Validation and Psychometric Analysis of the German Translation of the Appraisal of Self-Care Agency Scale-Revised
Abstract
1. Introduction
2. Materials and Methods
2.1. Translation
2.2. Data Collection and Participants
- Age in years, marital status (married, single, widowed/divorced), education as corresponding to the German education system (low: no school to 8 years, medium: 10 years, high: a-levels (at least 12 years) or higher educational degree)
- Number and type of diagnoses, selected from a list of 15 choices as specified in the Survey of Health, Ageing and Retirement in Europe (SHARE) dataset (http://www.share-project.org/home0.html, accessed on 29 July 2022) and the option to add further diagnoses, and year of main diagnosis to calculate the variable disease duration (2022—answer given in Year of Diagnosis)
- Number of medications taken daily
- Restrictions in daily activities (ADLs) based on the item used in the DEAS dataset, a large nationwide assessment of elderly patients in Germany: “In the last 6 months or longer, have you been restricted in your daily activities for health-related reasons?” (1 = yes, strongly, 2 = yes, a bit, 3 = no) [43]
2.3. Statistical Analysis
3. Results
3.1. Participants
3.2. Properties of the ASAS-R
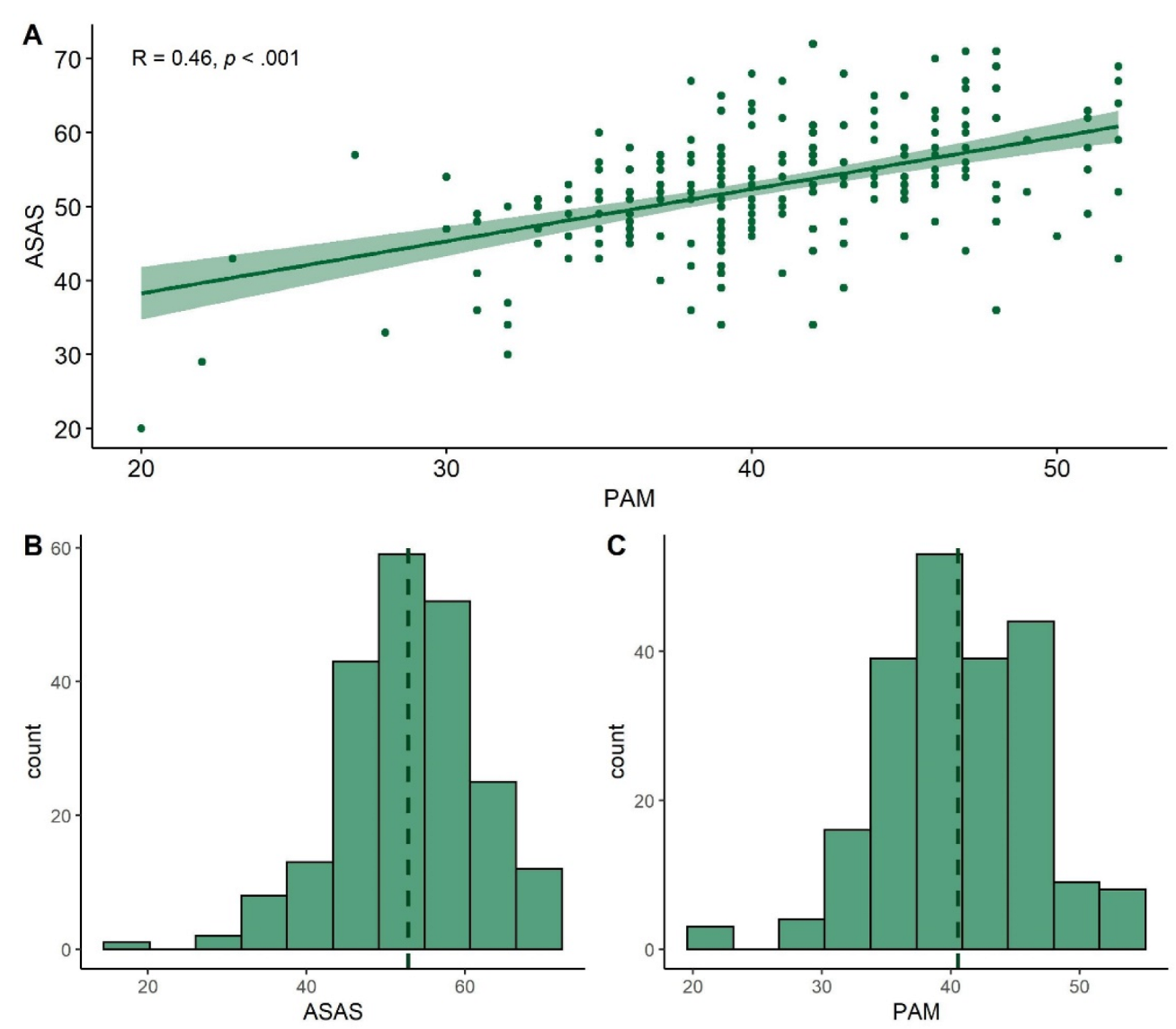
3.3. Comparison of ASAS and PAM
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allegrante, J.P.; Wells, M.T.; Peterson, J.C. Interventions to Support Behavioral Self-Management of Chronic Diseases. Annu. Rev. Public Health 2019, 40, 127–146. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, S.; Mc Carthy, V.J.C.; Savage, E. Frameworks for self-management support for chronic disease: A cross-country comparative document analysis. BMC Health Serv. Res. 2018, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Udlis, K.A. Self-management in chronic illness: Concept and dimensional analysis. J. Nurs. Healthc. Chronic Illn. 2011, 3, 130–139. [Google Scholar] [CrossRef]
- Packer, T.L.; Fracini, A.; Audulv, Å.; Alizadeh, N.; van Gaal, B.G.I.; Warner, G.; Kephart, G. What we know about the purpose, theoretical foundation, scope and dimensionality of existing self-management measurement tools: A scoping review. Patient Educ. Couns. 2018, 101, 579–595. [Google Scholar] [CrossRef]
- Bandura, A.; Freeman, W.H.; Lightsey, R. Self-Efficacy: The Exercise of Control. J. Cogn. Psychother. 1997, 13, 158–166. [Google Scholar] [CrossRef]
- Klompstra, L.; Jaarsma, T.; Strömberg, A. Self-efficacy Mediates the Relationship Between Motivation and Physical Activity in Patients With Heart Failure. J. Cardiovasc. Nurs. 2018, 33, 211–216. [Google Scholar] [CrossRef]
