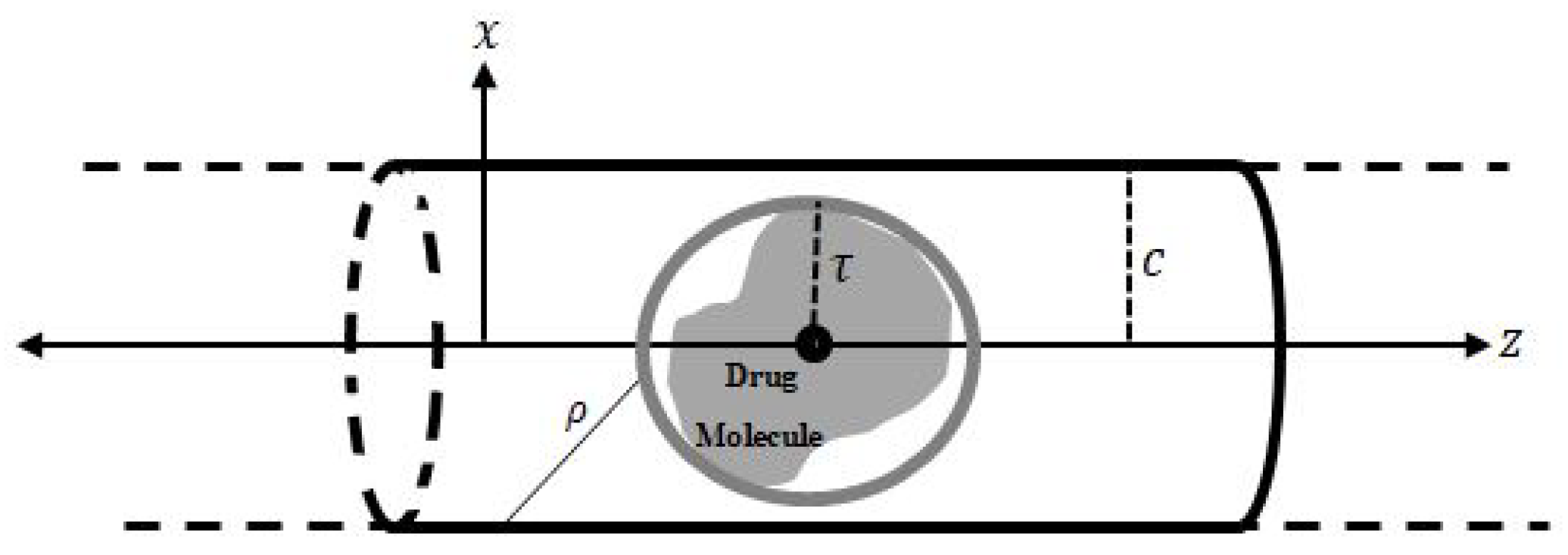
Figure 1, the Lennard–Jones potential and a continuum approach are used to determine the interaction energies of the drug molecule inside the nanotube, where the centre of the molecular mass is assumed to be situated on the tube axis (
z-axis). A mathematical model for a possible configuration of the drug molecule that is a sphere is proposed. In addition, the tubes are assumed to be in a cylindrical shell configuration with a radius of
c, which can be parametrised by
, where
and
. Firstly, I calculate the interaction energies of an atom at point
Q into infinite nanotubes, as shown in
Figure 2; subsequently, I assume that the atom is within the volume of the drug molecules. Moreover, the atom at point
Q is assumed to have coordinates
, where
; thus, the distance
from a typical surface element of the nanotube surface to point
Q may given by
. The continuum approach assumes that the atoms are uniformly distributed throughout the volume or over the surface of the molecule, and the molecular interatomic interaction energy is determined through integration over the volume or surface of each molecule [
31]. Thus, the energies interacting between the nanotube and the atom at point
Q can be obtained by carrying out a surface integral equation of the Lennard–Jones function over the surface of the nanotube, namely
where
is the mean surface density of the nanotubes, and they are given by
,
and
Å
[
18,
19]. Define the integral
as
Now, this integral must be evaluated by letting
and substituting
, which gives
where
is the beta function, and it may be evaluated using
where
is the gamma function. Making the change of the variable
, the integral
became
where
, and now the integral is in the Euler form. Therefore, special functions, such as the hypergeometric function
and gamma function
, may be used to solve this integral, and then by utilizing the quadratic transformation from [
32],
becomes
where
is the Pochhammer symbol. Here, the energy
for the interaction between an internal atom
Q and an infinite nanotube can be obtained by substituting (
3) into (
1). Next, the atom at point
Q is assumed to be within the volume element of the drug molecule. Thus, the total interaction energies arising from the drug molecules can be calculated by applying a volume integral of
over the volume of the molecule, which can be given as
where
is the distance from a typical volume element of the molecule to the nanotube axis, and
is the mean volume density of the drug. Note that the radius for each drug is obtained from the references that are shown in
Table 2, and then the volume of the sphere is determined using the formula (
), which is used to calculate the atomic volume density as
. In addition,
is given by (
3) and
Moreover, as mentioned above, the drug molecule can be modelled as a sphere centred at the origin with radius
, as shown in
Figure 1. The relation between the rectangular and spherical coordinates is given by
,
and
. Thus, the spherical coordinate system of the drug molecule is given by
where
,
and
. Therefore, the distance
is given by
and the volume element
. Based on (
3), to determine
, it may be necessary to calculate the integral of the form
where
m is a positive integer, which appears in the summation from (
3). Now, Equation (
6) is evaluated
where this integral can be evaluated using
In this case,
and
. Thus,
becomes
Thus, from (
3) and (
5), I obtain
and Expression (
8) completes Expression (
4), which can be given as
3. Discussion and Numerical Results
