The Relationship between Patellofemoral Pain Syndrome and Hip Biomechanics: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
- Knee joint musculature strength in PFPS
- -
- Studies including subjects with a PFPS diagnosed
- -
- Studies evaluated the effects of knee muscular strengthening in subjects with PFPS
- Hip joint musculature strength in PFPS
- -
- Studies including subjects with a PFPS diagnosed
- -
- Studies evaluated the effects of hip muscular strengthening in subjects with PFPS
- Hip joint kinematic variation in PFPS
- -
- Studies including subjects with PFPS diagnosed
- -
- Studies examined the effects of hip joint kinematics on subjects with PFPS.
- Knee joint kinematic variation in PFPS
- -
- Studies including subjects with PFPS diagnosed
- -
- Studies examined the effects of knee joint kinematic characteristics on subjects with PFPS.
2.2. Quality Assessment of Selected Studies
2.3. Data Extraction and Analysis
3. Results
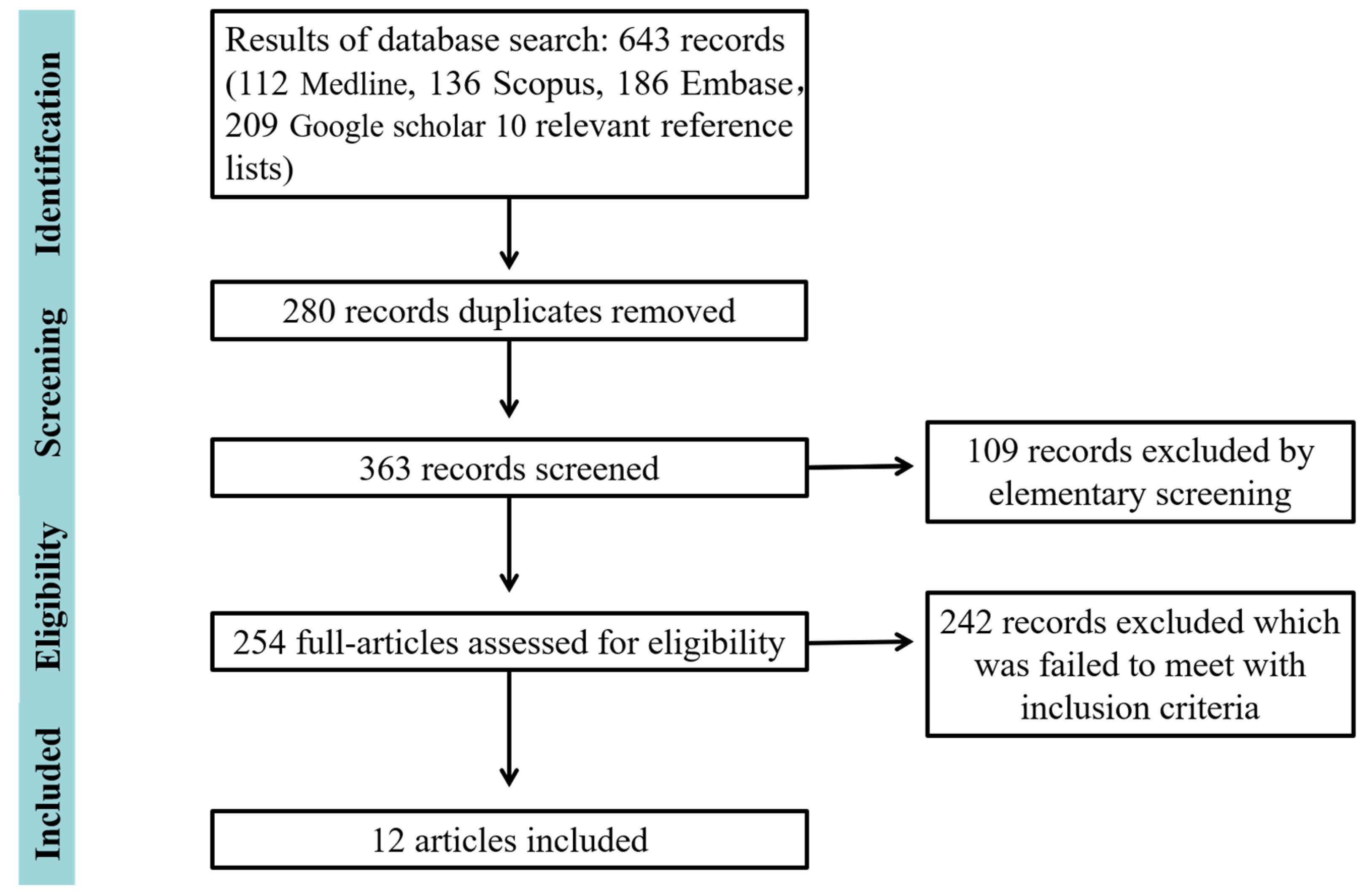
3.1. Search Results
3.2. Quality Assessment of Included Studies
3.3. Studies Characteristic
3.4. Kinematic Variation
3.5. Hip Strength and Torque
3.6. Muscle Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McClinton, S.; Donatell, G.; Weir, J.; Heiderscheit, B. Influence of step height on quadriceps onset timing and activation during stair ascent in individuals with patellofemoral pain syndrome. J. Orthop. Sport. Phys. Ther. 2007, 37, 239–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gaitonde, D.Y.; Ericksen, A.; Robbins, R.C. Patellofemoral Pain Syndrome. Am. Fam. Physician. 2019, 15, 88–94. [Google Scholar]
- Taunton, J.E.; Ryan, M.B.; Clement, D.B.; McKenzie, D.C.; Lloyd-Smith, D.R.; Zumbo, B.D. A retrospective case-control analysis of 2002 running injuries. Br. J. Sport. Med. 2002, 36, 95–101. [Google Scholar] [CrossRef]
- Davis, I.S.; Tenforde, A.S.; Neal, B.S.; Roper, J.L.; Willy, R.W. Gait retraining as an intervention for patellofemoral pain. Curr. Rev. Musculoskelet. Med. 2020, 13, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Puzzitiello, R.N.; Waterman, B.; Agarwalla, A.; Zuke, W.; Cole, B.J.; Verma, N.N.; Yanke, A.B.; Forsythe, B. Primary medial patellofemoral ligament repair versus reconstruction: Rates and risk factors for instability recurrence in a young, active patient population. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 2909–2915. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.; Whatman, C.; Sheerin, K.; Hume, P.; Johnson, M.I. The proportion of lower limb running injuries by gender, anatomical location and specific pathology: A systematic review. J. Sport. Sci. Med. 2019, 18, 21. [Google Scholar]
