Clinical Changes of Respiratory Parameters in Institutionalized Older Adults after a Physiotherapy Program Combining Respiratory and Musculoskeletal Exercises
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Randomization and Blinding
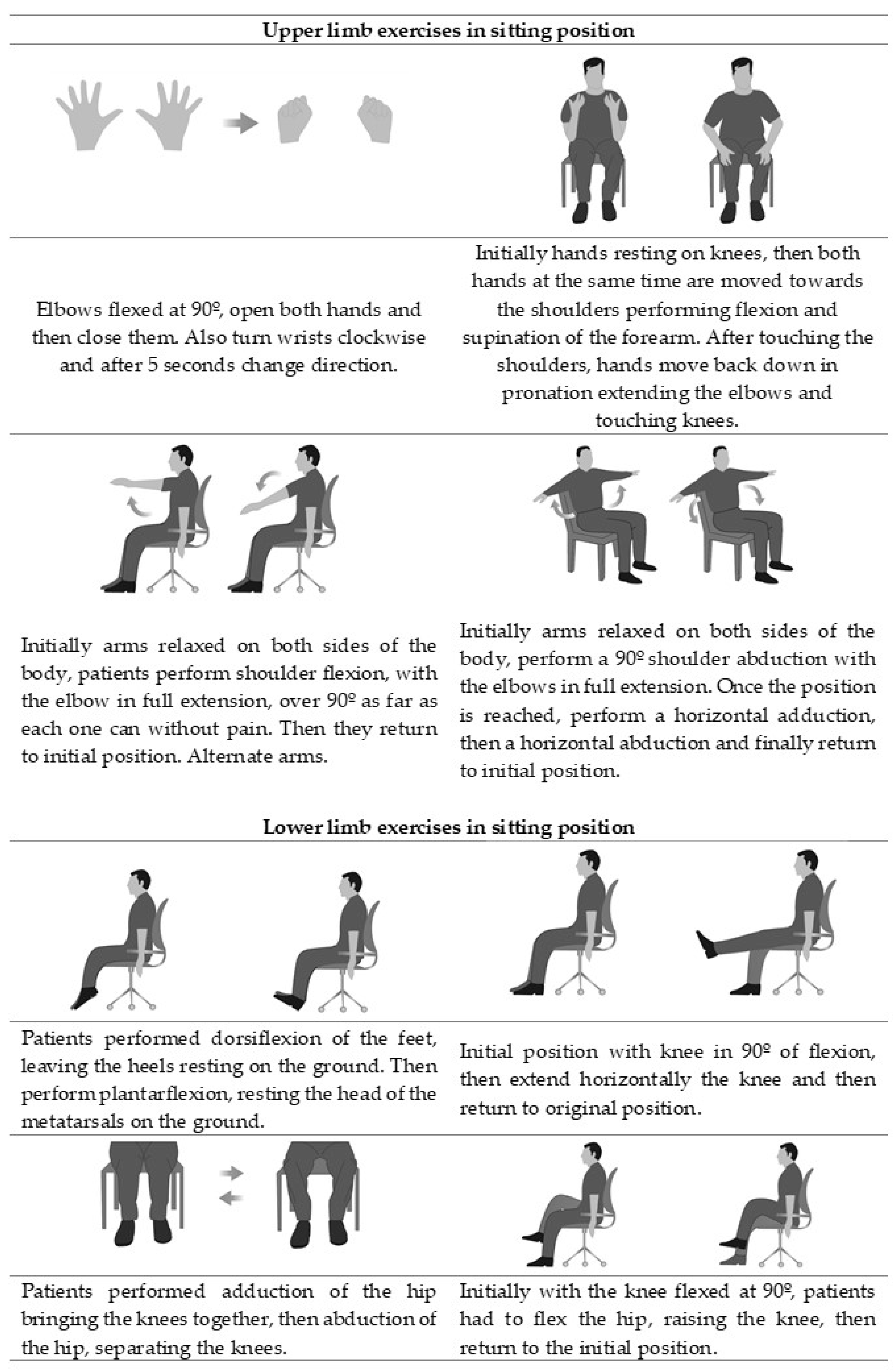
2.4. Interventions
2.5. Outcomes
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- United Nations Department of Economic and Social Affairs. Population Division. World Population Prospects: The 2017 Revision: Key Findings and Advance Tables. 2017. Available online: https://population.un.org/wpp/publications/files/wpp2017_keyfindings.pdf (accessed on 25 January 2019).
