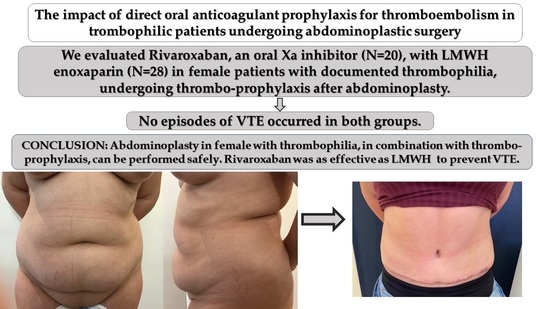
The Impact of Direct Oral Anticoagulant Prophylaxis for Thromboembolism in Thrombophilic Patients Undergoing Abdominoplastic Surgery
Abstract
1. Introduction
2. Materials and Methods
- −
- Rivaroxaban Group: rivaroxaban 10 mg oral 6–8 h post-operatively and then once a day for 7 days post-operatively.
- −
- Enoxaparin Group: enoxaparin 4000 UI subcutaneously 8–12 h pre-operatively and then once a day for 7 days post-operatively.
3. Results
4. Discussion
5. Conclusions
6. Patents
Statement of Ethics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Folsom, A.R.; Wu, K.K.; Rosamond, W.D.; Sharrett, A.R.; Chambless, L.E. Prospective study of hemostatic factors and incidence of coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 1997, 96, 1102–1108. [Google Scholar] [CrossRef]
- Catto, A.J.; Carter, A.M.; Barrett, J.H.; Bamford, J.; Rice, P.J.; Grant, P.J. Von Willebrand factor and factor VIII:C in acute cerebrovascular disease: Relationship to stroke subtype and mortality. Thromb. Haemost. 1997, 77, 1104–1108. [Google Scholar] [PubMed]
- Cushman, M.; Tsai, A.W.; White, R.H.; Heckbert, S.R.; Wayne, D.; Enright, R.P.; Folsom, A.R. Deep Vein Thrombosis and Pulmonary Embolism in Two Cohorts: The Longitudinal Investigation of Thromboembolism Etiology. Am. J. Med. 2004, 117, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Oger, E. Incidence of venous thromboembolism: A community-based study in Western France. EPI-GETBP Study Group. Groupe d’Etude de la Thrombose de Bretagne Occidentale. Thromb. Haemost. 2000, 83, 657–660. [Google Scholar]
- Ferraro, G.; Grella, R.; D’Andrea, F. Abdominoplasty: Thromboembolic risks for both sexes. Aesthetic Plast. Surg. 2004, 28, 412–416. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miszkiewicz, K.; Perreault, I.; Landes, G.; Harris, P.G.; Sampalis, J.S.; Dionyssopoulos, A.; Nikolis, A. Venous thromboembolism in plastic surgery: Incidence, current practice and recommendations. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Cohn, D.M.; Roshani, S.; Middeldorp, S. Thrombophilia and venous thromboembolism: Implications for testing. Semin. Thromb. Hemost. 2007, 33, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Rott, H. Prevention and treatment of venous thromboembolism during HRT: Current perspectives. Int. J. Gen. Med. 2014, 7, 433–440. [Google Scholar] [CrossRef][Green Version]
- Sode, B.F.; Allin, K.H.; Dahl, M.; Gyntelberg, F.; Nordestgaard, B.G. Risk of venous thromboembolism and myocardial infarction associated with factor V Leiden and prothrombin mutations and blood type. CMAJ 2013, 185, E229–E237. [Google Scholar] [CrossRef]
- Marturano, A.; Bury, L.; Gresele, P. Possible incorrect genotyping of heterozygous factor V Leiden and Prothrombin 20210 gene mutations by the GeneXpert assay. Clin. Chim. Acta 2014, 435, 36–39. [Google Scholar] [CrossRef]
- Kujovich, J.L. Factor V Leiden thrombophilia. Genet. Med. 2011, 13, 1–16. [Google Scholar] [CrossRef]
- Moll, S. Thrombophilia: Clinical–practical aspects. J. Thromb. Thrombolysis 2015, 39, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Poort, S.R.; Rosendaal, F.R.; Reitsma, P.H.; Bertina, R.M. A common genetic variation in the 3’-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood 1996, 88, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M. Thrombophilia Testing and Venous Thrombosis. N. Engl. J. Med. 2017, 377, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Dahlbäck, B. Advances in understanding pathogenic mechanisms of thrombophilic disorders. Blood 2008, 112, 19–27. [Google Scholar] [CrossRef]