- Lee, T.Q.; Yang, B.Y.; Sandusky, M.D.; McMahon, P.J. The effects of tibial rotation on the patellofemoral joint: Assessment of the changes in in situ strain in the peripatellar retinaculum and the patellofemoral contact pressures and areas. J. Rehabil. Res. Dev. 2014, 38, 463–469. [Google Scholar]
- Emamvirdi, M.; Letafatkar, A.; Khaleghi Tazji, M. The effect of valgus control instruction exercises on pain, strength, and functional. Sport. Health 2019, 11, 223–237. [Google Scholar] [CrossRef]
- Fagan, V.; Delahunt, E. Patellofemoral pain syndrome: A review on the associated neuromuscular deficits and current treatment options. Br. J. Sport. Med. 2008, 42, 789–795. [Google Scholar] [CrossRef]
- Barton, C.J.; Lack, S.; Malliaras, P.; Morrissey, D. Gluteal muscle activity and patellofemoral pain syndrome: A systematic review. Br. J. Sport. Med. 2013, 47, 207–214. [Google Scholar] [CrossRef]
- Neptune, R.R.; Wright, I.C.; van den Bogert, A.J. The influence of orthotic devices and vastus medialis strength and timing on patellofemoral loads during running. Clin. Biomech. 2000, 15, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Van Tulder, M.; Furlan, A.; Bombardier, C.; Bouter, L.; Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine 2003, 28, 1290–1299. [Google Scholar] [CrossRef]
- Dierks, T.A.; Manal, K.T.; Hamill, J.; Davis, I.S. Proximal and distal influences on hip and knee kinematics in runners with patellofemoral pain during a prolonged run. J. Orthop. Sport. Phys. Ther. 2008, 38, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Lack, S.; Barton, C.; Sohan, O.; Crossley, K.; Morrissey, D. Proximal muscle rehabilitation is effective for patellofemoral pain: A systematic review with meta-analysis. Br. J. Sport. Med. 2015, 49, 1365–1376. [Google Scholar] [CrossRef]
- Noehren, B.; Pohl, M.B.; Sanchez, Z.; Cunningham, T.; Lattermann, C. Proximal and distal kinematics in female runners with patellofemoral pain. Clin. Biomech. 2012, 27, 366–371. [Google Scholar] [CrossRef]
- Willson, J.D.; Davis, I.S. Lower extremity mechanics of females with and without patellofemoral pain across activities with progressively greater task demands. Clin. Biomech. 2008, 23, 203–211. [Google Scholar] [CrossRef]
- Nakagawa, T.H.; Moriya, É.T.; Maciel, C.D.; Serrão, F.V. Trunk, pelvis, hip, and knee kinematics, hip strength, and gluteal muscle activation during a single-leg squat in males and females with and without patellofemoral pain syndrome. J. Orthop. Sport. Phys. Ther. 2012, 42, 491–501. [Google Scholar] [CrossRef]
- Barton, C.J.; Levinger, P.; Webster, K.E.; Menz, H.B. Walking kinematics in individuals with patellofemoral pain syndrome: A case–control study. Gait Posture 2011, 33, 286–291. [Google Scholar] [CrossRef]
- Paoloni, M.; Mangone, M.; Fratocchi, G.; Murgia, M.; Saraceni, V.M.; Santilli, V. Kinematic and kinetic features of normal level walking in patellofemoral pain syndrome: More than a sagittal plane alteration. J. Biomech. 2010, 43, 1794–1798. [Google Scholar] [CrossRef]