- Abrahin, O.; Rodrigues, R.P.; Nascimento, V.C.; Da Silva-Grigoletto, M.E.; Sousa, E.C.; Marçal, A.C. Single- and Multiple-Set Resistance Training Improves Skeletal and Respiratory Muscle Strength in Elderly Women. Clin. Interv. Aging 2014, 9, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Mercado Rus, M. Manual de Fisioterapia Respiratoria; Ergon: Madrid, Spain, 2003. [Google Scholar]
- World Health Organization. World Health Organization World Health Statistics Overview 2019: Monitoring Health for the SDGs, Sustainable Development Goals. 2019. Available online: https://www.who.int/publications-detail-redirect/9789241565707 (accessed on 13 March 2019).
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Baldwin, C.; Bissett, B.; Boden, I.; Gosselink, R.; Granger, C.L.; Hodgson, C.; Jones, A.Y.; Kho, M.E.; Moses, R.; et al. Physiotherapy Management for COVID-19 in the Acute Hospital Setting: Clinical Practice Recommendations. J. Physiother. 2020, 66, 73–82. [Google Scholar]
- Sarkar, J.; Chakrabarti, P. A Machine Learning Model Reveals Older Age and Delayed Hospitalization as Predictors of Mortality in Patients with COVID-19; Infectious Diseases (except HIV/AIDS). 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.03.25.20043331v1 (accessed on 2 January 2021).
- Giménez, M.; Servera, E.; Vergara, P. Prevención y Rehabilitación En Patología Respiratoria Crónica: Fisioterapia, Entrenamiento y Cuidados Respiratorios, 2nd ed.; Editorial Médica Panamericana S.A.: Madrid, Spain, 2001. [Google Scholar]
- Celli, B.R.; MacNee, W.; Agusti, A.; Anzueto, A.; Berg, B.; Buist, A.S.; Calverley, P.M.A.; Chavannes, N.; Dillard, T.; Fahy, B.; et al. Standards for the Diagnosis and Treatment of Patients with COPD: A Summary of the ATS/ERS Position Paper. Eur. Respir. J. 2004, 23, 932–946. [Google Scholar] [CrossRef]
- Cebrià i Iranzo, M.d.À.; Arnall, D.A.; Igual Camacho, C.; Tomás, J.M.; Meléndez, J.C. Physiotherapy intervention for preventing the respiratory muscle deterioration in institutionalized older women with functional impairment. Arch. Bronconeumol. 2013, 49, 1–9. [Google Scholar] [CrossRef]
- Fonseca, M.d.A.; Cader, S.A.; Dantas, E.H.M.; Bacelar, S.C.; Silva, E.B.d.; Leal, S.M.d.O. Programas de Treinamento Muscular Respiratório: Impacto Na Autonomia Funcional de Idosos. Rev. Assoc. Med. Bras. 2010, 56, 642–648. [Google Scholar] [CrossRef]
- Watsford, M.; Murphy, A. The Effects of Respiratory-Muscle Training on Exercise in Older Women. J. Aging Phys. Act. 2008, 16, 245–260. [Google Scholar] [CrossRef]