- Lorig, K.R.; Holman, H. Self-management education: History, definition, outcomes, and mechanisms. Ann. Behav. Med. 2003, 26, 1–7. [Google Scholar] [CrossRef]
- Van de Velde, D.; De Zutter, F.; Satink, T.; Costa, U.; Janquart, S.; Senn, D.; De Vriendt, P. Delineating the concept of self-management in chronic conditions: A concept analysis. BMJ Open 2019, 9, e027775. [Google Scholar] [CrossRef]
- Pearce, G.; Parke, H.L.; Pinnock, H.; Epiphaniou, E.; Bourne, C.L.; Sheikh, A.; Taylor, S.J. The PRISMS taxonomy of self-management support: Derivation of a novel taxonomy and initial testing of its utility. J. Health Serv. Res. Policy 2016, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Grady, P.A.; Gough, L.L. Self-management: A comprehensive approach to management of chronic conditions. Am. J. Public Health 2014, 104, e25–e31. [Google Scholar] [CrossRef]
- WHO. Self Care for Health; WHO Regional Office for South-East Asia: New Delhi, India, 2014. [Google Scholar]
- WHO. Guideline on Self-Care Interventions for Health and Well-Being, 2022 Revision; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Sousa, V.D.; Zauszniewski, J.A.; Musil, C.M.; Price Lea, P.J.; Davis, S.A. Relationships among self-care agency, self-efficacy, self-care, and glycemic control. Res. Theory Nurs. Pract. 2005, 19, 217–230. [Google Scholar] [CrossRef]
- Riegel, B.; Moser, D.K.; Anker, S.D.; Appel, L.J.; Dunbar, S.B.; Grady, K.L.; Gurvitz, M.Z.; Havranek, E.P.; Lee, C.S.; Lindenfeld, J.; et al. State of the science: Promoting self-care in persons with heart failure: A scientific statement from the American Heart Association. Circulation 2009, 120, 1141–1163. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, M.; Allotey, P.; Hardon, A. Self care interventions to advance health and wellbeing: A conceptual framework to inform normative guidance. BMJ 2019, 365, l688. [Google Scholar] [CrossRef]
- Matarese, M.; Lommi, M.; De Marinis, M.G.; Riegel, B. A Systematic Review and Integration of Concept Analyses of Self-Care and Related Concepts. J. Nurs. Sch. 2018, 50, 296–305. [Google Scholar] [CrossRef] [PubMed]
- RKI. Gesundheit in Deutschland. Gesundheitsberichterstattung des Bundes. Gemeinsam Getragen von RKI und Destatis; Robert Koch Institut: Berlin, Germany, 2015. [Google Scholar]
- Nowossadeck, E. Einfluss der demografischen Alterung auf die Inanspruchnahme der medizinischen Rehabilitation in Deutschland bis 2040. Rehabilitation 2019, 58, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Fryer, C.E.; Luker, J.A.; McDonnell, M.N.; Hillier, S.L. Self management programmes for quality of life in people with stroke. Cochrane Database Syst. Rev. 2016, 2016, Cd010442. [Google Scholar] [CrossRef]