- Bolgla, L.A.; Malone, T.R.; Umberger, B.R.; Uhl, T.L. Hip strength and hip and knee kinematics during stair descent in females with and without patellofemoral pain syndrome. J. Orthop. Sport. Phys. Ther. 2008, 38, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Salsich, G.B.; Brechter, J.H.; Powers, C.M. Lower extremity kinetics during stair ambulation in patients with and without patellofemoral pain. Clin. Biomech. 2001, 16, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Ireland, M.L.; Willson, J.D.; Ballantyne, B.T.; Davis, I.M. Hip strength in females with and without patellofemoral pain. J. Orthop. Sport. Phys. Ther. 2003, 33, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.L.; Nee, R.J. Analysis of hip strength in females seeking physical therapy treatment for unilateral patellofemoral pain syndrome. J. Orthop. Sport. Phys. Ther. 2007, 37, 232–238. [Google Scholar] [CrossRef]
- Glaviano, N.R.; Saliba, S. Differences in gluteal and quadriceps muscle activation during weight-bearing exercises between female subjects with and without patellofemoral pain. J. Strength Cond. Res. 2022, 36, 55–62. [Google Scholar] [CrossRef]
- Davis, I.S.; Powers, C. Patellofemoral pain syndrome: Proximal, distal, and local factors—International research retreat, April 30–may 2, 2009, Baltimore, Maryland. J. Orthop. Sport. Phys. Ther. 2010, 40, A1–A48. [Google Scholar] [CrossRef]
- Arazpour, M.; Bahramian, F.; Abutorabi, A.; Nourbakhsh, S.T.; Alidousti, A.; Aslani, H. The effect of patellofemoral pain syndrome on gait parameters: A literature review. Arch. Bone Jt. Surg. 2016, 4, 298. [Google Scholar]
- Fulkerson, J.P. Diagnosis and treatment of patients with patellofemoral pain. Am. J. Sport. Med. 2002, 30, 447–456. [Google Scholar] [CrossRef]
- Osborne, J.D.; Luczak, S.B.; Acker, W.B.; Bicos, J. Patellofemoral joint contact pressures: Current concepts and use in patellar instability studies. Orthopedics 2019, 42, e172–e179. [Google Scholar] [CrossRef]
- Mizuno, Y.; Kumagai, M.; Mattessich, S.M.; Elias, J.J.; Ramrattan, N.; Cosgarea, A.J.; Chao, E.Y. Q-angle influences tibiofemoral and patellofemoral kinematics. J. Orthop. Res. 2001, 19, 834–840. [Google Scholar] [CrossRef]
- Mascal, C.L.; Landel, R.; Powers, C. Management of patellofemoral pain targeting hip, pelvis, and trunk muscle function: 2 case reports. J. Orthop. Sport. Phys. Ther. 2003, 33, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.M. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: A theoretical perspective. J. Orthop. Sport. Phys. Ther. 2003, 33, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Nyland, J.; Kuzemchek, S.; Parks, M.; Caborn, D.N.M. Femoral anteversion influences vastus medialis and gluteus medius EMG amplitude: Composite hip abductor EMG amplitude ratios during isometric combined hip abduction-external rotation. J. Electromyogr. Kinesiol. 2004, 14, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lankhorst, N.E.; Bierma-Zeinstra, S.M.; van Middelkoop, M. Factors associated with patellofemoral pain syndrome: A systematic review. Br. J. Sport. Med. 2013, 47, 193–206. [Google Scholar] [CrossRef]
