Pilot Study of Use of Nitric Oxide in Monitoring Multiple Dental Foci in Oral Cavity—A Case Report
Abstract
:1. Introduction
2. Aims and Objectives of the Study
3. Materials and Methods
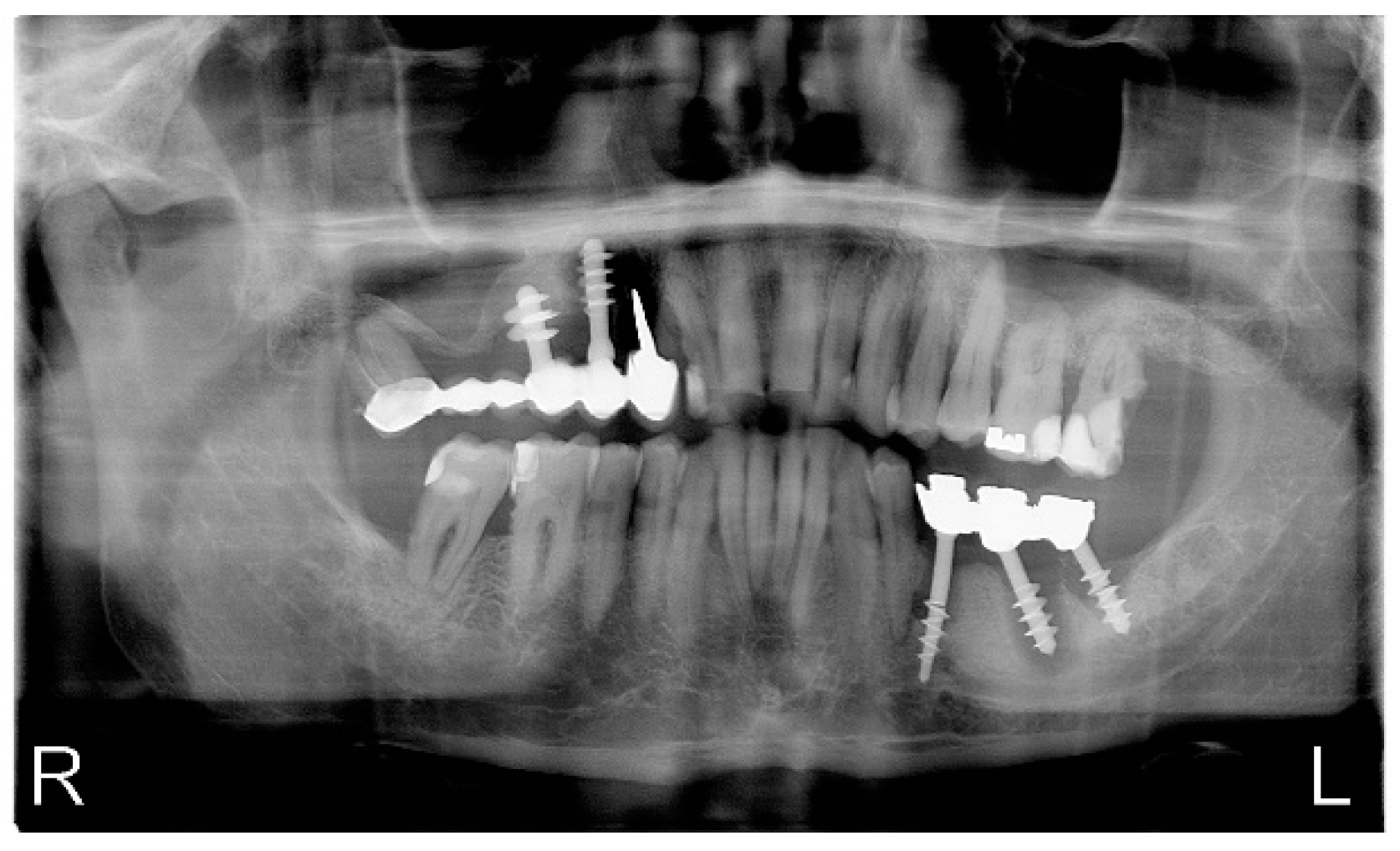
4. Case Report
4.1. First Measurement of NO in Exhaled Air
4.2. Second Measurement of NO in Exhaled Air
4.3. Histopathological Examination
4.4. Third Measurement of NO in Exhaled Air
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-W. Periimplantitis. J. Periodontol. 2018, 89 (Suppl. 1), S267–S290. [Google Scholar] [CrossRef]
- Kumar, S.; Muthulingam, V.; Tharani, P. Factors Involving In Dental Implant Failure—A Review. Eur. J. Mol. Clin. Med. 2020, 7, 1621–1625. [Google Scholar]
- An Do, T.; Son Le, H.; Shen, Y.-W.; Huang, H.-L.; Fuh, L.-J. Risk Factors related to Late Failure of Dental Implant—A Systematic Review of Recent Studies. Int. J. Environ. Res. Public Health 2020, 17, 3931. [Google Scholar] [CrossRef]
- Fathima, M.; Sinha, N.; Mazhar Ali, S. Failures in Dental Implants: A Review. Int. J. Adv. Health Sci. 2017, 4, 5–9. [Google Scholar]
- De Angelis, F.; Papi, P.; Mencio, F.; Rosella, D.; Di Carlo, S.; Pompa, G. Implant survival and success rates in patients with risk factors: Results from a long-term retrospective study with a 10 to 18 years follow-up. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 433–437. [Google Scholar] [PubMed]
- Khammissa, R.A.G.; Feller, L.; Meyerov, R.; Lemmer, J. Peri-implant mucositis and peri-implantitis: Clinical and histopathological characteristics and treatment. S. Afr. Dent. J. 2012, 67, 124–126. [Google Scholar]
- Elemek, E.; Almas, K. Peri-implantitis: Etiology, diagnosis and treatment: An update. N. Y. State Dent. J. 2014, 80, 26–32. [Google Scholar] [PubMed]
- Wilson, V. An insight into peri-implantitis: A systematic literature review. Prim. Dent. J. 2013, 2, 69–73. [Google Scholar] [CrossRef]
- Garaicoa-Pazmino, C.; Sinjab, K.; Wang, H.-L. Current Protocols for the Treatment of Peri-implantitis. Curr. Oral Health Rep. 2019, 6, 209–217. [Google Scholar] [CrossRef]
- Polymieri, A.; Loos, B.G.; Aronovich, S.; Inglehart, M.R. Risk factors, diagnosis and treatment of peri-implantitis: A cross-cultural comparison of U.S. and European periodontists’ considerations. J. Periodontol. 2021, 1–12. [Google Scholar] [CrossRef]
- Mombelli, A.; Muller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral. Implant. Res. 2012, 23 (Suppl. 6), 67–76. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, C.H.; Bragger, U.; Burgin, W.; Lang, N.P. The effect of subcrestal placement of the polished surface of ITI implants on marginal soft and hard tissues. Clin. Oral Implants Res. 1996, 7, 111–119. [Google Scholar] [CrossRef]
- Bullon, P.; Fioroni, M.; Goteri, G.; Rubini, C.; Battino, M. Immunohistochemical analysis of soft tissues in implants with healthy and peri-implantitis condition, and aggressive periodontitis. Clinical Oral Implants Res. 2004, 15, 553–559. [Google Scholar] [CrossRef]