- Lenferink, A.; Brusse-Keizer, M.; van der Valk, P.D.; Frith, P.A.; Zwerink, M.; Monninkhof, E.M.; van der Palen, J.; Effing, T.W. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2017, 8, Cd011682. [Google Scholar] [CrossRef]
- Luciani, M.; De Maria, M.; Page, S.D.; Barbaranelli, C.; Ausili, D.; Riegel, B. Measuring self-care in the general adult population: Development and psychometric testing of the Self-Care Inventory. BMC Public Health 2022, 22, 598. [Google Scholar] [CrossRef]
- Andrews, D.R.; Richard, D.; Aroian, K. Factor Structure of the Denyes Self Care Practice Instrument (DSCPI-90). West J. Nurs. Res. 2009, 31, 799–811. [Google Scholar] [CrossRef]
- Räsänen, P.; Backman, K.; Kyngäs, H. Development of an instrument to test the middle-range theory for the self-care of home-dwelling elderly. Scand J. Caring Sci. 2007, 21, 397–405. [Google Scholar] [CrossRef]
- Kephart, G.; Packer, T.L.; Audulv, Å.; Warner, G. The structural and convergent validity of three commonly used measures of self-management in persons with neurological conditions. Qual. Life Res. 2019, 28, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Orem, D.E. Nursing Concepts of Practice, 5th ed.; Mosby: Boston, MA, USA, 1995. [Google Scholar]
- Tanaka, M. Orem’s nursing self-care deficit theory: A theoretical analysis focusing on its philosophical and sociological foundation. Nurs. Forum. 2022, 57, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Evers, G.C.M.; Isenberg, M.; Philipsen, H.; Brouns, G.; Halfens, R.; Smeets, H. The appraisal of self-care agency’s ASA-Scale: Research program to test reliability and validity. In Proceedings of the International Nursing Research Conference New Frontiers in Nursing Research; University of Alberta: Edmond, AB, Canada, 1986. [Google Scholar]
- Sousa, V.D.; Zauszniewski, J.A.; Bergquist-Beringer, S.; Musil, C.M.; Neese, J.B.; Jaber, A.F. Reliability, validity and factor structure of the Appraisal of Self-Care Agency Scale-Revised (ASAS-R). J. Eval. Clin. Pract. 2010, 16, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Evers, G.C.; Isenberg, M.A.; Philipsen, H.; Senten, M.; Brouns, G. Validity testing of the Dutch translation of the appraisal of the self-care agency A.S.A.-scale. Int. J. Nurs. Stud. 1993, 30, 331–342. [Google Scholar] [CrossRef]
