Effect of Bariatric Surgery on Metabolic Syndrome, Framingham Risk Scores and Thyroid Function during One-Year Follow-Up: A Saudi Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Obesity and Overweight. 2021. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 July 2021).
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Montesi, L.; Ghoch, M.E.; Brodosi, L.; Calugi, S.; Marchesini, G.; Grave, R.D. Long-term weight loss maintenance for obesity: A multidisciplinary approach. Diabetes Metab. Syndr. Obes. Targets Ther. 2016, 9, 37. [Google Scholar]
- ASMBS. Bariatric Surgery Procedures|ASMBS. 2021. Available online: https://asmbs.org/patients/bariatric-surgery-procedures (accessed on 28 July 2021).
- Singh, A.K.; Singh, R.; Kota, S.K. Bariatric surgery and diabetes remission: Who would have thought it? Indian J. Endocrinol. Metab. 2015, 19, 563. [Google Scholar] [CrossRef] [PubMed]
- SchSchiavon, C.A.; Bersch-Ferreira, A.C.; Santucci, E.V.; Oliveira, J.D.; Torreglosa, C.R.; Bueno, P.T.; Frayha, J.C.; Santos, R.N.; Damiani, L.P.; Noujaim, P.M.; et al. Effects of Bariatric Surgery in Obese Patients with Hypertension: The GATEWAY Randomized Trial (Gastric Bypass to Treat Obese Patients with Steady Hypertension). Circulation 2018, 137, 1132. [Google Scholar] [CrossRef] [PubMed]
- Benraoune, F.; Litwin, S.E. Reductions in Cardiovascular Risk after Bariatric Surgery. Curr. Opin. Cardiol. 2011, 26, 555. [Google Scholar] [CrossRef] [PubMed]
- Aftab, H.; Risstad, H.; Søvik, T.T.; Bernklev, T.; Hewitt, S.; Kristinsson, J.A.; Mala, T. Five-year outcome after gastric bypass for morbid obesity in a Norwegian cohort. Surg. Obes. Relat. Dis. 2014, 10, 71–78. [Google Scholar] [CrossRef] [PubMed]
- English, W.J.; Spann, M.D.; Aher, C.V.; Williams, D.B. Cardiovascular risk reduction following metabolic and bariatric surgery. Ann. Transl. Med. 2020, 8 (Suppl. 1), S12. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355. [Google Scholar] [CrossRef]
- Svare, A.; Nilsen, T.I.L.; Bjøro, T.; Åsvold, B.O.; Langhammer, A. Serum TSH related to measures of body mass: Longitudinal data from the HUNT study, norway. Clin. Endocrinol. 2011, 74, 769–775. [Google Scholar] [CrossRef]
- Sanyal, D.; Raychaudhuri, M. Hypothyroidism and obesity: An intriguing link. Indian J. Endocrinol. Metab. 2016, 20, 554–557. [Google Scholar] [CrossRef]
- Granzotto, P.C.D.; Junior, C.O.M.; Strobel, R.; Radominski, R.; Graf, H.; de Carvalho, G.A. Thyroid function before and after Roux-en-Y gastric bypass: An observational study. Surg. Obes. Relat. Dis. 2020, 16, 261–269. [Google Scholar] [CrossRef] [PubMed]
- IDF. Consensus Statements. 2006. Available online: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html (accessed on 28 July 2021).
- Framingham Risk Score for Hard Coronary Heart Disease—MDCalc. Available online: https://www.mdcalc.com/calc/38/framingham-risk-score-hard-coronary-heart-diseas (accessed on 18 November 2022).