- Gosselink, R. Controlled Breathing and Dyspnea in Patients with Chronic Obstructive Pulmonary Disease (COPD). J. Rehabil. Res. Dev. 2003, 40, 25. [Google Scholar] [CrossRef]
- Pomidori, L.; Campigotto, F.; Amatya, T.M.; Bernardi, L.; Cogo, A. Efficacy and Tolerability of Yoga Breathing in Patients with Chronic Obstructive Pulmonary Disease: A Pilot Study. J. Cardiopulm. Rehabil. Prev. 2009, 29, 133–137. [Google Scholar]
- Nield, M.A.; Hoo, G.W.S.; Roper, J.M.; Santiago, S. Efficacy of Pursed-Lips Breathing: A Breathing Pattern Retraining Strategy for Dyspnea Reduction. J. Cardiopulm. Rehabil. Prev. 2007, 27, 237–244. [Google Scholar]
- Collins, E.G.; Langbein, W.E.; Fehr, L.; O’Connell, S.; Jelinek, C.; Hagarty, E.; Edwards, L.; Reda, D.; Tobin, M.J.; Laghi, F. Can Ventilation–Feedback Training Augment Exercise Tolerance in Patients with Chronic Obstructive Pulmonary Disease? Am. J. Respir. Crit. Care Med. 2008, 177, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar]
- Rous, M.R.G.; Lobato, S.D.; Trigo, G.R.; Vélez, F.M.; San Miguel, M.; Cejudo, P.; Ruiz, F.O.; Munoz, A.; Iturri, J.B.G.; García, A. Pulmonary Rehabilitation. Arch. Bronconeumol. 2014, 50, 332–344. [Google Scholar]
- Mador, M.J.; Bozkanat, E.; Aggarwal, A.; Shaffer, M.; Kufel, T.J. Endurance and Strength Training in Patients With COPD. Chest 2004, 125, 2036–2045. [Google Scholar] [CrossRef]
- Rodríguez-Molinero, A.; Narvaiza, L.; Ruiz, J.; Gálvez-Barrón, C. Normal Respiratory Rate and Peripheral Blood Oxygen Saturation in the Elderly Population. J. Am. Geriatr. Soc. 2013, 61, 2238–2240. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Saz, P.; Marcos, G.; Día, J.L.; de la Cámara, C.; Ventura, T.; Morales Asín, F.; Fernando Pascual, L.; Montañés, J.A.; Aznar, S. Revalidation and standardization of the cognition mini-exam (first Spanish version of the Mini-Mental Status Examination) in the general geriatric population. Med. Clin. 1999, 112, 767–774. [Google Scholar]
- The CONSORT Group; Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. Trials 2010, 11, 32. [Google Scholar] [CrossRef]
- Dhanak, M.; Penhall, R. Australian and New Zealand Society for Geriatric Medicine: Position Statement-Exercise Guidelines for Older Adults. Australas J. Ageing 2014, 33, 287–294. [Google Scholar] [CrossRef]