- Konttinen , Y.T.; Ma, J.; Lappalainen, R.; Laine, P.; Kitti, U.; Santavirta, S.; Teronen, O. Immunohistochemical Evaluation of Inflammatory Mediators in Failing Implants. Int. J. Periodontics Restor. Dentistry 2006, 26, 135–141. [Google Scholar] [PubMed]
- Figuero, E.; Graziani, F.; Sanz, I.; Herrera, D.; Sanz, M. Management of peri-implant mucositis and peri-implantitis. Periodontology 2000 2014, 66, 255–273. [Google Scholar]
- Parwani, S.R.; Parwani, R.N. Nitric oxide and inflammatory periodontal disease. Gen. Dent. 2015, 63, 34–40. [Google Scholar]
- Nazaryan, R.; Kryvenko, L.; Gargin, V. The role of nitric oxide synthase in the modulation of the immune response in atopic disease. New Armen. Med. J. 2017, 11, 52–57. [Google Scholar]
- Maniscalco, M.; Lundberg, J.O. Hand-held nitric oxide sensor NIOX MINO® for the monitoring of respiratory disorders. Expert Rev. Respir. Med. 2010, 4, 715–721. [Google Scholar] [CrossRef]
- Wyszyńska, M.; Czelakowska, A.; Rafał, R.; Zając, M.; Mielnik, M.; Kasperski, J.; Skucha-Nowak, M. Measurement of the Level of Nitric Oxide in Exhaled Air in Patients Using Acrylic Complete Dentures and with Oral Pathologies. Coatings 2021, 11, 169. [Google Scholar] [CrossRef]
- Jasinska, T.; Wyszyńska-Chlap, M.; Kasperski, J.; Kasperska-Zajac, A. Plasma soluble CD40 concentration in patients with delayed pressure urticaria. Eur. J. Inflamm. 2015, 13, 126–129. [Google Scholar] [CrossRef]
- Kasperska-Zając, A.; Grzanka, A.; Jarząb, J.; Misiołek, M.; Wyszyńska-Chłap, M.; Kasperski, J.; Machura, E. The Association between Platelet Count and Acute Phase Response in Chronic Spontaneous Urticaria. BioMed Res. Int. 2014, 2014, 650913. [Google Scholar] [CrossRef]
- Kendall, H.K.; Marshall, R.I.; Bartold, P.M. Nitric oxide and tissue destruction. Oral Dis. 2001, 7, 2–10. [Google Scholar] [CrossRef]
- Batista, A.C.; Silva, T.A.; Chun, J.H.; Lara, V.S. Nitric oxide synthesis and severity of human periodontal disease. Oral Dis. 2002, 8, 254–260. [Google Scholar] [CrossRef]
- Levine, A.B.; Punihaole, D.; Levine, T.B. Characterization of the Role of Nitric Oxide and Its Clinical Applications. Cardiology 2012, 122, 55–68. [Google Scholar] [CrossRef]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic resistance in human peri-implantitis microbiota. Clin. Oral Implant. Res. 2013, 25, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Charalampakis, G.; Leonhardt, A.; Rabe, P.; Dahlen, G. Clinical and microbiological characteristics of peri-implantitis cases: A retrospective multicentre study. Clin. Oral Implant. Res. 2012, 23, 1045–1054. [Google Scholar] [CrossRef]
- Salvi, G.E.; Fürst, M.M.; Lang, N.P.; Persson, G.R. One-year bacterial colonization patterns of Staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin. Oral Implant. Res. 2008, 19, 242–248. [Google Scholar] [CrossRef]
- James, A.H.; Tsoia Flávia, K.H.; Rodrigues, P.; Leprincec, J.G.; Paline, W.M. Bacterial adhesion mechanisms on dental implant surfaces and the influencing factors. Int. J. Adhes. Adhes. 2016, 69, 58–71. [Google Scholar]
- Sorsa, T.; Hernández, M.; Leppilahti, J.; Munjal, S.; Netuschil, L.; Mäntylä, P. Detection of gingival crevicular fluid MMP-8 levels with different laboratory and chair-side methods. Oral Dis. 2010, 16, 39–45. [Google Scholar] [CrossRef]
