Manual Therapy versus Localisation (Tactile, Sensory Training) in Patients with Non-Specific Neck Pain: A Randomised Clinical Pilot Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sample Size
2.3. Randomization
2.4. Blinding
2.5. Trial Registry
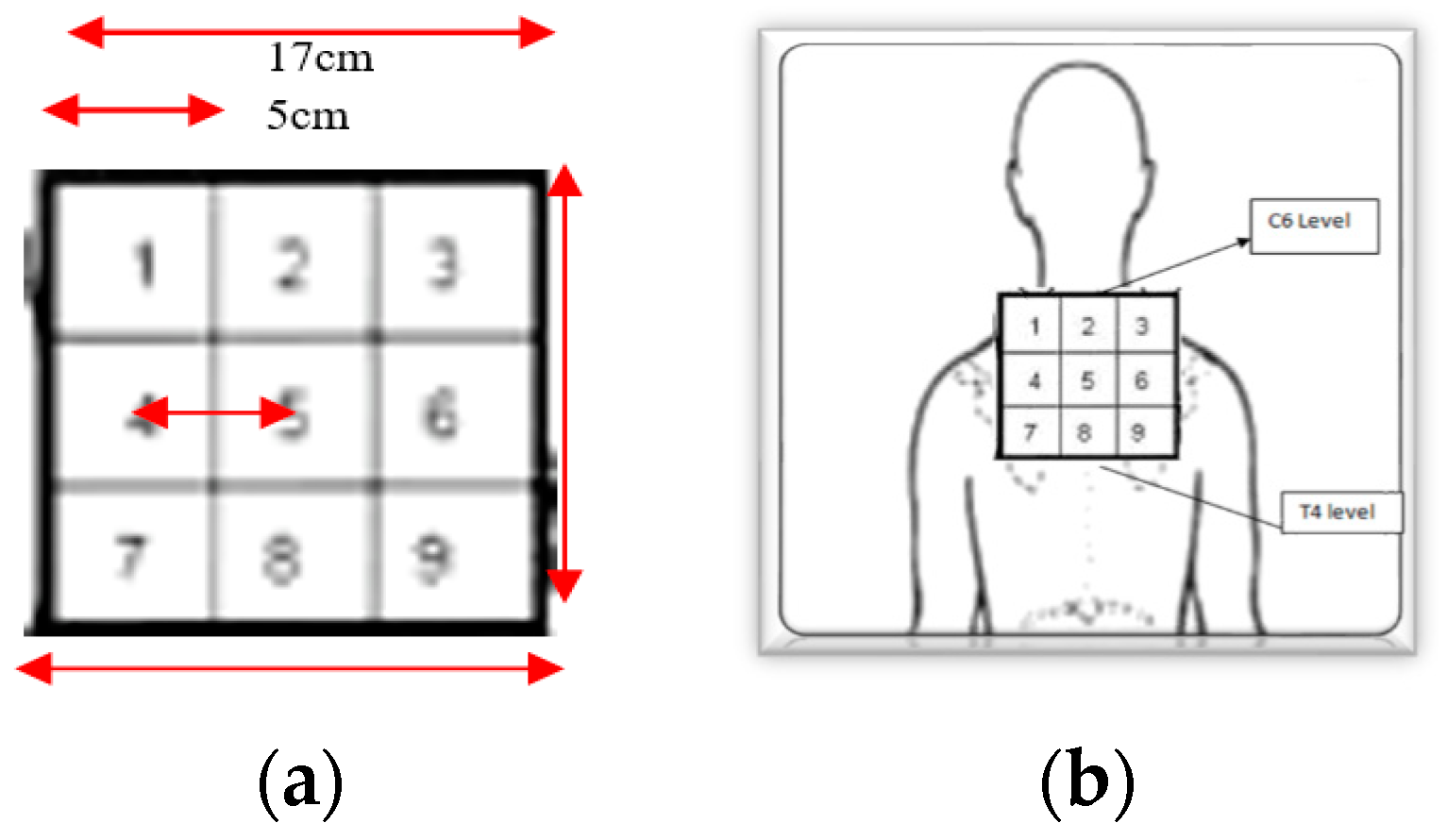
2.6. Outcome Measures and Measurements
2.7. Assessors
2.8. Interventions
2.8.1. Manual Therapy (MT) Group
2.8.2. Localisation Training (LT) Group
2.9. Data Analysis
3. Results
3.1. Participants
3.2. Within Group Analyses
3.3. Between Group Analyses
3.4. Within-Subjects Interaction Time * Group Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, S.P. Epidemiology, diagnosis, and treatment of neck pain. Mayo Clin. Proc. 2015, 90, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Kolahi, A.A.; Hoy, D.; Buchbinder, R.; Mansournia, M.A.; Bettampadi, D.; Ashrafi-Asgarabad, A.; Almasi-Hashiani, A.; Smith, E.; Sepidarkish, M.; et al. Global, regional, and national burden of neck pain in the general population 1990–2017: Systematic analysis of the Global Burden of Disease Study 2017. BMJ 2020, 368, m791. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, J.L.; Cao, J.; Chapin, A.; Chen, C.; Li, Z.; Liu, A.; Horst, C.; Kaldjian, A.; Matyasz, T.; Scott, K.W.; et al. US health care spending by payer and health condition, 1996–2016. JAMA 2020, 323, 863–884. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Andersson, G.; Watkins-Castillo, S. United States Bone and Joint Initiative: The Burden of Musculoskeletal Diseases in the United States (BMUS), 4th Ed.; Rosemont, IL. 2020. Available online: https://www.boneandjointburden.org/fourth-edition/ii0/spine-disorders (accessed on 1 May 2023).
- Butler, D.; Moseley, G. Explain Pain, 2nd ed.; Noi Group: Adelaide, Australia, 2003. [Google Scholar]
- Flor, H. Cortical reorganisation and chronic pain: Implications for rehabilitation. J. Rehabil. Med. 2003, 35, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Moseley, L.G. I can’t find it! Distorted body image and tactile dysfunction in patients with chronic back pain. Pain 2008, 140, 239–243. [Google Scholar] [CrossRef]
- Van Rijn, M.; van Hilten, J.; van Dijk, J. Spatiotemporal integration of sensory stimuli in complex regional pain syndrome and dystonia. J. Neural Transm. 2009, 116, 559–565. [Google Scholar] [CrossRef]
- Catley, M.; O’Connell, N.; Berryman, C.; Ayhan, F.; Moseley, G. Is Tactile Acuity Altered in People with Chronic Pain? A Systematic Review and Meta-analysis. J. Pain 2014, 15, 985–1000. [Google Scholar] [CrossRef]
- Harvie, D.; Edmond-Hank, G.; Smith, A. Tactile acuity is reduced in people with chronic neck pain. Musculoskelet. Sci. Pract. 2018, 33, 61–66. [Google Scholar] [CrossRef]
- Gross, A.; Miller, J.; D’Sylva, J.; Burnie, S.; Goldsmith, C.; Graham, N.; Haines, T.; Brønfort, G.; Hoving, J. Manipulation or mobilisation for neck pain: A Cochrane Review. Man. Ther. 2010, 15, 315–333. [Google Scholar] [CrossRef]