- Dechamps, A. Effects of Exercise Programs to Prevent Decline in Health-Related Quality of Life in Highly Deconditioned Institutionalized Elderly Persons: A Randomized Controlled Trial. Arch. Intern. Med. 2010, 170, 162. [Google Scholar] [CrossRef]
- Rebelatto, J.R.; da Silva Morelli, J.G.; Cassa, F.B.; García, S.M. Fisioterapia Geriátrica: Practica Asistencial En El Anciano; McGraw-Hill Interamericana: Madrid, Spain, 2005. [Google Scholar]
- Cahalin, L.P.; Braga, M.; Matsuo, Y.; Hernandez, E.D. Efficacy of Diaphragmatic Breathing in Persons with Chronic Obstructive Pulmonary Disease: A Review of the Literature. J. Cardiopulm. Rehabil. 2002, 22, 7–21. [Google Scholar] [CrossRef]
- Migliore, A. Improving Dyspnea Management in Three Adults with Chronic Obstructive Pulmonary Disease. Am. J. Occup. Ther. 2004, 58, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Pellegrino, R. Interpretative Strategies for Lung Function Tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Villar, A.; Represas-Represas, C.; Mouronte-Roibás, C.; Ramos-Hernández, C.; Priegue-Carrera, A.; Fernández-García, S.; López-Campos, J.L. Reliability and Usefulness of Spirometry Performed during Admission for COPD Exacerbation. PLoS ONE 2018, 13, e0194983. [Google Scholar] [CrossRef]
- Torp, K.D.; Modi, P.; Simon, L.V. Pulse Oximetry. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Jubran, A. Pulse Oximetry. Crit Care 2015, 19, 272. [Google Scholar] [CrossRef]
- Cohen, J. Eta-Squared and Partial Eta-Squared in Fixed Factor Anova Designs. Educ. Psychol. Meas. 1973, 33, 107–112. [Google Scholar] [CrossRef]
- Gorman, R.B.; McKenzie, D.K.; Pride, N.B.; Tolman, J.F.; Gandevia, S.C. Diaphragm Length during Tidal Breathing in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2002, 166, 1461–1469. [Google Scholar] [CrossRef]
- Borge, C.R.; Hagen, K.B.; Mengshoel, A.M.; Omenaas, E.; Moum, T.; Wahl, A.K. Effects of Controlled Breathing Exercises and Respiratory Muscle Training in People with Chronic Obstructive Pulmonary Disease: Results from Evaluating the Quality of Evidence in Systematic Reviews. BMC Pulm. Med. 2014, 14, 184. [Google Scholar] [CrossRef]
- Hyatt, R.E.; Cowl, C.T.; Bjoraker, J.A.; Scanlon, P.D. Conditions Associated with an Abnormal Nonspecific Pattern of Pulmonary Function Tests. Chest 2009, 135, 419–424. [Google Scholar] [CrossRef]
- Enright, P.L.; Adams, A.B.; Boyle, P.J.R.; Sherrill, D.L. Spirometry and Maximal Respiratory Pressure References from Healthy Minnesota 65- to 85-Year-Old Women and Men. Chest 1995, 108, 663–669. [Google Scholar] [CrossRef]