In the current study, the numerical results showing the interaction energies of the three encapsulated drug molecules inside CNTs, BNNTs, and SiNTs are presented. Using Maple packages with the aforementioned constant values together with the values given in
Table 1 and
Table 2, the relationship between the total interaction energies
for the drugs situated in the nanotubes and the radius
c is plotted, as shown in
Figure 3. The optimal radii of the nanotubes that minimize the energies for each drugs are given in
Table 3. From
Table 3 and
Figure 3, the sizes of the radii of the nanotubes that encapsulate each drug can be deduced. For the three nanotubes examined, i.e., CNTs, BNNTs, and SiNTs, to encapsulate the drug molecules, the tube radius must be at least 5.493 Å, 5.497 Å, and 5.691 Å for CIS; 6.452 Å, 6.459 Å, and 6.643 Å for CAR; and 10.208 Å, 10.209 Å, and 10.389 Å for DOX, respectively. In addition, the results show that the profile of encapsulation for SiNTs is significantly different from that for CNTs and BNNTs. That is, the radii of CNTs and BNNTs that are required to encapsulate anticancer drugs are smaller than the radius of SiNTs; thus, a fewer amount of materials are required for the delivery of drug, which means a reduced level of toxicity in the system. Moreover, the results show that there is minimal difference in the interaction between the drugs and CNTs and that between the drugs and BNNTs. The interaction between each drug and BNNTs is slightly stronger than that between the drugs and CNTs, as the former reaches the lowest minimum energy. Overall, to demonstrate the validity of our study and calculation method of the encapsulation of the drugs into nanotubes, the results were compared with those of other research works. As shown in
Table 4, our results show good agreement in comparison with the results of other studies and are well within the spectrum of results in the literature. Notably, the difference in the results in some cases can be attributed to the geometric structure and the physical parameters adopted here, and the fact that the model does not take into account all interactions must also be considered, such as the solvent which affects the interactions. Further research should investigate the acceptance of these drugs entering a semi-infinite tube when they are outside the nanotube as well as the different configurations of a number of drug molecules inside the tube, such as line chain, zigzag, and spiral configurations.
4. Conclusions
In this paper, a mathematical model was developed to examine the encapsulation of three anticancer drugs into CNTs, BNNTs, and SiNTs. The study resulted in the development of the use of mathematical modelling to investigate the use of nanotechnology in the area of medicine, specifically in drug delivery, to reduce the side effects and toxicity of drugs. The results suggest that the encapsulation of drug molecules inside large nanotubes depends on the location where the drug model with a large size, such as doxorubicin, tends to be placed.
In addition, the results show that the optimal radii c of the carbon, silicon, and boron nitride nanotubes for encapsulating cisplatin, carboplatin, and doxorubicin are approximately ≥5.493 Å, ≥6.452 Å, and ≥10.208 Å, respectively. Knowledge of the ideal size of the tube is necessary for the design of nanotubes for the purpose of loading genes and drug molecules; therefore, our results may be of assistance in experimental and simulation studies designing nanotubes to serve as biocompatible transporters. However, more studies are required to elucidate several of the mechanisms associated with the use of nanotubes in medicine.