- Hidalgo, B.; Hall, T.; Bossert, J.; Dugeny, A.; Cagnie, B.; Pitance, L. The efficacy of manual therapy and exercise for treating non-specific neck pain: A systematic review. J. Back Musculoskelet. Rehabil. 2017, 30, 1149–1169. [Google Scholar] [CrossRef]
- McCarthy, C.J. Combined Movement Theory: Rational Mobilization and Manipulation of the Vertebral Column; Elsevier Science: Oxford, UK, 2010. [Google Scholar]
- Louw, A.; Diener, I.; Butler, D.S.; Puentedura, E.J. The Effect of Neuroscience Education on Pain, Disability, Anxiety, and Stress in Chronic Musculoskeletal Pain. Arch Phys. Med. Rehabil. 2011, 92, 2041–2056. [Google Scholar] [CrossRef] [PubMed]
- Flor, H. The functional organization of the brain in chronic pain. Prog. Brain Res. 2000, 129, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Stavrinou, M.L.; Della Penna, S.; Pizzella, V.; Torquati, K.; Cianflone, F.; Franciotti, R.; Bezerianos, A.; Romani, G.L.; Rossini, P.M. Temporal dynamics of plastic changes in human primary somatosensory cortex after finger webbing. Cereb. Cortex 2007, 17, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.; Braun, C.; Elbert, T.; Birbaumer, N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci. Lett. 1997, 224, 5–8. [Google Scholar] [CrossRef]
- Flor, H.; Denke, C.; Schaefer, M.; Grüsser, S. Effect of sensory discrimination training on cortical reorganisation and phantom limb pain. Lancet 2001, 357, 1763–1764. [Google Scholar] [CrossRef]
- Wand, B.; O’Connell, N.; Di Pietro, F.; Bulsara, M. Managing Chronic Nonspecific Low Back Pain with a Sensorimotor Retraining Approach: Exploratory Multiple-Baseline Study of 3 Participants. Phys. Ther. 2011, 91, 535–546. [Google Scholar] [CrossRef]
- Dewitte, V.; Beernaert, A.; Vanthillo, B.; Barbe, T.; Danneels, L.; Cagnie, B. Articular dysfunction patterns in patients with mechanical neck pain: A clinical algorithm to guide specific mobilization and manipulation techniques. Man. Ther. 2014, 19, 2–9. [Google Scholar] [CrossRef]
- Hutting, N.; Kerry, R.; Coppieters, M.; Scholten-Peeters, G. Considerations to improve the safety of cervical spine manual therapy. Musculoskelet. Sci. Pract. 2018, 33, 41–45. [Google Scholar] [CrossRef]
- Haldeman, S.; Carroll, L.; Cassidy, J. Findings from The Bone and Joint Decade 2000 to 2010 Task Force on Neck Pain and Its Associated Disorders. J. Occup. Environ. Med. 2010, 52, 424–427. [Google Scholar] [CrossRef]
- Barker, K.; Elliott, C.; Sackley, C.; Fairbank, J. Treatment of chronic back pain by sensory discrimination training. A Phase I RCT of a novel device (FairMed) vs. TENS. BMC Musculoskelet. Disord. 2008, 9, 97. [Google Scholar] [CrossRef]
- Louw, A.; Farrell, K.; Wettach, L.; Uhl, J.; Majkowski, K.; Welding, M. Immediate effects of sensory discrimination for chronic low back pain: A case series. N. Z. J. Phys. 2015, 43, 58–63. [Google Scholar] [CrossRef]