- Geerts, W.H.; Heit, J.A.; Clagett, G.P.; Pineo, G.F.; Colwell, C.W.; Anderson, F.A., Jr.; Wheeler, H.B. Prevention of venous thromboembolism. Chest 2001, 119 (Suppl. 1), 132S–175S. [Google Scholar] [CrossRef]
- Flordal, P.A.; Bergqvist, D.; Ljungstrom, K.G.; Törngren, S. Clinical relevance of the fibrinogen uptake test in patients undergoing elective general abdominal surgery–relation to major thromboembolism and mortality. Thromb. Res. 1995, 80, 491–497. [Google Scholar] [CrossRef]
- Wu, O.; Robertson, L.; Twaddle, S.; Lowe, G.D.; Clark, P.; Greaves, M.; Walker, I.D.; Langhorne, P.; Brenkel, I.; Regan, L.; et al. Screening for thrombophilia in high-risk situations: Systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study. Health Technol. Assess. 2006, 10, 1–110. [Google Scholar] [CrossRef]
- Molitor, M.; Mestak, O.; Popelka, P.; Vitova, L.; Hromadkova, V.; Mestak, J. Pulmunary embolism after abdominoplasty- are we really able to avoid all complications? Case reports and literature review. Acta Chir. Plast. 2016, 58, 35–38. [Google Scholar]
- Grazer, F.M.; Golwin, M. Abdominoplasty assessed by survey, with emphasis on complications. Plast. Reconstr. Surg. 1977, 59, 513–517. [Google Scholar] [CrossRef]
- Friedman, T.; O’Brien Coon, D.; Michaels, V.J.; Bontempo, F.; Young, V.L.; Clavijo, J.A.; Rubin, J.P. Hereditary coagulopathies: Practical diagnosis and management for the plastic surgeon. Plast. Reconstr. Surg. 2010, 125, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Shermak, M.A.; Chang, D.C.; Heller, J. Factors implicating thromboembolism aftyer bariatric body contouring surgery. Plast. Reconstr. Surg. 2007, 119, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Neaman, K.C.; Hansen, J.E. Analysis of complications from abdominoplasty: A review of 206 cases at university hospital. Ann. Plast. Surg. 2007, 58, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Heuft, T.; Richter, D.E.; Wieder, M. Venous thromboembolism (VTE) prophylaxis after abdominoplasty and liposuction: A review of literature. Aesthet. Plast. Surg. 2020, 44, 473–482. [Google Scholar] [CrossRef]
- Kraft, C.T.; Janis, J.E. Deep Venous Thrombosis Prophylaxis. Clin. Plast. Surg. 2020, 47, 409–414. [Google Scholar] [CrossRef]
- Bates, S.M.; Greer, I.A.; Middeldorp, S.; Veenstra, D.L.; Prabulos, A.M.; Vandvik, P.O. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e691S–e736S. [Google Scholar] [CrossRef]
- Caprini, J.A.; Reyna, J.J. Effective risk stratification of surgical and non-surgical patients for venous thromboembolic disease. Semin. Hematol. 2001, 38 (Suppl. 5), 12–19. [Google Scholar] [CrossRef]
- Caprini, J.A. Risk assessment as a guide to thrombosis prophylaxis. Curr. Opin. Pulm. Med. 2010, 16, 448–452. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, Y.; Li, X.; Wang, L.; Wang, M.; Xiao, J.; Yi, Q. Assessment of the Risk of Venous Thromboembolism in Medical Inpatients using the Padua Prediction Score and Caprini Risk Assessment Model. J. Atheroscler. Thromb. 2018, 25, 1091–1104. [Google Scholar] [CrossRef]
- Pannucci, C.J.; MacDonald, J.K.; Ariyan, S.; Gutowski, K.A.; Kerrigan, C.L.; Kim, J.Y.; Chung, K.C. Benefits and Risks of Prophylaxis for Deep Venous Thrombosis and Pulmonary Embolus in Plastic Surgery: A Systematic Review and Meta-Analysis of Controlled Trials and Consensus Conference. Plast. Reconstr. Surg. 2016, 137, 709–730. [Google Scholar] [CrossRef]