- van Achterberg, T.; Lorensen, M.; Isenberg, M.A.; Evers, G.C.; Levin, E.; Philipsen, H. The Norwegian, Danish and Dutch version of the Appraisal of Self-care Agency Scale; comparing reliability aspects. Scand J. Caring Sci. 1991, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Söderhamn, O.; Cliffordson, C. The internal structure of the Appraisal of Self-care Agency (ASA) Scale. J. Nurs. Theory 2001, 10, 5–12. [Google Scholar]
- Hudon, É.; Hudon, C.; Lambert, M.; Bisson, M.; Chouinard, M.-C. Generic Self-Reported Questionnaires Measuring Self-Management: A Scoping Review. Clin. Nurs. Res. 2021, 30, 855–865. [Google Scholar] [CrossRef]
- Freund, T.; Gensichen, J.; Goetz, K.; Szecsenyi, J.; Mahler, C. Evaluating self-efficacy for managing chronic disease: Psychometric properties of the six-item Self-Efficacy Scale in Germany. J. Eval. Clin. Pract. 2013, 19, 39–43. [Google Scholar] [CrossRef]
- Ritter, P.L.; Lorig, K. The English and Spanish Self-Efficacy to Manage Chronic Disease Scale measures were validated using multiple studies. J. Clin. Epidemiol. 2014, 67, 1265–1273. [Google Scholar] [CrossRef]
- Sousa, V.D.; Rojjanasrirat, W. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: A clear and user-friendly guideline. J. Eval. Clin. Pract. 2011, 17, 268–274. [Google Scholar] [CrossRef]
- Cramm, J.M.; Strating, M.M.; de Vreede, P.L.; Steverink, N.; Nieboer, A.P. Validation of the self-management ability scale (SMAS) and development and validation of a shorter scale (SMAS-S) among older patients shortly after hospitalisation. Health Qual Life Outcomes 2012, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Incirkuş, K.; Nahcivan, N. Validity and reliability study of the Turkish version of the self-efficacy for managing chronic disease 6-item scale. Turk. J. Med. Sci. 2020, 50, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Goetz, K.; Freund, T.; Gensichen, J.; Miksch, A.; Szecsenyi, J.; Steinhaeuser, J. Adaptation and psychometric properties of the PACIC short form. Am. J. Manag. Care 2012, 18, e55–e60. [Google Scholar]
- Marconcin, P.; Tomé, G.; Carnide, F.; Yázigi, F.; Campos, P.; Pais, S.; Espanha, M. Translation, cultural adaptation and validation of the self-efficacy to manage chronic disease 6-item scale for european Portuguese. Acta Reumatol. Port. 2021, 46, 15–22. [Google Scholar] [PubMed]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis; Pearson Education: London, UK, 2009. [Google Scholar]
- Stevens, J.P. Applied Multivariate Statistics for the Social Sciences; Routledge: London, UK, 2012. [Google Scholar]
- Leiner, D.J. SoSci Survey (Version 3.1.06) [Computer Software]. 2019. Available online: https://www.soscisurvey.de (accessed on 3 August 2022).