- Ryan, C.; Harland, N.; Drew, B.; Martin, D. Tactile acuity training for patients with chronic low back pain: A pilot randomised controlled trial. BMC Musculoskelet. Disord. 2014, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Harvie, D.; Kelly, J.; Buckman, H.; Chan, J.; Sutherland, G.; Catley, M.; Novak, J.; Tuttle, N.; Sterling, M. Tactile acuity testing at the neck: A comparison of methods. Musculoskelet. Sci. Pract. 2017, 32, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Moseley, L. Combined physiotherapy and education is efficacious for chronic low back pain. Aust. J. Physiother. 2002, 48, 297–302. [Google Scholar] [CrossRef]
- Cleland, J.A.; Childs, J.D.; Whitman, J.M. Psychometric Properties of the Neck Disability Index and Numeric Pain Rating Scale in Patients with Mechanical Neck Pain. Arch. Phys. Med. Rehabil. 2008, 89, 69–74. [Google Scholar] [CrossRef]
- Potter, L.; McCarthy, C.; Oldham, J. Algometer reliability in measuring pain pressure threshold over normal spinal muscles to allow quantification of anti-nociceptive treatment effects. Inter. J. Osteop. Med. 2006, 9, 113–119. [Google Scholar] [CrossRef]
- Chesterton, L.; Sim, J.; Wright, C.; Foster, N. Interrater Reliability of Algometry in Measuring Pressure Pain Thresholds in Healthy Humans, Using Multiple Raters. Clin. J. Pain 2007, 23, 760–766. [Google Scholar] [CrossRef]
- Walton, D.M.; MacDermid, J.C.; Nielson, W.; Teasell, R.W.; Chiasson, M.; Brown, L. Reliability, Standard Error, and Minimum Detectable Change of Clinical Pressure Pain Threshold Testing in People With and Without Acute Neck Pain. J. Orthop. Sports Phys. Ther. 2011, 41, 644–650. [Google Scholar] [CrossRef]
- Williams, M.A.; McCarthy, C.J.; Chorti, A.; Cooke, M.W.; Gates, S. A Systematic Review of Reliability and Validity Studies of Methods for Measuring Active and Passive Cervical Range of Motion. J. Manip. Physiol. Ther. 2010, 33, 138–155. [Google Scholar] [CrossRef]
- Vernon, H.; Mior, S. The Neck Disability Index: A study of reliability and validity. J. Manip. Physiol. Ther. 1991, 14, 409–415. [Google Scholar]
- Trouli, M.; Vernon, H.; Kakavelakis, K.; Antonopoulou, M.; Paganas, A.; Lionis, C. Translation of the Neck Disability Index and validation of the Greek version in a sample of neck pain patients. BMC Musculoskelet. Disord. 2008, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Bicer, A.; Yazici, A.; Camdeviren, H.; Erdogan, C. Assessment of pain and disability in patients with chronic neck pain: Reliability and construct validity of the Turkish version of the neck pain and disability scale. Disabil. Rehabil. 2004, 26, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, I.; Douzenis, A.; Kalkavoura, C.; Christodoulou, C.; Michalopoulou, P.; Kalemi, G.; Fineti, K.; Patapis, P.; Protopapas, K.; Lykouras, L. Hospital Anxiety and Depression Scale (HADS): Validation in a Greek general hospital sample. Ann. Gen. Psychiatry 2008, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Exelby, L. The Mulligan concept: Its application in the management of spinal conditions. Man. Ther. 2002, 7, 64–70. [Google Scholar] [CrossRef]