- Hall, J.; Britse, A.O.; Jemt, T.; Friberg, B. A controlled clinical exploratory study on genetic markers for peri-implantitis. Eur. J. Oral Implantol. 2011, 4, 371–382. [Google Scholar]
- Roos-Jansåker, A.-M.; Renvert, H.; Lindahl, C.; Renvert, S. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: A prospective cohort study. J. Clin. Periodontol. 2007, 34, 625–632. [Google Scholar] [CrossRef]
- Levine, R.A.; Wilson, T.G., Jr.; Weber, H.P. The ITI Dental Implant System. In Craniomaxillofacial Reconstructive and Corrective Bone Surgery; Springer: Berlin/Heidelberg, Germany, 1994; pp. 138–154. [Google Scholar]
- Vervaeke, S.; Collaert, B.; Cosyn, J.; Deschepper, E.; De Bruyn, H. A multifactorial analysis to identify predictors of implant failure and peri-implant bone loss. Clin. Implant Dent. Relat. Res. 2013, 17, e298–e307. [Google Scholar] [CrossRef]
- Renvert, S.; Aghazadeh, A.; Hallstrom, H.; Persson, G.R. Factors related to peri-implantitis—A retrospective study. Clin. Oral Implants Res. 2014, 25, 522–529. [Google Scholar] [CrossRef]
- Ferreira, S.D.; Silva, G.L.; Cortelli, J.R.; Costa, J.E.; Costa, F.O. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J. Clin. Periodontol. 2006, 33, 929–935. [Google Scholar] [CrossRef]
- Clementini, M.; Rossetti, P.H.; Penarrocha, D.; Micarelli, C.; Bonachela, W.C.; Canullo, L. Systemic risk factors for peri-implant bone loss: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2014, 43, 323–334. [Google Scholar] [CrossRef]
- Kasat, V.; Ladda, R. Smoking and dental implants. J. Int. Soc. Prev. Commun. Dent. 2012, 2, 38–41. [Google Scholar] [CrossRef] [Green Version]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Gatto, R.; Monaco, A. Smoking and the risk of peri-implantitis. A systematic review and meta-analysis. Clin. Oral Implants Res. 2014, 26, e62–e67. [Google Scholar] [CrossRef]
- Koldsland, O.C.; Scheie, A.A.; Aass, A.M. The association between selected risk indicators and severity of peri-implantitis using mixed model analyses. J. Clin. Periodontol. 2011, 38, 285–292. [Google Scholar] [CrossRef]
- Ayna, M.; Gulses, A.; Ziebart, T.; Neff, A.; Açil, Y. Histopathological and microradiological features of peri-implantitis. A case report. Stomatol. Balt. Dent. Maxillofac. J. 2017, 19, 97–100. [Google Scholar]
- Galárraga-Vinueza, M.E.; Tangl, S.; Bianchini, M.; Magini, R.; Obreja, K.; Gruber, R.; Schwarz, F. Histological characteristics of advanced peri-implantitis bone defects in humans. Int. J. Implant. Dent. 2020, 6, 12. [Google Scholar] [CrossRef]
- Berglundh, T.; Gislason, Ö.; Lekholm, U.; Sennerby, L.; Lindhe, J. Histopathological observations of human periimplantitis lesions. J. Clin. Periodontol. 2004, 31, 341–347. [Google Scholar] [CrossRef]