- Engstler, H.; Hameister, N.; Schwichtenberg-Hilmert, B. German Ageing Survey (DEAS): User Manual SUF DEAS2014, Version 3.0.; Deutsches Zentrum für Altersfragen: Berlin, Germany, 2020. [Google Scholar]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Bullinger, M.; Kirchberger, I.; Ware, J. Der deutsche SF-36 Health Survey Übersetzung und psychometrische Testung eines krankheitsübergreifenden Instruments zur Erfassung der gesundheitsbezogenen Lebensqualität. Z. Für Gesundh. 1995, 3, 21–36. [Google Scholar] [CrossRef]
- Zill, J.M.; Dwinger, S.; Kriston, L.; Rohenkohl, A.; Härter, M.; Dirmaier, J. Psychometric evaluation of the German version of the Patient Activation Measure (PAM13). BMC Public Health 2013, 13, 1027. [Google Scholar] [CrossRef]
- Hibbard, J.H.; Mahoney, E.R.; Stockard, J.; Tusler, M. Development and testing of a short form of the patient activation measure. Health Serv. Res. 2005, 40, 1918–1930. [Google Scholar] [CrossRef]
- Hibbard, J.H.; Stockard, J.; Mahoney, E.R.; Tusler, M. Development of the Patient Activation Measure (PAM): Conceptualizing and measuring activation in patients and consumers. Health Serv. Res. 2004, 39, 1005–1026. [Google Scholar] [CrossRef]
- Brenk-Franz, K.; Hibbard, J.H.; Herrmann, W.J.; Freund, T.; Szecsenyi, J.; Djalali, S.; Steurer-Stey, C.; Sönnichsen, A.; Tiesler, F.; Storch, M.; et al. Validation of the German version of the patient activation measure 13 (PAM13-D) in an international multicentre study of primary care patients. PLoS ONE 2013, 8, e74786. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Revelle, W. Psych: Procedures for Personality and Psychological Research Version 2.1.9; Northwestern University: Evanston, IL, USA, 2021. [Google Scholar]
- Prinsen, C.A.C.; Mokkink, L.B.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; de Vet, H.C.W.; Terwee, C.B. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual. Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Blanz, M. Forschungsmethoden und Statistik für die Soziale Arbeit: Grundlagen und Anwendungen; Kohlhammer: Stuttgart, Germany, 2015. [Google Scholar]
- Everitt, B.; Skrondal, A. The Cambridge Dictionary of Statistics, 4th ed.; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Piedmont, R.L. Inter-item Correlations. In Encyclopedia of Quality of Life and Well-Being Research; Michalos, A.C., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 3303–3304. [Google Scholar]
- Rosseel, Y. lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Costello, A.B.; Osborne, J. Best Practices in Exploratory Factor Analysis: Four Recommendations for Getting the Most From Your Analysis. Pract. Assess. Res. Eval. 2005, 10, 1–9. [Google Scholar]