- Pannucci, C.J.; Barta, R.J.; Portschy, P.R.; Dreszer, G.; Hoxworth, R.E.; Kalliainen, L.K.; Wilkins, E.G. Assessment of postoperative venous thromboembolism risk in plastic surgery patients using the 2005 and 2010 Caprini Risk score. Plast. Reconstr. Surg. 2012, 130, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Pannucci, C.J.; Bailey, S.H.; Dreszer, G.; Fisher Wachtman, C.; Zumsteg, J.W.; Jaber, R.M.; Hamill, J.B.; Hume, K.M.; Rubin, J.P.; Neligan, P.C.; et al. Validation of the Caprini risk assessment model in plastic and reconstructive surgery patients. J. Am. Coll. Surg. 2011, 212, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kocayigit, I.; Can, Y.; Sahinkus, S.; Aydin, E.; Vatan, M.B.; Kilic, H.; Gunduz, H. Spontaneous rectus sheath hematoma during rivaroxaban therapy. Indian J. Pharmacol. 2014, 46, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, M.; Jeanneret, B.; Schaeren, S. Spontaneous spinal epidural hematoma during factor xa inhibitor treatment(rivaroxaban). Eur. Spin. J. 2011, 21, 1–3. [Google Scholar]
- Sierakowski, K.L.; Dean, N.R. Rivaroxaban-associated delayed spontaneous periprosthetic hematoma. Plast. Reconstr. Surg. Glob. Open 2015, 3, e421. [Google Scholar] [CrossRef]
- Kim, J.H.; Seo, S.; Kim, K.P.; Chang, H.M.; Royo, B.Y.; Yoo, C.; Jeong, J.H.; Lee, J.L.; Im, H.S.; Jeong, H.; et al. Rivaroxaban versus low-molecular- weight heparin for venous thromboembolism in advanced upper gastrointestinal tract and hepatopancreatobiliary cancer. In Vivo 2020, 34, 829–837. [Google Scholar] [CrossRef]
- Hunstad, J.P.; Krochmal, D.J.; Flugstad, N.A.; Kortesis, B.G.; Augenstein, A.C.; Culbertson, G.R. Rivaroxaban for Venous Thromboembolism Prophylaxis in Abdominoplasty: A Multicenter Experience. Aesthet. Surg. J. 2016, 36, 60–66. [Google Scholar] [CrossRef]
- Dini, G.; Ferreira, M.; Albuquerque, L.; Ferreira, L. How safe is chemoprophylaxis after abdominoplasty? Plast. Reconstr. Surg. 2012, 130, 851e–857e. [Google Scholar] [CrossRef]
- Thomas, T.F.; Ganetsky, V.; Spinler, S.A. Rivaroxaban: An oral factor Xa inhibitor. Clin. Ther. 2013, 35, 4–27. [Google Scholar] [CrossRef]
- Linnemann, B.; Hart, C. Laboratory Diagnostics in Thrombophilia. Hamostaseologie 2019, 39, 49–61. [Google Scholar]
- Armstrong, E.M.; Bellone, J.M.; Hornsby, L.B.; Treadway, S.; Phillippe, H.M. Acquired Thrombophilia. J. Pharm. Pract. 2014, 27, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.M.; Woller, S.C.; Bauer, K.A.; Kasthuri, R.; Cushman, M.; Streiff, M.; Lim, W.; Douketis, J.D. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J. Thromb. Thrombolysis 2016, 41, 154–164. [Google Scholar] [CrossRef]
- Metcalfe, S.A.; Barlow-Stewart, K.; Campbell, J.; Emery, J. Genetics and blood--haemoglobinopathies and clotting disorders. Aust. Fam. Phys. 2007, 36, 812–819. [Google Scholar]
- Couturaud, F.; Leroyer, C.; Julian, J.A.; Kahn, S.R.; Ginsberg, J.S.; Wells, P.S.; Douketis, J.D.; Mottier, D.; Kearon, C. Factors that predict risk of thrombosis in relatives of patients with unprovoked venous thromboembolism. Chest 2009, 136, 1537–15459. [Google Scholar] [CrossRef] [PubMed]
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Iverson, R.E.; Gomez, J.L. Deep venous thrombosis: Prevention and management. Clin. Plast. Surg. 2013, 40, 389–398. [Google Scholar] [CrossRef]