- Al-Rubeaan, K.; Bawazeer, N.; Al Farsi, Y.; Youssef, A.M.; Al-Yahya, A.A.; AlQumaidi, H.; Al-Malki, B.M.; Naji, K.A.; Al-Shehri, K.; Al Rumaih, F.I. Prevalence of metabolic syndrome in Saudi Arabia—A cross sectional study. BMC Endocr. Disord. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, C.; Tang, X.; Yan, X.; Zhou, H.-D.; Liu, J.; Ji, L.; Yang, X.; Zhou, Z. Prevalence of Metabolic Syndrome and Its Determinants in Newly-Diagnosed Adult-Onset Diabetes in China: A Multi-Center, Cross-Sectional Survey. Front. Endocrinol. 2019, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Ammar, W.; Basset, H.A.; Al Faramawy, A.; Hegazy, T.; Sharaf, Y. Bariatric surgery and cardiovascular outcome. Egypt. Heart J. 2020, 72, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alomar, A.O.; Shaheen, M.F.; Almaneea, A.S.; Althaqeb, E.K.; Alshahrani, Z.M.; Jarman, Y.A.; Alhabdan, S. The Effect of Bariatric Surgery on Metabolic Syndrome: A Three-Center Experience in Saudi Arabia. Obes. Surg. 2021, 31, 3630–3636. [Google Scholar] [CrossRef]
- Martini, F.; Anty, R.; Schneck, A.-S.; Casanova, V.; Iannelli, A.; Gugenheim, J. Predictors of metabolic syndrome persistence 1 year after laparoscopic Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2015, 11, 1054–1060. [Google Scholar] [CrossRef]
- Cruz-Muñoz, N.D.L.; Lopez-Mitnik, G.; Arheart, K.L.; Miller, T.L.; Lipshultz, S.E.; Messiah, S.E. Effectiveness of Bariatric Surgery in Reducing Weight and Body Mass Index Among Hispanic Adolescents. Obes. Surg. 2013, 23, 150. [Google Scholar] [CrossRef][Green Version]
- Hasan, N.A.; Freije, A.; Abualsel, A.; Al-Saati, H.; Perna, S. Effect of Bariatric Surgery on Weight Loss, Nutritional Deficiencies, Postoperative Complications and Adherence to Dietary and Lifestyle Recommendations: A retrospective cohort study from Bahrain. Sultan Qaboos Univ. Med. J. 2020, 20, 344–351. [Google Scholar] [CrossRef]
- Jeon, D.J.; Kim, S.H.; Kim, J.H.; Kim, Y.J. Impact of Bariatric Surgery on Cardiovascular Risk Reduction in Korean Obese Patients. J. Metab. Bariatr. Surg. 2019, 8, 1–7. [Google Scholar] [CrossRef][Green Version]