- Simpson, C.F.; Punjabi, N.M.; Wolfenden, L.; Shardell, M.; Shade, D.M.; Fried, L.P. Relationship Between Lung Function and Physical Performance in Disabled Older Women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2005, 60, 350–354. [Google Scholar] [CrossRef]
- Chiner, E.; Giner, G. Manual SEPAR de Procedimientos; Sistemas de Oxigenoterapia; Editorial Respira: Barcelona, Spain, 2014. [Google Scholar]
- Sharma, G.; Goodwin, J. Effect of Aging on Respiratory System Physiology and Immunology. Clin. Interv. Aging 2006, 1, 253–260. [Google Scholar] [CrossRef]
- Peterson, D.D.; Pack, A.I.; Silage, D.A.; Fishman, A.P. Effects of Aging on Ventilatory and Occlusion Pressure Responses to Hypoxia and Hypercapnia. Am. Rev. Respir. Dis. 1981, 124, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Black, L.F.; Hyatt, R.E. Maximal Respiratory Pressures: Normal Values and Relationship to Age and Sex. Am. Rev. Respir. Dis. 1969, 99, 696–702. [Google Scholar] [CrossRef]
- Enright, P.L.; Kronmal, R.A.; Manolio, T.A.; Schenker, M.B.; Hyatt, R.E. Respiratory Muscle Strength in the Elderly. Correlates and Reference Values. Cardiovascular Health Study Research Group. Am. J. Respir. Crit. Care Med. 1994, 149, 430–438. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
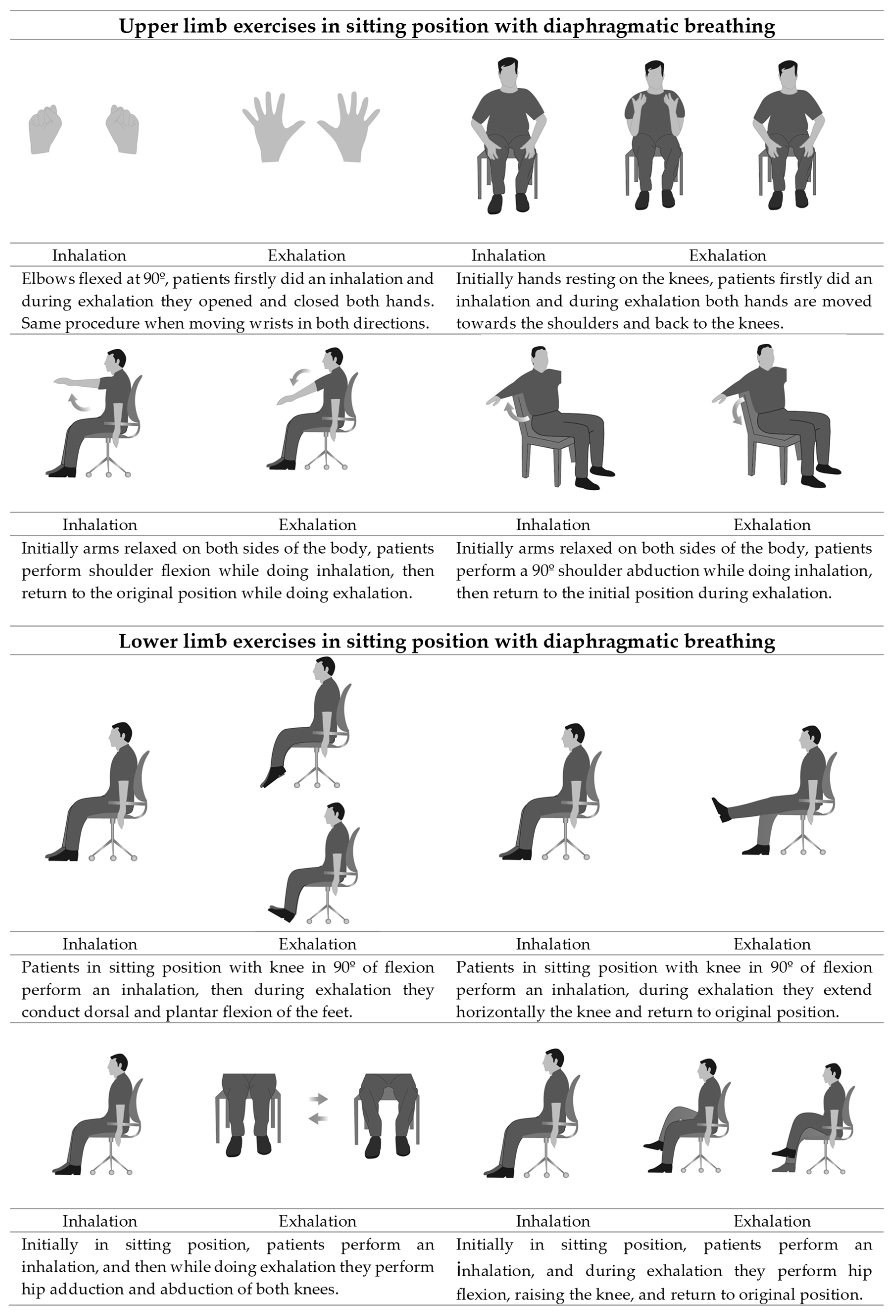

| Week | Session | Step |
|---|---|---|
| 1 | 1 | 1st Comfortable body position and breathing awareness: with appropriate position of the pelvis, neck, eyes, and upper and lower extremities, participants took deep breaths and observed each other’s breathing chest and/or abdominal movements. |
| 2 | ||
| 2 | 3 | 2nd Learning and applying diaphragmatic breathing: participants were instructed to apply nasal inhalation and oral exhalation for promoting a more even inhalation, and patency of small airways during exhalation, respectively. Participants hands were placed on the abdomen (level of the umbilicus) and upper chest (level of the manubrium) for tactile stimulation of movements, and for visual stimulation, participants were instructed to observe increased motion of hand over the abdomen and decreased motion of hand over the upper chest. |
| 4 | ||
| 5 | 3rd Diaphragmatic breathing for coughing: participants were instructed to inhale through the nose with the abdominal-diaphragmatic breathing pattern and then incorporate an abdominal contraction when coughing. | |
| 6 | ||