- Bolgla, L.A.; Uhl, T.L. Electromyographic analysis of hip rehabilitation exercises in a group of healthy subjects. J. Orthop. Sport. Phys. Ther. 2005, 35, 487–494. [Google Scholar] [CrossRef]
- Dolak, K.L.; Silkman, C.; Medina McKeon, J.; Hosey, R.G.; Lattermann, C.; Uhl, T.L. Hip strengthening prior to functional exercises reduces pain sooner than quadriceps strengthening in females with patellofemoral pain syndrome: A randomized clinical trial. J. Orthop. Sport. Phys. Ther. 2011, 41, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.Y.; Rossetto, F.M.; Magalhães, E.; Bryk, F.F.; Lucareli, P.R.; de Almeida Aparecida Carvalho, N. Short-term effects of hip abductors and lateral rotators strengthening in females with patellofemoral pain syndrome: A randomized controlled clinical trial. J. Orthop. Sport. Phys. Ther. 2010, 40, 736–742. [Google Scholar] [CrossRef]
- Fukuda, T.Y.; Melo, W.P.; Zaffalon, B.M.; Rossetto, F.M.; Magalhães, E.; Bryk, F.F.; Martin, R.L. Hip posterolateral musculature strengthening in sedentary women with patellofemoral pain syndrome: A randomized controlled clinical trial with 1-year follow-up. J. Orthop. Sport. Phys. Ther. 2012, 42, 823–830. [Google Scholar] [CrossRef]
- Khayambashi, K.; Mohammadkhani, Z.; Ghaznavi, K.; Lyle, M.A.; Powers, C.M. The effects of isolated hip abductor and external rotator muscle strengthening on pain, health status, and hip strength in females with patellofemoral pain: A randomized controlled trial. J. Orthop. Sport. Phys. Ther. 2012, 42, 22–29. [Google Scholar] [CrossRef]
- Dempster, J.; Dutheil, F.; Ugbolue, U.C. The Prevalence of Lower Extremity Injuries in Running and Associated Risk Factors: A Systematic Review. Phys. Act. Health 2021, 5, 133–145. [Google Scholar] [CrossRef]





| Study | 1 | 2 | 3 | 5 | 6 | 7 | 10 | 11 | 12 | 15 | 16 | 18 | 20 | 21 | 25 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dierks et al. 2008 [14] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | U | U | 1 | 1 | 1 | 1 | U | 11 |
| Lack et al. 2009 [15] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | U | U | 1 | 1 | 1 | 1 | U | 10 |
| Noehren et al. 2012 [16] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | U | U | 1 | 1 | 1 | 1 | U | 11 |
| Willson et al. 2008 [17] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | U | U | 1 | 1 | U | 1 | U | 9 |
| Nakagawa et al. 2012 [18] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | U | U | 1 | 1 | 1 | 1 | U | 11 |