- Doumouras, A.G.; Wong, J.A.; Paterson, J.M.; Lee, Y.; Sivapathasundaram, B.; Tarride, J.-E.; Thabane, L.; Hong, D.; Yusuf, S.; Anvari, M. Bariatric Surgery and Cardiovascular Outcomes in Patients with Obesity and Cardiovascular Disease: A Population-Based Retrospective Cohort Study. Circulation 2021, 143, 1468–1480. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Picca, A.; Tosato, M.; Martone, A.M.; Ortolani, E.; Sisto, A.; D’Angelo, E.; Serafini, E.; Desideri, G.; et al. Body Mass Index is Strongly Associated with Hypertension: Results from the Longevity Check-Up 7+ Study. Nutrients 2018, 10, 1976. [Google Scholar] [CrossRef] [PubMed]
- Gilardini, L.; Redaelli, G.; Croci, M.; Conti, A.; Pasqualinotto, L.; Invitti, C. Effect of a Modest Weight Loss in Normalizing Blood Pressure in Obese Subjects on Antihypertensive Drugs. Obes. Facts 2016, 9, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.B. Hypertension in obesity and the impact of weight loss. Curr. Cardiol. Rep. 2017, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Young, J.; Kale-Pradhan, P. Effect of Bariatric Surgery on Hypertension: A Meta-analysis. Ann. Pharmacother. 2014, 48, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; El-Syed, M.K.; Saad, R.M. Effects of Laparoscopic Sleeve Gastrectomy on Hypertensive Morbidly Obese Patients. Egypt. J. Hosp. Med. 2019, 74, 1804–1808. [Google Scholar] [CrossRef]
- Ding, L.; Zhuo, C.; Fan, Y.; Zhang, Y.; Li, H.; Qi, D.; Tang, S.; Cui, J.; He, Q.; Liu, M. Comparative long-term effectiveness and safety of primary bariatric surgeries in treating type 2 diabetes mellitus in adults: A protocol for systematic review and network meta-analysis of randomised controlled trials. BMJ Open 2019, 9, e028430. [Google Scholar] [CrossRef]
- McGlone, E.R.; Carey, I.; Veličković, V.; Chana, P.; Mahawar, K.; Batterham, R.L.; Hopkins, J.; Walton, P.; Kinsman, R.; Byrne, J.; et al. Bariatric surgery for patients with type 2 diabetes mellitus requiring insulin: Clinical outcome and cost-effectiveness analyses. PLoS Med. 2020, 17, e1003228. [Google Scholar] [CrossRef]