| 3 | 7 | 4th Diaphragmatic breathing during exercises: for upper limb exercises patients alternated one arm and the other, firstly doing an inhalation while rising the arms and then lowered it in exhalation; for lower limb exercises patients firstly did an inhalation and then they applied the strenuous movement of the exercise while doing exhalation; physiotherapist provided audible stimulation by inhaling loudly with the inspiratory maneuver of the patient and exhaling loudly with the expiratory maneuver of the patient. |
| 8 | ||
| 9 | ||
| 4 | 10 | |
| 11 | 5th Diaphragmatic breathing during activities of daily living: participants were instructed to apply the new pattern gradually in their activities, by performing inhalation when pulling movements and elevation of the arms and exhalation with the strenuous parts of an activity (tying shoelaces) and with pushing movements and lowering of the arms. | |
| 12 | ||
| 5–8 | 13–24 | 6th Consolidation and resolution of doubts: In order to consolidate the breathing technique, during the last four weeks, the diaphragmatic breathing was applied during upper and lower limb exercises and in activities of daily living. |
| CG (N = 15) | EG (N = 15) | P | |
|---|---|---|---|
| Anthropometric variables | |||
| Age, mean (SD) | 75.8 (9.6) | 80.06 (8.2) | 0.180 |
| Sex, frequency (%) | 0.690 | ||
| Male | 5 (33.3) | 4 (26.7) | |
| Female | 10 (66.7) | 11 (73.3) | |
| Weight (kg), mean (SD) | 59.5 (11.3) | 61.0 (8.8) | 1 |
| Height (m), mean (SD) | 1.6 (0.1) | 1.6 (0.1) | 0.441 |
| BMI (kg/m2), mean (SD) | 24.5 (3.8) | 24.6 (2.9) | 0.775 |
| Social and clinical variables | |||
| Marital status, frequency (%) | |||
| Unmarried | 2 (13.3) | 3 (20.0) | 0.856 |
| Married | 5 (33.3) | 4 (26.7) | |
| Widower | 8 (53.3) | 8 (53.3) | |
| Educational level, frequency (%) | |||
| Non-complete primary level | 3 (20.0) | 4 (26.7) | 0.828 |
| Primary level | 7 (46.7) | 4 (26.7) | |
| Secondary level | 2 (13.3) | 3 (20.0) | |
| Professional training | 2 (13.3) | 2 (13.3) | |
| University degree | 1 (6.7) | 1 (6.7) | |
| Number of days per week of exercise, in the last 10 years, frequency (%) | |||
| 0 | 4 (26.7) | 5 (33.3) | 0.649 |
| 1–2 | 4 (26.7) | 3 (20.0) | |
| 3 or more | 5 (33.3) | 7 (46.7) | |
| Medication, frequency (%) | |||
| 0 | 1 (6.7) | 1 (6.7) | 0.746 |
| 1–2 | 3 (20.0) | 5 (33.3) | |
| ≥3 | 11 (73.3) | 9 (60.0) | |
| Hospitalization stays, last 5 years, frequency (%) | |||
| Yes | 4 (26.7) | 7 (46.7) | 0.256 |
| No | 11 (73.3) | 8 (53.3) | |
| Pulmonary function tests | |||