- Gaskin, C.J.; Happell, B. On exploratory factor analysis: A review of recent evidence, an assessment of current practice, and recommendations for future use. Int. J. Nurs. Stud. 2014, 51, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.D.; Grieder, S.G. EFAtools: An R package with fast and flexible implementations of exploratory factor analysis tools. J. Open Source Softw. 2020, 5, 252. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 03139. [Google Scholar] [CrossRef]
- Hayes, A.F.; Cai, L. Using heteroskedasticity-consistent standard error estimators in OLS regression: An introduction and software implementation. Behav. Res. Methods 2007, 39, 709–722. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kamradt, M.; Bozorgmehr, K.; Krisam, J.; Freund, T.; Kiel, M.; Qreini, M.; Flum, E.; Berger, S.; Besier, W.; Szecsenyi, J.; et al. Assessing self-management in patients with diabetes mellitus type 2 in Germany: Validation of a German version of the Summary of Diabetes Self-Care Activities measure (SDSCA-G). Health Qual. Life Outcomes 2014, 12, 185. [Google Scholar] [CrossRef]
- Graffigna, G.; Barello, S.; Bonanomi, A.; Lozza, E.; Hibbard, J. Measuring patient activation in Italy: Translation, adaptation and validation of the Italian version of the patient activation measure 13 (PAM13-I). BMC Med. Inform. Deci. Mak. 2015, 15, 109. [Google Scholar] [CrossRef]
- Maindal, H.T.; Sokolowski, I.; Vedsted, P. Translation, adaptation and validation of the American short form Patient Activation Measure (PAM13) in a Danish version. BMC Public Health 2009, 9, 209. [Google Scholar] [CrossRef]
- Sousa, V.D.; Zauszniewski, J.A.; Zeller, R.A.; Neese, J.B. Factor analysis of the Appraisal of Self-care Agency Scale in American adults with diabetes mellitus. Diabetes Educ. 2008, 34, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Fok, M.S.; Alexander, M.F.; Wong, T.K.; McFayden, A.K. Contextualising the appraisal of Self-care Agency Scale in Hong Kong. Contemp. Nurse. 2002, 12, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Stacciarini, T.S.; Pace, A.E. Confirmatory factor analysis of the Appraisal of Self-Care Agency Scale-Revised. Rev. Lat. Am. 2017, 25, e2856. [Google Scholar] [CrossRef] [PubMed]
- Colomer-Pérez, N.; Useche, S.A. Self-care appraisal in nursing assistant students: Adaptation, validation and psychometric properties of the Spanish ASAS. PLoS ONE 2021, 16, e0260827. [Google Scholar] [CrossRef]
- RKI. Welche Auswirkungen hat der demografische Wandel auf Gesundheit und Gesundheitsversorgung? Robert Koch Institut: Berlin, Germany, 2015. [Google Scholar]
- Greene, A.L.; Eaton, N.R.; Forbes, M.K.; Fried, E.I.; Watts, A.L.; Kotov, R.; Krueger, R.F. Model fit is a fallible indicator of model quality in quantitative psychopathology research: A reply to Bader and Moshagen. J. Psychopathol. Clin. Sci. 2022, 131, 696–703. [Google Scholar] [CrossRef]
| Variable | M (SD) | Median (IQR) | Range | n |
|---|---|---|---|---|
| Age | 51.633 (14.686) | 47 (59–42) | 92–19 | 215 |
| Number of Medications | 3.219 (2.802) | 3 (4–1) | 18–0 | 215 |
| Number of Diagnoses | 1.986 (1.190) | 2 (2–1) | 8–1 | 215 |