- Park, J.Y. Prediction of Type 2 Diabetes Remission after Bariatric or Metabolic Surgery. J. Obes. Metab. Syndr. 2018, 27, 213–222. [Google Scholar] [CrossRef]
- Holst, J.J.; Madsbad, S.; Bojsen-Møller, K.N.; Svane, M.S.; Jørgensen, N.B.; Dirksen, C.; Martinussen, C. Mechanisms in bariatric surgery: Gut hormones, diabetes resolution, and weight loss. Surg. Obes. Relat. Dis. 2018, 14, 708–714. [Google Scholar] [CrossRef]
- Schmidt, J.B.; Pedersen, S.D.; Gregersen, N.T.; Vestergaard, L.; Nielsen, M.S.; Ritz, C.; Madsbad, S.; Worm, D.; Hansen, D.L.; Clausen, T.R.; et al. Effects of RYGB on energy expenditure, appetite and glycaemic control: A randomized controlled clinical trial. Int. J. Obes. 2016, 40, 281–290. [Google Scholar] [CrossRef]
- Lee, W.-J.; Almalki, O. Mechanism of diabetes control after metabolic surgery. Ann. Laparosc. Endosc. Surg. 2017, 2, 128. [Google Scholar] [CrossRef]
- Jiménez, J.-M.; Carbajo, M.-A.; López, M.; Cao, M.-J.; Rúiz-Tovar, J.; García, S.; Castro, M.-J. Changes in Lipid Profile, Body Weight Variables and Cardiovascular Risk in Obese Patients Undergoing One-Anastomosis Gastric Bypass. Int. J. Environ. Res. Public Health 2020, 17, 5858. [Google Scholar] [CrossRef] [PubMed]
- Liaskos, C.; Koliaki, C.; Alexiadou, K.; Argyrakopoulou, G.; Tentolouris, N.; Diamantis, T.; Alexandrou, A.; Katsilambros, N.; Kokkinos, A. Roux-en-Y Gastric Bypass Is More Effective than Sleeve Gastrectomy in Improving Postprandial Glycaemia and Lipaemia in Non-diabetic Morbidly Obese Patients: A Short-term Follow-up Analysis. Obes. Surg. 2018, 28, 3997–4005. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Jones, P.H.; Jacobson, T.A.; Cohen, D.E.; Orringer, C.E.; Kothari, S.; Azagury, D.E.; Morton, J.; Nguyen, N.T.; Westman, E.C.; et al. Lipids and bariatric procedures part 1 of 2: Scientific statement from the National Lipid Association, American Society for Metabolic and Bariatric Surgery, and Obesity Medicine Association: Executive Summary. J. Clin. Lipidol. 2015, 10, 15–32. [Google Scholar] [CrossRef]
- Nickel, F.; Tapking, C.; Benner, L.; Sollors, J.; Billeter, A.T.; Kenngott, H.G.; Bokhary, L.; Schmid, M.; Von Frankenberg, M.; Fischer, L.; et al. Bariatric Surgery as an Efficient Treatment for Non-Alcoholic Fatty Liver Disease in a Prospective Study with 1-Year Follow-up: BariScan Study. Obes. Surg. 2018, 28, 1342–1350. [Google Scholar] [CrossRef]