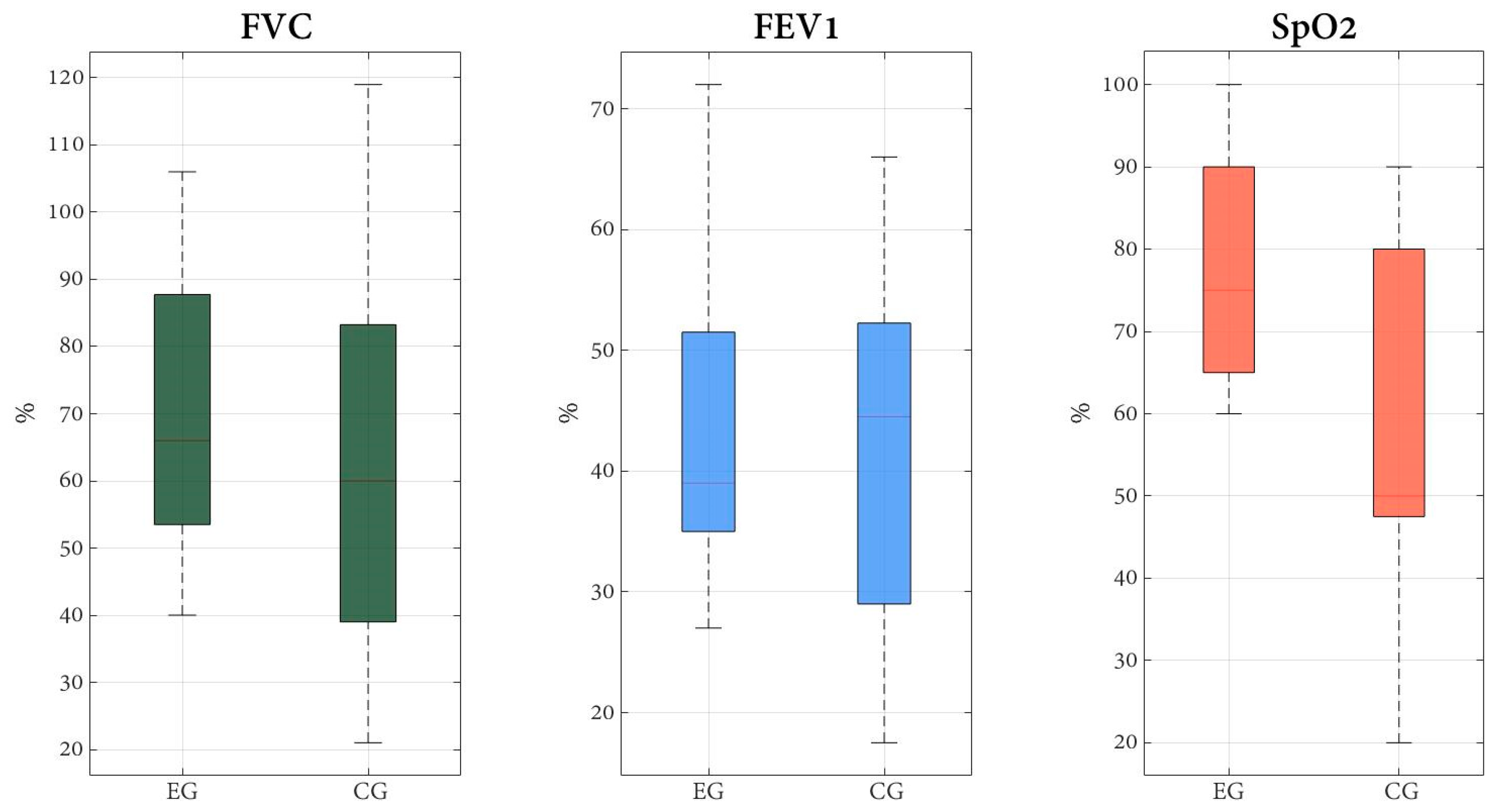
| FVC (%), mean (SD) | 61.5 (28.2) | 65.8 (19.1) | 0.632 |
| FEV1 (%), mean (SD) | 63.7 (28.0) | 61.3 (19.2) | 0.780 |
| FEV1/FVC ratio (%), mean (SD) | 108.9 (13.6) | 105.7 (15.5) | 0.544 |
| Pulse oximetry | |||
| SpO2 (%) | 96.7 (3.0) | 97.1 (2.1) | 0.718 |
| Time Mean (SD) | Mean Difference (95%CI); Effect Size (d) | ||||
|---|---|---|---|---|---|
| Pre | Post | Within-Group Differences | Between-Groups Differences (Post) | ||
| Pulmonary function tests | |||||
| FVC (%) | EG | 65.8 (19.2) | 77.7 (29.9) | 11.9 b(−23.2 to −0.7) | 11.8 (−8.9 to 32.5) |
| CG | 61.5 (28.2) | 65.9 (25.2) | 4.4 (−10.1 to 1.3) | ||
| FEV1 (%) | EG | 61.3 (19.2) | 74.7 (27.8) | 13.4 b(−30.6 to 3.8) | 12.1(−9.5 to 31.6) |
| CG | 63.7 (28.0) | 62.6 (27,00) | −1.1 (−5.2 to 5.5) | ||
| FEV1/FVC ratio (%) | EG | 105.7 (15.5) | 99.5 (21.2) | −6.2 (−7.1 to 10.7) | −0.1(−11.1 to 17.6) |
| CG | 108.9 (13.6) | 99.5 (21.2) | −9.4 (−1.4 to 18.0) | ||
| Pulse oximetry | |||||
| SpO2 (%) | EG | 97.1 (2.1) | 97.9 (1.9) | 0.8 (−1.3 to −0.3) | 1.67 a,b(−0.5 to 1.5); d = 0.8 |
| CG | 96.7 (3.0) | 96.2 (2.3) | −0.5 (−0.2 to 1.3) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnal-Gómez, A.; Saavedra-Hernández, M.; Martinez-Millana, A.; Espí-López, G.V. Clinical Changes of Respiratory Parameters in Institutionalized Older Adults after a Physiotherapy Program Combining Respiratory and Musculoskeletal Exercises. Healthcare 2022, 10, 1680. https://doi.org/10.3390/healthcare10091680
Arnal-Gómez A, Saavedra-Hernández M, Martinez-Millana A, Espí-López GV. Clinical Changes of Respiratory Parameters in Institutionalized Older Adults after a Physiotherapy Program Combining Respiratory and Musculoskeletal Exercises. Healthcare. 2022; 10(9):1680. https://doi.org/10.3390/healthcare10091680
Chicago/Turabian StyleArnal-Gómez, Anna, Manuel Saavedra-Hernández, Antonio Martinez-Millana, and Gemma V. Espí-López. 2022. "Clinical Changes of Respiratory Parameters in Institutionalized Older Adults after a Physiotherapy Program Combining Respiratory and Musculoskeletal Exercises" Healthcare 10, no. 9: 1680. https://doi.org/10.3390/healthcare10091680
APA StyleArnal-Gómez, A., Saavedra-Hernández, M., Martinez-Millana, A., & Espí-López, G. V. (2022). Clinical Changes of Respiratory Parameters in Institutionalized Older Adults after a Physiotherapy Program Combining Respiratory and Musculoskeletal Exercises. Healthcare, 10(9), 1680. https://doi.org/10.3390/healthcare10091680