- Sica, A.; Vitiello, P.; Ronchi, A.; Casale, B.; Calogero, A.; Sagnelli, E.; Costa, N.G.; Troiani, T.; Franco, R.; Argenziano, G.; et al. Primary Cutaneous Anaplastic Large Cell Lymphoma (pcALCL) in the Elderly and the Importance of Sport Activity Training. Int. J. Environ. Res. Public Health 2020, 17, 839. [Google Scholar] [CrossRef]
- Gould, M.K.; Garcia, D.A.; Wren, S.M.; Karanicolas, P.J.; Arcelus, J.I.; Heit, J.A.; Samama, C.M. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. 2), e227S–e277S. [Google Scholar] [CrossRef]
- Falck-Ytter, Y.; Francis, C.W.; Johanson, N.A.; Curley, C.; Dahl, O.E.; Schulman, S.; Ortel, T.L.; Pauker, S.G.; Colwell, C.W.J. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. 2), e278S–e325S. [Google Scholar] [CrossRef]
- Caprini, J.A. Thrombosis risk assessment as a guide to quality patient care. Dis. Mon. 2005, 51, 70–78. [Google Scholar] [CrossRef]
- Bahl, V.; Hu, H.M.; Henke, P.K.; Wakefield, T.W.; Campbell, D.A.J.; Caprini, J.A. A validation study of a retrospective venous thromboembolism risk scoring method. Ann. Surg. 2010, 251, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Hatef, D.A.; Kenkel, J.M.; Nguyen, M.Q.; Farkas, J.P.; Abtahi, F.; Rohrich, R.J.; Brown, S.A. Thromboembolic risk assessment and the efficacy of enoxaparin prophylaxis in excisional body contouring surgery. Plast. Reconstr. Surg. 2008, 122, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Zakai, N.A.; Wright, J.; Cushman, M. Risk factors for venous thrombosis in medical inpatients: Validation of a thrombosis risk score. J. Thromb. Haemost. 2004, 2, 2156–2161. [Google Scholar] [CrossRef]
- Arcelus, J.I.; Candocia, S.; Traverso, C.I.; Fabrega, F.; Caprini, J.A.; Hasty, J.H. Venous thromboembolism prophylaxis and risk assessment in medical patients. Semin. Thromb. Hemost. 1991, 17 (Suppl. 3), 313–318. [Google Scholar]
- Keyes, G.R.; Singer, R.; Iverson, R.E.; Nahai, F. Incidence and predictors of venous thromboembolism in abdominoplasty. Aesthet. Surg. J. 2018, 38, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Mavrakanas, T.; Bounameaux, H. The potential role of new oral anticoagulants in the prevention and treatment of thromboembolism. Pharmacol. Ther. 2011, 130, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Elsebaie, M.A.T.; van Es, N.; Langston, A.; Büller, H.R.; Gaddh, M. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: A systematic review and meta-analysis. J. Thromb. Haemost. 2019, 17, 645–656. [Google Scholar] [CrossRef]
- Undas, A.; Góralczyk, T. Direct Oral Anticoagulants in Patients with Thrombophilia: Challenges in Diagnostic Evaluation and Treatment. Adv. Clin. Exp. Med. 2016, 25, 1321–1330. [Google Scholar] [CrossRef]
- Sica, A.; Vitiello, P.; Papa, A.; Sagnelli, C.; Calogero, A.; Casale, D.; Mottola, M.; Svanera, G.; Dodaro, C.; Martinelli, E.; et al. Use of rituximab in NHL malt type affected pregnant woman, during the first trimester for two times. Open Med. 2019, 14, 757–760. [Google Scholar] [CrossRef]
- Morales, R.J.; Ruff, E.; Patronella, C.; Mentz, H.; Newall, G.; Hustak, K.L.; Fortes, P.; Bush, A. Safety and Efficacy of Novel Oral Anticoagulants vs Low Molecular Weight Heparin for Thromboprophylaxis in Large-Volume Liposuction and Body Contouring Procedures. Aesthet. Surg. J. 2016, 36, 440–449. [Google Scholar] [CrossRef]