| Disease Duration | 13.778 (11.071) | 12 (20–5) | 57–0 | 207 |
| ASAS-R Sum | 52.805 (8.386) | 53 (58–48) | 72–20 | 215 |
| PAM Sum | 40.576 (5.925) | 40 (45–37) | 52–20 | 215 |
| Value | Count | % | n | |
| Gender | 215 | |||
| Male | 64 | 29.767 | ||
| Female | 151 | 70.233 | ||
| Education | 214 | |||
| Low | 16 | 7.477 | ||
| Medium | 110 | 51.402 | ||
| High | 88 | 41.121 | ||
| Marital State | 215 | |||
| Married | 139 | 64.651 | ||
| Single | 50 | 23.256 | ||
| Divorced/Widowed | 26 | 12.093 | ||
| Current Health | 215 | |||
| 1 Excellent | 5 | 2.326 | ||
| 2 Very Good | 29 | 13.488 | ||
| 3 Good | 78 | 36.279 | ||
| 4 Not Good | 90 | 41.860 | ||
| 5 Poor | 13 | 6.047 | ||
| ADL | 215 | |||
| 1 Very restricted | 46 | 21.395 | ||
| 2 A Bit Restricted | 113 | 52.558 | ||
| 3 Not Restricted | 56 | 26.047 |
| Response Frequencies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | r * | cor.r * | Mean (SD) | 1 | 2 | 3 | 4 | 5 | Floor | Ceiling |
| 1 | 0.59 | 0.67 | 4.2 (0.85) | 0.01 | 0.05 | 0.08 | 0.47 | 0.39 | 2 (0.9%) | 83 (17.7%) |
| 2 | 0.63 | 0.74 | 4.0 (0.83) | 0.01 | 0.04 | 0.13 | 0.54 | 0.27 | 3 (1.4%) | 58 (27.0%) |
| 3 | 0.60 | 0.69 | 4.1 (0.76) | 0.00 | 0.04 | 0.10 | 0.56 | 0.29 | 1 (0.5%) | 63 (29.3%) |
| 4 | 0.40 | 0.43 | 2.6 (1.31) | 0.22 | 0.35 | 0.10 | 0.24 | 0.09 | 48 (22.3%) | 19 (8.8%) |
| 5 | 0.31 | 0.38 | 3.8 (0.98) | 0.02 | 0.10 | 0.18 | 0.46 | 0.25 | 4 (1.9%) | 53 (24.7%) |
| 6 | 0.56 | 0.59 | 3.5 (1.13) | 0.03 | 0.24 | 0.12 | 0.43 | 0.19 | 6 (2.8%) | 40 (18.6%) |
| 7 | 0.39 | 0.44 | 3.4 (1.27) | 0.07 | 0.23 | 0.12 | 0.34 | 0.23 | 16 (7.4%) | 50 (23.5%) |
| 8 | 0.43 | 0.50 | 3.7 (1.00) | 0.03 | 0.11 | 0.21 | 0.45 | 0.20 | 6 (2.8%) | 44 (20.5%) |
| 9 | 0.31 | 0.41 | 3.7 (0.91) | 0.02 | 0.08 | 0.27 | 0.47 | 0.15 | 5 (2.3%) | 33 (15.3%) |
| 10 | 0.48 | 0.58 | 3.6 (0.88) | 0.01 | 0.12 | 0.29 | 0.46 | 0.12 | 2 (0.9%) | 26 (12.1%) |
| 11 | 0.48 | 0.46 | 2.7 (1.11) | 0.11 | 0.41 | 0.18 | 0.25 | 0.05 | 23 (10.7%) | 11 (5.1%) |
| 12 | 0.33 | 0.39 | 4.1 (0.89) | 0.01 | 0.06 | 0.08 | 0.48 | 0.37 | 3 (1.4%) | 79 (36.7%) |
| 13 | 0.38 | 0.40 | 3.9 (0.98) | 0.02 | 0.11 | 0.09 | 0.54 | 0.24 | 5 (2.3%) | 51 (23.7%) |
| 14 | 0.46 | 0.50 | 3.0 (1.26) | 0.13 | 0.30 | 0.18 | 0.27 | 0.13 | 27 (12.6%) | 27 (21.6%) |
| 15 | 0.47 | 0.51 | 2.5 (1.26) | 0.22 | 0.38 | 0.16 | 0.15 | 0.10 | 47 (21.9%) | 21 (9.8%) |
| A | Std. α | Mean (SD) | 95% CI | Range | Floor | Ceiling | ||||
| Sum | 0.82 | 0.83 | 52.81 (8.39) | 0.79–0.86 | 72–20 | 0 (0%) | 0 (0%) | |||
| Fac1 | 0.80 | 0.80 | 2.90 (0.91) | 0.76–0.85 | 25–5 | 2 (0.93) | 2 (0.93) | |||
| Fac2 | 0.78 | 0.79 | 4.0 (0.63) | 0.73–0.82 | 25–5 | 1 (0.47) | 11 (5.12) | |||
| Fac3 | 0.67 | 0.69 | 3.60 (0.72) | 0.59–0.74 | 20–4 | 1 (0.47) | 8 (3.72) | |||
| Model Fit | ||||||
|---|---|---|---|---|---|---|
| Model | 𝜒2 | Df | p | |||
| Baseline | 1119.79 | 91 | ||||
| Factor Model | 155.563 | 74 | <0.001 | |||
| CFI | TLI | AIC | RMSEA | 95% CI | p | |