- Gualini, F.; Berglundh, T. Immunohistochemical characteristics of inflammatory lesions at implants. J. Clin. Periodontol. 2003, 30, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Berglundh, T.; Marinello, C.P.; Marinello, L. Experimental peri-implant mucositis in man. J. Clin. Periodontol. 2001, 28, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Polyzois, I.; Claffey, N. Surgical therapy for the control of peri-implantitis. Clin. Oral Implant. Res. 2012, 23 (Suppl. 6), 84–94. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Paulo, M.; Araújo, A.V.; Rodrigues, G.J.; Bendhack, L.M. Nitric oxide synthesis and biological functions of nitric oxide released from ruthenium compounds. Braz. J. Med. Biol. Res. 2011, 44, 947–957. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Wu, Z.; Yang, Y.; Wang, J.; Carey Satterfield, M.; Meininger, C.J.; Bazer, F.W.; Wu, G. Review Article Nitric oxide and energy metabolism in mammals. BioFactors 2013, 39, 383–391. [Google Scholar] [CrossRef]
- Schiller, B.; Hammer, J.; Barben, J.; Trachsel, D. Comparability of a hand-held nitric oxide analyser with online and offline chemiluminescence-based nitric oxide measurement. Pediatr. Allergy Immunol. 2009, 20, 679–685. [Google Scholar] [CrossRef]
- Price, D.; Berg, J.; Lindgren, P. An economic evaluation of NIOX MINO airway inflammation monitor in the United Kingdom. Allergy 2009, 64, 431–438. [Google Scholar] [CrossRef]
- Boot, J.D.; de Ridder, L.; de Kam, M.L.; Calderon, C.; Mascelli, M.A.; Diamant, Z. Comparison of exhaled nitric oxide measurements between NIOX MINO electrochemical and Ecomedics chemiluminescence analyzer. Respir. Med. 2008, 102, 1667–1671. [Google Scholar] [CrossRef] [Green Version]
- Pizzimenti, S.; Bugiani, M.; Piccioni, P.; Heffler, E.; Carosso, A.; Guida, G.; Rolla, G. Exhaled nitric oxide measurements: Correction equation to compare hand-held device to stationary analyzer. Respir. Med. 2008, 102, 1272–1275. [Google Scholar] [CrossRef] [Green Version]
- de Laurentiis, G.; Maniscalco, M.; Cianciulli, F.; Stanziola, A.; Marsico, S.; Lundberg, J.O.; Weitzberg, E.; Sofia, M. Exhaled nitric oxide monitoring in COPD using a portable analyzer. Pulm Pharm. Ther. 2008, 21, 689–693. [Google Scholar] [CrossRef] [Green Version]
- Maniscalco, M.; de Laurentiis, G.; Weitzberg, E.; Lundberg, J.O.; Sofia, M. Validation study of nasal nitric oxide measurements using a hand-held electrochemical analyser. Eur. J. Clin. Investig. 2008, 38, 197–200. [Google Scholar] [CrossRef]
- Khalili, B.; Boggs, P.B.; Bahna, S.L. Reliability of a new hand-held device for the measurement of exhaled nitric oxide. Allergy 2007, 62, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Adachi, Y.; Itazawa, T.; Okabe, Y.; Adachi, Y.S.; Katsumuma, T.; Miyawaki, T. Comparison of exhalation time methods (6 sec vs. 10 sec) of a hand-held exhaled nitric oxide analyzer. Pediatr. Pulmonol. 2010, 45, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Antus, B.; Horvath, I.; Barta, I. Assessment of exhaled nitric oxide by a new hand-held device. Respir. Med. 2010, 104, 1377–1380. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, J.O.; Farkas-Szallasi, T.; Weitzberg, E.; Rinder, J.; Lidholm, J.; Änggåard, A.; Hökfelt, T.; Lundberg, J.M.; Alving, K. High nitric oxide production in human paranasal sinuses. Nat. Med. 1995, 1, 370–373. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am. J. Respir. Crit. Care. Med. 2005, 171, 912–930. [Google Scholar] [CrossRef] [PubMed]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. Am. J. Respir. Crit Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [Green Version]