- Sica, A.; Casale, D.; Rossi, G.; Casale, B.; Ciccozzi, M.; Fasano, M.; Ciotti, M.; Sagnelli, E.; Papa, A.; Sagnelli, C. The impact of the SARS-CoV-2 infection, with special reference to the haematological setting. J. Med. Virol. 2021, 93, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Sagnelli, C.; Vitiello, P.; Franco, R.; Argenziano, G.; Ciccozzi, M.; Sagnelli, E.; Ronchi, A. Rescue therapy of refractory DLBCL BCL2 with venetoclax: Case report. Chemotherapy 2021, 21, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Swanson, E. Concerns Regarding the Use of Oral Anticoagulants (Rivaroxaban and Apixaban) for Venous Thromboembolism Prophylaxis in Plastic Surgery Patients. Aesthet. Surg. J. 2016, 36, NP262–NP264. [Google Scholar] [CrossRef]
- Sagnelli, C.; Sica, A.; Gallo, M.; Peluso, G.; Varlese, F.; D’Alessandro, V.; Ciccozzi, M.; Crocetto, F.; Garofalo, C.; Fiorelli, A.; et al. Renal involvement in COVID-19: Focus on kidney transplant sector. Infection 2021, 49, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.I.; Borris, L.C.; Friedman, R.J.; Haas, S.; Huisman, M.V.; Kakkar, A.K.; Bandel, T.J.; Beckmann, H.; Muehlhofer, E.; Misselwitz, F.; et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N. Engl. J. Med. 2008, 358, 2765–2775. [Google Scholar] [CrossRef]
- Fisher, W.D.; Eriksson, B.I.; Bauer, K.A.; Borris, L.; Dahl, O.E.; Gent, M.; Haas, S.; Homering, M.; Huisman, M.V.; Kakkar, A.K.; et al. Rivaroxaban for thromboprophylaxis after orthopaedic surgery: Pooled analysis of two studies. Thromb. Haemost. 2007, 97, 931–937. [Google Scholar] [PubMed]
- Chan, N.C.; Siegal, D.; Lauw, M.N.; Ginsberg, J.S.; Eikelboom, J.W.; Guyatt, G.H.; Hirsh, J. A systematic review of contemporary trials of anticoagulants in orthopaedic thromboprophylaxis: Suggestions for a radical reappraisal. J. Thromb. Thrombolysis 2015, 40, 231–239. [Google Scholar] [CrossRef]
| Characteristics | Rivaroxaban Group n = 20 Patients | Enoxaparin Group n = 28 Patients | p-Value |
|---|---|---|---|
| Age, years, (Mean ± SD) | 46.75 ± 10.08 | 48.67 ± 9.36 | 0.5144 |
| BMI, kg/m2, (Mean ± SD) | 27.10 ± 5.67 | 27.07 ± 4.80 | 0.9838 |
| Smoking, N (%) | 9 (45%) | 12 (42.85%) | 0.8827 |
| Caprini Score (Mean ± SD) | 8.55 ± 1.86 | 8.53 ± 0.77 | 0.9547 |
| Drain Permanence, days (Mean ± SD) | 7.15 ± 1.91 | 7.00 ± 0.745 | 0.842 |
| Complications, n (%): | |||
| Hematoma | 0 | 2 | 0.680 |
| Thromboembolic Events | 0 | 0 | 0.992 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verolino, P.; Sagnelli, C.; Grella, R.; Nicoletti, G.F.; Sica, A.; Faenza, M. The Impact of Direct Oral Anticoagulant Prophylaxis for Thromboembolism in Thrombophilic Patients Undergoing Abdominoplastic Surgery. Healthcare 2022, 10, 476. https://doi.org/10.3390/healthcare10030476
Verolino P, Sagnelli C, Grella R, Nicoletti GF, Sica A, Faenza M. The Impact of Direct Oral Anticoagulant Prophylaxis for Thromboembolism in Thrombophilic Patients Undergoing Abdominoplastic Surgery. Healthcare. 2022; 10(3):476. https://doi.org/10.3390/healthcare10030476
Chicago/Turabian StyleVerolino, Pasquale, Caterina Sagnelli, Roberto Grella, Giovanni Francesco Nicoletti, Antonello Sica, and Mario Faenza. 2022. "The Impact of Direct Oral Anticoagulant Prophylaxis for Thromboembolism in Thrombophilic Patients Undergoing Abdominoplastic Surgery" Healthcare 10, no. 3: 476. https://doi.org/10.3390/healthcare10030476
APA StyleVerolino, P., Sagnelli, C., Grella, R., Nicoletti, G. F., Sica, A., & Faenza, M. (2022). The Impact of Direct Oral Anticoagulant Prophylaxis for Thromboembolism in Thrombophilic Patients Undergoing Abdominoplastic Surgery. Healthcare, 10(3), 476. https://doi.org/10.3390/healthcare10030476