| Barton et al. 2011 [19] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | U | U | 1 | 1 | 1 | 1 | U | 11 |
| Paoloni et al. 2010 [20] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | U | U | 1 | 1 | 0 | 1 | U | 9 |
| Bolgla et al. 2008 [21] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | U | U | 1 | 1 | 1 | 1 | U | 11 |
| Salsich et a. 2001 [22] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | U | U | 1 | 1 | U | 1 | U | 10 |
| Ireland et al. 2003 [23] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | U | 1 | 1 | 0 | 1 | U | 11 |
| Robinson and Nee 2007 [24] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | U | U | 1 | 1 | 1 | 1 | U | 11 |
| Glaviano and Saliba 2022 [25] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | U | U | 1 | 1 | 1 | 1 | U | 11 |
| Study | Sample Size | Gender (F:M) | Age Range (Mean Age) | Height (m), Weight (kg) | ||||
|---|---|---|---|---|---|---|---|---|
| PFPS | CON | PFPS | CON | PFPS | CON | PFPS | CON | |
| Dierks et al. 2008 [14] | 20 | 20 | 15:5 | 15:5 | NR (24.1 ± 7.4) | NR (22.7 ± 5.6) | 1.71 ± 0.1 m 65.75 ± 12.56 kg | 1.70 ± 0.08 m 63.02 ± 9.15 kg |
| Lack et al. 2009 [15] | 21 | 20 | 21:0 | 20:0 | 18-45 (27 ± 6) | 18-45 (26 ± 5) | 1.70 ± 0.06 m 65 ± 10 kg | 1.70 ± 0.05 m 63 ± 7.0 kg |
| Noehren et al. 2012 [16] | 16 | 16 | 16:0 | 16:0 | 18–45 (27 ± 6) | 18–45 (25 ± 4) | 1.64 ± 0.05 m 57.4 ± 4.6 kg | 1.65 ± 0.07 m 58.7 ± 6.5 kg |
| Willson et al. 2008 [17] | 20 | 20 | 20:0 | 20:0 | NR (23.3 ± 3.1) | NR (23.7 ± 3.6) | 1.66 ± 0.08 m 61.7 ± 10.6 kg | 1.66 ± 0.06 m 61.1 ± 5.4 kg |
| Nakagawa et al. 2012 [18] | 20 | 20 | 20:20 | 20:20 | F: NR (22.3 ± 3.1) M: NR (24.2 ± 4.4) | F: NR (21.8 ± 2.6) M: NR (23.5 ± 3.8) | F: 1.66 ± 0.59 m 61.1 ± 7.5 kg M: 1.80 ± 0.51 m 77.0 ± 9.6 kg | F: 1.63 ± 0.73 m 59.4 ± 7.3 kg M: 1.76 ± 0.6 m 74.6 ± 9.1kg |
| Barton et al. 2011 [19] | 26 | 20 | 21:5 | 16:4 | 18–35 (25.1 ± 4.6) | 18–35 (23.4 ± 2.3) | 1.6 ± 8.4 m 66.7 ± 12.8 kg | 1.7 ± 8.4 m 66.0 ± 15.4 kg |
| Paoloni et al. 2010 [20] | 9 | 9 | 7:2 | 7:2 | 19–45 (28.1 ± 8.1) | 21–38 (18.3 ± 5.9) | 1.71 ± 0.09 m 64.4 ± 9.5 kg | 1.70 ± 0.09 m 64.2 ± 10.8 kg |
| Bolgla et al. 2008 [21] | 18 | 18 | 18:0 | 18:0 | NR (24.5 ± 3.2) | NR (23.9 ± 2.8) | 1.7 ± 0.1m 63.1 ± 9.1kg | 1.7 ± 0.1m 62.1 ± 8.5 kg |
| Salsich et a. 2001 [22] | 10 | 10 | 5:5 | 5:5 | 22–55 (36.5 ± 11.1) | 21–42 (31.9 ± 7.3) | 1.73 ± 10.3 m 70.9 ± 13.3 kg | 1.70 ± 11.3 m 14.5 ± 67.7 kg |
| Ireland et al. 2003 [23] | 15 | 15 | 15:0 | 15:0 | 12–21 (15.7 ± 2.7) | 12–21 (15.7 ± 2.7) | 63.1 ± 16.5 kg | 56.6 ± 12.5 kg |
| Robinson and Nee 2007 [24] | 10 | 10 | 10:0 | 10:0 | 12–34 (21.0) | 16–35 (26.6) | 63.5 kg | 66.5 kg |
| Glaviano and Saliba 2022 [25] | 20 | 20 | 20:0 | 20:0 | NR (21.3 ± 2.7) | NR (20.7 ± 2.1) | 1.68 ± 6.4 m 20.7 ± 21.0 kg | 1.67 ± 6.5 m 64.2 ± 9.5 kg |