- Aksoy, E.K.; Göktaş, Z.; Albuz, Ö.; Akpınar, M.Y.; Öztürk, D.; Buluş, H.; Uzman, M. Effects of sleeve gastrectomy on liver enzymes, non-alcoholic fatty liver disease-related fibrosis and steatosis scores in morbidly obese patients: First year follow-up. J. Lab. Med. 2019, 43, 115–122. Available online: https://www.degruyter.com/document/doi/10.1515/labmed-2018-0181/html (accessed on 5 September 2021). [CrossRef]
- Motamedi, M.A.K.; Khalaj, A.; Mahdavi, M.; Valizadeh, M.; Hosseinpanah, F.; Barzin, M. Longitudinal Comparison of the Effect of Gastric Bypass to Sleeve Gastrectomy on Liver Function in a Bariatric Cohort: Tehran Obesity Treatment Study (TOTS). Obes. Surg. 2019, 29, 511–518. Available online: https://pubmed.ncbi.nlm.nih.gov/30298459/ (accessed on 5 September 2021). [CrossRef]
- Wang, B.; Song, R.; He, W.; Yao, Q.; Li, Q.; Jia, X.; Zhang, J.-A. Sex Differences in the Associations of Obesity with Hypothyroidism and Thyroid Autoimmunity among Chinese Adults. Front. Physiol. 2018, 9, 1397. [Google Scholar] [CrossRef]
- Song, R.-H.; Wang, B.; Yao, Q.-M.; Li, Q.; Jia, X.; Zhang, J.-A. The Impact of Obesity on Thyroid Autoimmunity and Dysfunction: A Systematic Review and Meta-Analysis. Front. Immunol. 2019, 10, 2349. [Google Scholar] [CrossRef]
- Torlinska, B.; Nichols, L.; Mohammed, M.A.; McCabe, C.; Boelaert, K. Patients Treated for Hyperthyroidism Are at Increased Risk of Becoming Obese: Findings from a Large Prospective Secondary Care Cohort. Thyroid 2019, 29, 1380–1389. [Google Scholar] [CrossRef]
- Lips, M.A.; Pijl, H.; van Klinken, J.B.; de Groot, G.H.; Janssen, I.M.; Van Ramshorst, B.; Van Wagensveld, B.A.; Swank, D.J.; Van Dielen, F.; Smit, J.W.; et al. Roux-en-Y gastric bypass and calorie restriction induce comparable time-dependent effects on thyroid hormone function tests in obese female subjects. Eur. J. Endocrinol. 2013, 169, 339–347. Available online: https://eje.bioscientifica.com/view/journals/eje/169/3/339.xml (accessed on 5 September 2021). [CrossRef]