- Buyukturan, O.; Buyukturan, B.; Sas, S.; Karartı, C.; Ceylan, İ. The Effect of Mulligan Mobilization Technique in Older Adults with Neck Pain: A Randomised Controlled, Double-Blind Study. Pain Res. Manag. 2018, 15, 2856375. [Google Scholar] [CrossRef]
- Kanlayanaphotporn, R.; Chiradejnant, A.; Vachalathiti, R. The Immediate Effects of Mobilization Technique on Pain and Range of Motion in Patients Presenting with Unilateral Neck Pain: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2009, 90, 187–192. [Google Scholar] [CrossRef]
- Snodgrass, S.; Rivett, D.; Sterling, M.; Vicenzino, B. Dose Optimization for Spinal Treatment Effectiveness: A Randomized Controlled Trial Investigating the Effects of High and Low Mobilization Forces in Patients with Neck Pain. J. Orthop. Sport. Phys. Ther. 2014, 44, 141–152. [Google Scholar] [CrossRef]
- Izquierdo Pérez, H.; Alonso Perez, J.; Gil Martinez, A.; La Touche, R.; Lerma-Lara, S.; Commeaux Gonzalez, N.; Arribas Perez, H.; Bishop, M.; Fernández-Carnero, J. Is one better than another?: A randomized clinical trial of manual therapy for patients with chronic neck pain. Man. Ther. 2014, 19, 215–221. [Google Scholar] [CrossRef]
- Catley, M.J.; Tabor, A.; Wand, B.M.; Moseley, G.L. Assessing tactile acuity in rheumatology and musculoskeletal medicine—How reliable are two-point discrimination tests at the neck, hand, back and foot? Rheumatology 2013, 52, 1454–1461. [Google Scholar] [CrossRef]
- Sterling, M.; Jull, G.; Wright, A. Cervical mobilisation: Concurrent effects on pain, sympathetic nervous system activity and motor activity. Man. Ther. 2001, 6, 72–81. [Google Scholar] [CrossRef]
- Lopez-Lopez, A.; Alonso Perez, J.; González Gutierez, J.L.; La Touche, R.; Lerma Lara, S.; Izquierdo, H.; Fernández-Carnero, J. Mobilization versus manipulations versus sustain apophyseal natural glide techniques and interaction with psychological factors for patients with chronic neck pain: Randomised controlled trial. Eur. J. Phys. Rehabil. Med. 2015, 51, 121–132. [Google Scholar] [PubMed]
- Kälin, S.; Rausch-Osthoff, A.K.; Bauer, C.M. What is the effect of sensory discrimination training on chronic low back pain? A systematic review. BMC Musculoskelet. Disord. 2016, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Bialosky, J.; Bishop, M.; Price, D.; Robinson, M.; George, S. The mechanisms of manual therapy in the treatment of musculoskeletal pain: A comprehensive model. Man. Ther. 2009, 14, 531–538. [Google Scholar] [CrossRef]