| Study | Functional Activity | Pain Duration | Muscle Strength | Muscles | EMG Variable | Kinematics (Peak) |
|---|---|---|---|---|---|---|
| Dierks et al. 2008 [14] | Running; IST | More than 2 months | Hip abductor: Hip external rotator | |||
| Lack et al. 2009 [15] | Running Stair descent DLDJ | GMed GMax | The average magnitude of activity (%MVC - average over stance period) | Peak hip rotation Peak hip adduction Peak hip torque | ||
| Noehren et al. 2012 [16] | Walking | More than 6 weeks | Knee flexion; abduction; internal rotation Hip adduction; internal rotation | |||
| Willson et al. 2008 [17] | Stair descent Stair ascent | NR | Knee flexion Hip flexion | |||
| Nakagawa et al. 2012 [18] | SL squat | 14.4 ± 12.8 months | GMed VM VL | Maximal voluntary isometric contraction (MVIC) | Peak hip adduction Hip internal rotation Knee abduction | |
| Barton et al. 2011 [19] | walking | More than 6 weeks | Hip, knee, rearfoot, and forefoot movement in three planes | |||
| Paoloni et al. 2010 [20] | Single leg squat Single leg jump Running | NR | Knee internal rotation Hip internal rotation Hip adduction | |||
| Bolgla et al. 2008 [21] | IST Stair descent | 14.4 ± 12.8 months | Hip abductor: Hip external rotator | - | - | Hip internal rotation Hip adduction Knee varus |
| Salsich et a. 2001 [22] | IST running | More than 2 months | Hip abductor: Hip external rotator | Knee adduction Hip internal rotation Hip adduction | ||
| Ireland et al. 2003 [23] | IST | More than 3 three months | Hip abduction Hip external rotation | |||
| Robinson and Nee 2007 [24] | Running | More than 2 months | Hip adduction Hip internal rotation | |||
| Glaviano and Saliba 2022 [25] | SL squat Step up; Step down Lateral step-down Lunge | More than 3 months | GMed GMax VMO VL | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, P.; István, B.; Liang, M. The Relationship between Patellofemoral Pain Syndrome and Hip Biomechanics: A Systematic Review with Meta-Analysis. Healthcare 2023, 11, 99. https://doi.org/10.3390/healthcare11010099
Xie P, István B, Liang M. The Relationship between Patellofemoral Pain Syndrome and Hip Biomechanics: A Systematic Review with Meta-Analysis. Healthcare. 2023; 11(1):99. https://doi.org/10.3390/healthcare11010099
Chicago/Turabian StyleXie, Pingping, Bíró István, and Minjun Liang. 2023. "The Relationship between Patellofemoral Pain Syndrome and Hip Biomechanics: A Systematic Review with Meta-Analysis" Healthcare 11, no. 1: 99. https://doi.org/10.3390/healthcare11010099
APA StyleXie, P., István, B., & Liang, M. (2023). The Relationship between Patellofemoral Pain Syndrome and Hip Biomechanics: A Systematic Review with Meta-Analysis. Healthcare, 11(1), 99. https://doi.org/10.3390/healthcare11010099