- Neves, J.S.; AMTCO Group; Oliveira, S.C.; Souteiro, P.; Pedro, J.; Magalhães, D.; Guerreiro, V.; Bettencourt-Silva, R.; Costa, M.M.; Santos, A.C.; et al. Effect of Weight Loss after Bariatric Surgery on Thyroid-Stimulating Hormone Levels in Patients with Morbid Obesity and Normal Thyroid Function. Obes. Surg. 2018, 28, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Taylor, P.N.; Javaid, H.; Tennant, B.P.; Geen, J.; Aldridge, A.; Okosieme, O. Seasonal Variation of Vitamin D and Serum Thyrotropin Levels and Its Relationship in A Euthyroid Caucasian Population. Endocr. Pract. 2018, 24, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Bieler, B.M.; Gaughan, J.; Khan, M.; Rao, G.; Hunter, K.; Morgan, F.H. Lack of An Association between BMI and TSH in Treated Hypothyroid Patients and Euthyroid Controls. Endocr. Pract. 2016, 22, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Ranabir, S.; Archana, N.; Ipsita, R.; Naorem, S.; Prasad, L. Is there a correlation between body mass index and thyroid stimulating hormone? Endocrinol. Metab. Int. J. 2019, 7, 151–154. Available online: https://medcraveonline.com/EMIJ/EMIJ-07-00262.php (accessed on 5 September 2021). [CrossRef]
- Mwafy, S.; Yassin, M.; Mousa, R. Thyroid hormones, lipid profile and anthropometric changes after programmed weight loss in Palestinian obese adult females. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 269–273. [Google Scholar] [CrossRef]
- Chang, P.-C.; Chen, K.-H.; Jhou, H.-J.; Chen, P.-H.; Huang, C.-K.; Lee, C.-H.; Chang, T.-W. Promising effects of 33 to 36 Fr. bougie calibration for laparoscopic sleeve gastrectomy: A systematic review and network meta-analysis. Sci. Rep. 2021, 11, 15217. [Google Scholar] [CrossRef]

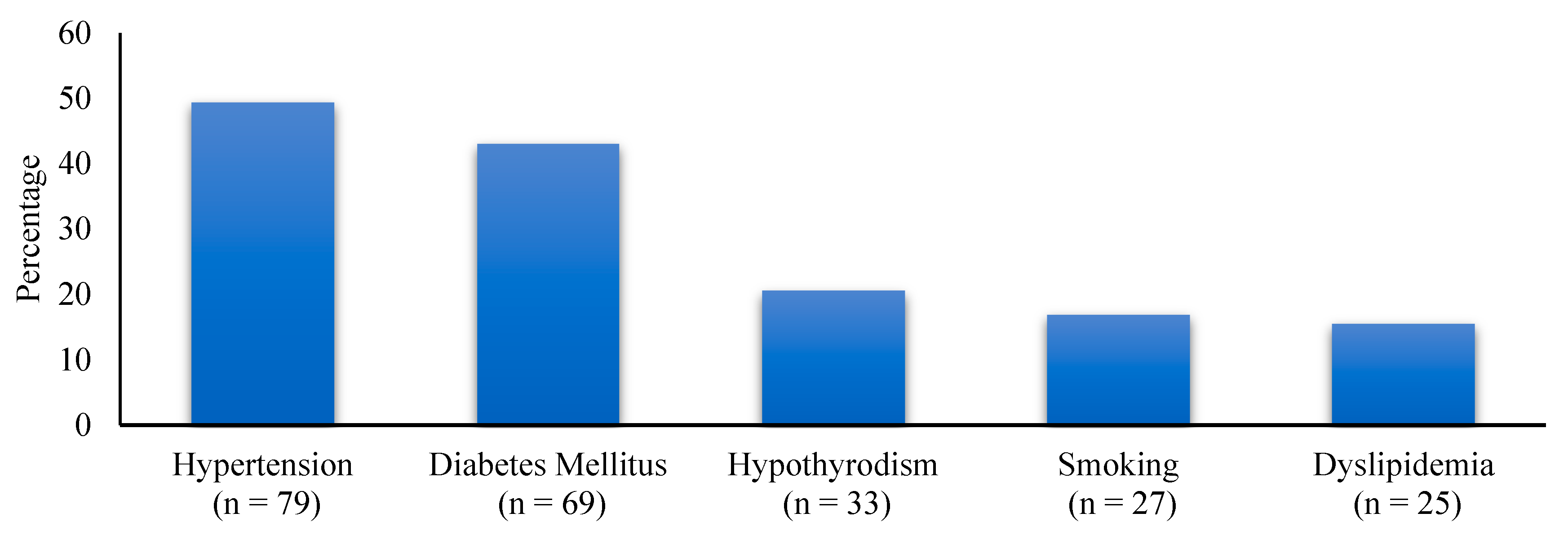
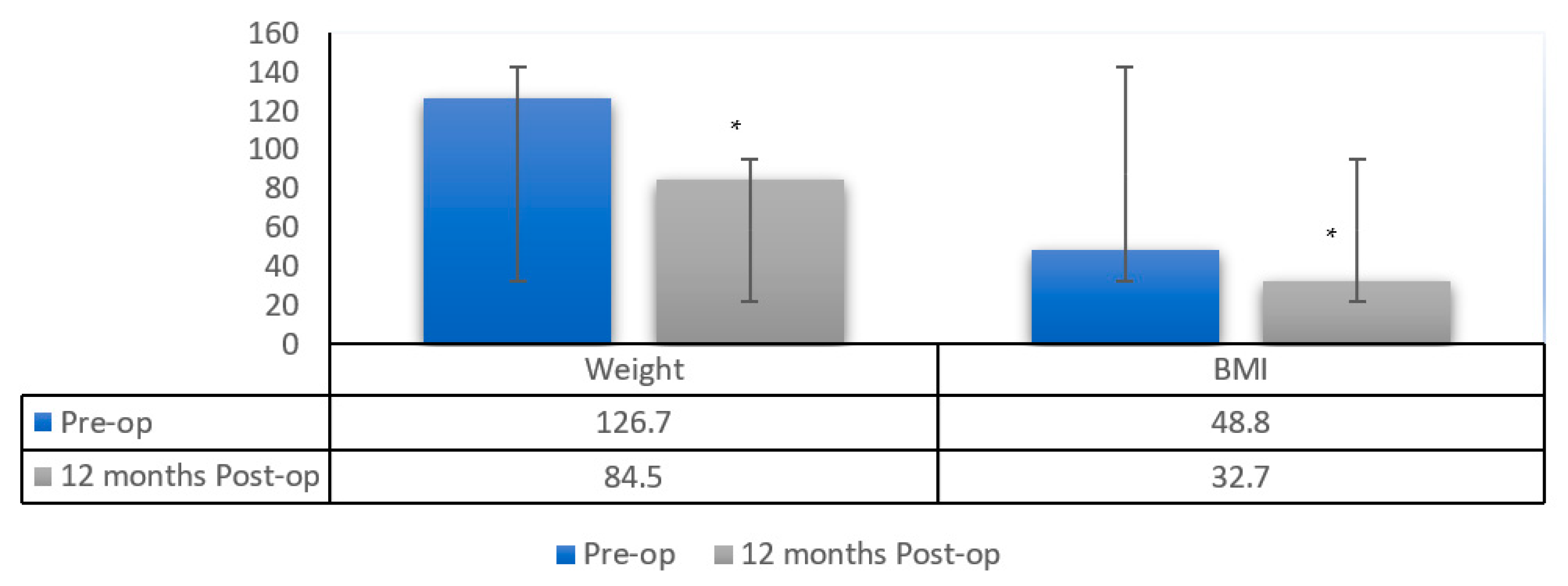
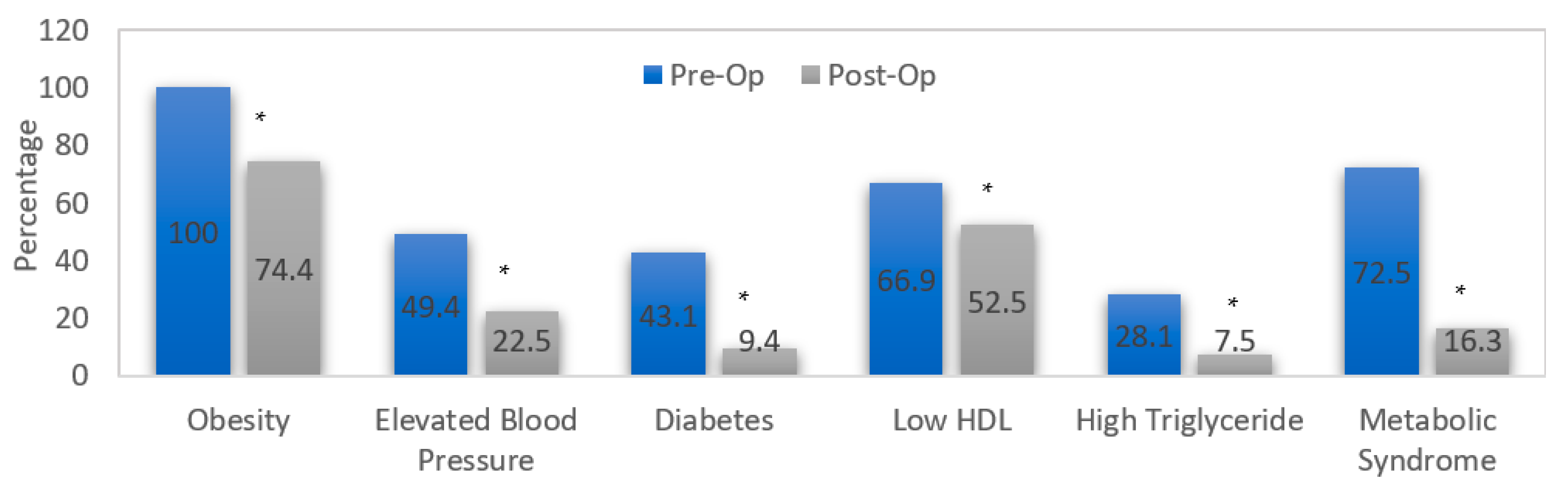
| Parameter | Preoperative | 3 Months | 12 Months | p-Value |
|---|---|---|---|---|
| Systolic Blood Pressure (mm Hg) | 139.5 a ± 13.7 | 130 b ± 9.9 | 125.1 c ± 7.6 | <0.001 * |
| Diastolic Blood Pressure (mm Hg) | 75.4 a ± 9.7 | 71.8 b ± 8.7 | 71.6 b ± 7.6 | <0.001 * |