- Duong-Quy, S. Clinical Utility Of The Exhaled Nitric Oxide (NO) Measurement with Portable Devices in The Management of Allergic Airway Inflammation and Asthma. J. Asthma Allergy 2019, 12, 331–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Türk, M.; Yılmaz, I. Measurement of exhaled nitric oxide in young children. Ann. Allergy Asthma Immunol. 2019, 122, 343–345. [Google Scholar]
- Reher, V.G.S.; Zenobio, E.G.; Costa, F.O.; Reher, P.; Soares, R.V. Nitric oxide levels in saliva increase with severity of chronic periodontitis. J. Oral Sci. 2007, 49, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Lappin, D.F.; Kjeldsen, M.; Sander, L.; Kinane, D.F. Inducible nitric oxide synthase expression in periodontitis. J. Periodont. Res. 2000, 35, 369–373. [Google Scholar] [CrossRef]
- Kazuyuki, S.; Warbington, M.L.; Gordon, B.J.; Kurihara, H.; Van Dyke, T.E. Nitric oxide synthase activity in neutrophils from patients with Localized Aggressive Periodontitis. J. Periodontol. 2001, 72, 1052–1058. [Google Scholar]
- Hirose, M.; Ishihiara, K.; Saito, A.; Nakagawa, T.; Yamada, S.; Okuda, K. Expression of cytokines and inducible nitric oxide synthase in inflamed gingival tissue. J. Periodontol. 2001, 72, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, M.; Shanmugam, S. Evaluation of salivary nitric oxide levels in oral mucosal diseases: A controlled clinical trial. Indian J. Dent. Res. 2006, 17, 117–120. [Google Scholar] [CrossRef] [PubMed]




| l.p. | * Weeks | Level of Exhaled NO (ppb) | * Drop in Level of Exhaled NO (%) |
|---|---|---|---|
| 1. | 3 | 72 | 42.86% |
| 2. | 7 | 31 | 75.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszyńska, M.; Rosak, P.; Czelakowska, A.; Białożyt-Bujak, E.; Kasperski, J.; Łopaciński, M.; Al Khatib, N.; Skucha-Nowak, M. Pilot Study of Use of Nitric Oxide in Monitoring Multiple Dental Foci in Oral Cavity—A Case Report. Healthcare 2022, 10, 195. https://doi.org/10.3390/healthcare10020195
Wyszyńska M, Rosak P, Czelakowska A, Białożyt-Bujak E, Kasperski J, Łopaciński M, Al Khatib N, Skucha-Nowak M. Pilot Study of Use of Nitric Oxide in Monitoring Multiple Dental Foci in Oral Cavity—A Case Report. Healthcare. 2022; 10(2):195. https://doi.org/10.3390/healthcare10020195
Chicago/Turabian StyleWyszyńska, Magdalena, Przemysław Rosak, Aleksandra Czelakowska, Ewa Białożyt-Bujak, Jacek Kasperski, Maciej Łopaciński, Nour Al Khatib, and Małgorzata Skucha-Nowak. 2022. "Pilot Study of Use of Nitric Oxide in Monitoring Multiple Dental Foci in Oral Cavity—A Case Report" Healthcare 10, no. 2: 195. https://doi.org/10.3390/healthcare10020195
APA StyleWyszyńska, M., Rosak, P., Czelakowska, A., Białożyt-Bujak, E., Kasperski, J., Łopaciński, M., Al Khatib, N., & Skucha-Nowak, M. (2022). Pilot Study of Use of Nitric Oxide in Monitoring Multiple Dental Foci in Oral Cavity—A Case Report. Healthcare, 10(2), 195. https://doi.org/10.3390/healthcare10020195