| 0.921 | 0.903 | 7721.316 | 0.072 | 0.056, 0.087 | 0.014 | |
| Parameter Estimates | ||||||
| Factor | Item | Est. (ß) | Std. Error | z-value | p | 95% CI |
| Factor 1 | ASAS1 | 0.614 | 0.053 | 11.607 | <0.001 | 0.510, 0.717 |
| ASAS2 | 0.721 | 0.048 | 14.980 | <0.001 | 0.627, 0.815 | |
| ASAS3 | 0.602 | 0.046 | 13.217 | <0.001 | 0.513, 0.692 | |
| ASAS8 | 0.511 | 0.068 | 7.550 | <0.001 | 0.378, 0.644 | |
| ASAS12 | 0.370 | 0.061 | 6.023 | <0.001 | 0.250, 0.490 | |
| Factor 2 | ASAS5 | 0.496 | 0.069 | 7.164 | <0.001 | 0.360, 0.632 |
| ASAS7 | 0.516 | 0.092 | 5.631 | <0.001 | 0.336, 0.696 | |
| ASAS9 | 0.628 | 0.061 | 10.236 | <0.001 | 0.508, 0.748 | |
| ASAS10 | 0.748 | 0.057 | 13.016 | <0.001 | 0.635, 0.861 | |
| Factor 3 | ASAS4 | 0.684 | 0.088 | 7.738 | <0.001 | 0.510, 0.857 |
| ASAS6 | 0.689 | 0.074 | 9.318 | <0.001 | 0.544, 0.384 | |
| ASAS11 | 0.633 | 0.074 | 8.579 | <0.001 | 0.488, 0.777 | |
| ASAS14 | 1.075 | 0.074 | 14.467 | <0.001 | 0.929, 1.221 | |
| ASAS15 | 1.020 | 0.076 | 13.459 | <0.001 | 0.871, 1.168 | |
| PAM | |||
|---|---|---|---|
| Predictors | Est. (ß) | CI | p |
| (Intercept) | 31.76 | 24.49–39.04 | <0.001 |
| ASAS-R | 0.28 | 0.20–0.36 | <0.001 |
| Age | −0.00 | −0.06–0.05 | 0.875 |
| Number of Diagnoses | −0.55 | −1.21–0.10 | 0.099 |
| Disease Duration | 0.07 | 0.01–0.13 | 0.020 |
| Gender: Male | 0.54 | −0.96–2.04 | 0.479 |
| Education: Medium | 1.98 | −0.59–4.54 | 0.130 |
| Education: High | 3.66 | 1.05–6.27 | 0.006 |
| Health: Very good | −7.20 | −11.61–−2.80 | 0.001 |
| Health: Good | −7.39 | −11.66–−3.13 | 0.001 |
| Health: Not good | −10.09 | −14.43–−5.75 | <0.001 |
| Health: Poor | −13.65 | −19.00–−8.30 | <0.001 |
| ADL: Lightly Restricted | −0.01 | −1.90–1.88 | 0.991 |
| ADL: Not Restricted | 0.36 | −1.99–2.71 | 0.761 |
| Number of Medications | 0.13 | −0.16–0.42 | 0.381 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönenberg, A.; Teschner, U.; Prell, T.; Mühlhammer, H.M. Validation and Psychometric Analysis of the German Translation of the Appraisal of Self-Care Agency Scale-Revised. Healthcare 2022, 10, 1785. https://doi.org/10.3390/healthcare10091785
Schönenberg A, Teschner U, Prell T, Mühlhammer HM. Validation and Psychometric Analysis of the German Translation of the Appraisal of Self-Care Agency Scale-Revised. Healthcare. 2022; 10(9):1785. https://doi.org/10.3390/healthcare10091785
Chicago/Turabian StyleSchönenberg, Aline, Ulrike Teschner, Tino Prell, and Hannah M. Mühlhammer. 2022. "Validation and Psychometric Analysis of the German Translation of the Appraisal of Self-Care Agency Scale-Revised" Healthcare 10, no. 9: 1785. https://doi.org/10.3390/healthcare10091785
APA StyleSchönenberg, A., Teschner, U., Prell, T., & Mühlhammer, H. M. (2022). Validation and Psychometric Analysis of the German Translation of the Appraisal of Self-Care Agency Scale-Revised. Healthcare, 10(9), 1785. https://doi.org/10.3390/healthcare10091785