| Hba1c(%) | 6.9 a ± 1.8 | 6 b ± 1.4 | 5.4 c ± 0.8 | <0.001 * |
| TSH (mIU/L) | 2.8 ± 2.4 | 2.3 ± 1.4 | 3.0 ± 1.6 | 0.388 |
| AST (units/L) | 23.6 a ± 16 | 23.2 a ± 15.4 | 19.6 b ± 11.8 | 0.022 * |
| ALT (IU/L) | 31.6 a ± 23.5 | 31.7 a ± 28.1 | 24.9 b ± 15.8 | 0.012 * |
| Parameter | Preoperative | 3 Months | 12 Months | p-Value |
|---|---|---|---|---|
| HDL (mg/dL) | 56 ± 14.8 | 56.6 ± 18 | 59 ± 13.4 | 0.199 |
| LDL (mg/dL) | 126 a ± 49.4 | 112.9 b ± 39.5 | 96.9 c ± 28.9 | <0.001 * |
| Triglycerides (mg/dL) | 126.6 a ± 53.9 | 107.7 b ± 40.4 | 92.4 c ± 36.4 | <0.001 * |
| Total Cholesterol (mg/dL) | 205.1 a ± 52.8 | 189.7 b ± 40.9 | 176.3 b ± 106.8 | 0.002 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamro, N.; Azhri, A.S.; Almuqati, A.; Azzeh, F.; Azhar, W.; Qadhi, A.; Almohmadi, N.H.; Abusudah, W.F.; Ghafouri, K. Effect of Bariatric Surgery on Metabolic Syndrome, Framingham Risk Scores and Thyroid Function during One-Year Follow-Up: A Saudi Retrospective Study. Healthcare 2022, 10, 2530. https://doi.org/10.3390/healthcare10122530
Alamro N, Azhri AS, Almuqati A, Azzeh F, Azhar W, Qadhi A, Almohmadi NH, Abusudah WF, Ghafouri K. Effect of Bariatric Surgery on Metabolic Syndrome, Framingham Risk Scores and Thyroid Function during One-Year Follow-Up: A Saudi Retrospective Study. Healthcare. 2022; 10(12):2530. https://doi.org/10.3390/healthcare10122530
Chicago/Turabian StyleAlamro, Nuha, Afnan S. Azhri, Asma Almuqati, Firas Azzeh, Wedad Azhar, Alaa Qadhi, Najlaa H. Almohmadi, Wafaa F. Abusudah, and Khloud Ghafouri. 2022. "Effect of Bariatric Surgery on Metabolic Syndrome, Framingham Risk Scores and Thyroid Function during One-Year Follow-Up: A Saudi Retrospective Study" Healthcare 10, no. 12: 2530. https://doi.org/10.3390/healthcare10122530
APA StyleAlamro, N., Azhri, A. S., Almuqati, A., Azzeh, F., Azhar, W., Qadhi, A., Almohmadi, N. H., Abusudah, W. F., & Ghafouri, K. (2022). Effect of Bariatric Surgery on Metabolic Syndrome, Framingham Risk Scores and Thyroid Function during One-Year Follow-Up: A Saudi Retrospective Study. Healthcare, 10(12), 2530. https://doi.org/10.3390/healthcare10122530