- Bishop, M.; Bialosky, J.; Cleland, J. Patient expectations of benefit from common interventions for low back pain and effects on outcome: Secondary analysis of a clinical trial of manual therapy interventions. J. Man. Manip. Ther. 2011, 19, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Wainner, R.; Whitman, J.; Cleland, J.; Flynn, T. Regional Interdependence: A Musculoskeletal Examination Model Whose Time Has Come. J. Orthop. Sport. Phys. Ther. 2007, 37, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Van Houdenhove, B.; Oostendorp, R. Recognition of central sensitization in patients with musculoskeletal pain: Application of pain neurophysiology in manual therapy practice. Man. Ther. 2010, 15, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Louw, A.; Zimney, K.; Puentedura, E.J.; Diener, I. The efficacy of pain neuroscience education on musculoskeletal pain: A systematic review of the literature. Physiother. Theory Pract. 2016, 32, 332–355. [Google Scholar] [CrossRef]
- Moseley, L.; Zalucki, N.; Wiech, K. Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain. Pain 2008, 137, 600–608. [Google Scholar] [CrossRef]
- Simmonds, M. Pain and the Placebo in Physiotherapy. Physiotherapy 2000, 86, 631–637. [Google Scholar] [CrossRef]
- De Oliveira, R.F.; Liebano, R.E.; Costa Lda, C.; Rissato, L.L.; Costa, L.O. Immediate effects of region-specific and non-region-specific spinal manipulative therapy in patients with chronic low back pain: A randomised controlled trial. Phys. Ther. 2013, 93, 748–756. [Google Scholar] [CrossRef]
- Donaldson, M.; Petersen, S.; Cook, C.; Learman, K. A Prescriptively selected Nonthrust manipulation versus a Therapist-Selected Nonthrust manipulation for treatment of individuals with low back pain: A randomised clinical trial. J. Orthop. Sport. Phys. Ther. 2016, 46, 243–250. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.J.; Potter, L.; Oldham, J.A. Comparing targeted thrust manipulation with general thrust manipulation in patients with low back pain. A general approach is as effective as a specific one. A randomised controlled trial. BMJ Open Sport Exerc. Med. 2019, 5, e000514. [Google Scholar] [CrossRef] [PubMed]
- Nim, C.G.; Downie, A.; O’Neill, S.; Kawchuk, G.N.; Perle, S.M.; Leboeuf-Yde, C. The importance of selecting the correct site to apply spinal manipulation when treating spinal pain: Myth or reality? A systematic review. Sci. Rep. 2021, 11, 23415. [Google Scholar] [CrossRef] [PubMed]


| Whole Sample (n = 30) | Localisation Group (n = 15) | Manual Therapy Group (n = 15) | Significance (p-Value) | ||
|---|---|---|---|---|---|
| Mean ± SD | |||||
| Age (years) | 28.63 ± 12.49 | 26.4 ± 9.66 | 30.87 ± 14.81 | 0.461 | |
| Height (m) | 1.74 ± 0.099 | 1.71 ± 0.09 | 1.70 ± 0.11 | 0.746 | |
| Weight (kg) | 78.5 ± 15.40 | 72.26 ± 17.80 | 70.6 ± 13.17 | 0.713 | |
| BMI (kg/m2) | 24.61 ± 3.61 | 24.40 ± 3.99 | 24.33 ± 3.33 | 0.958 | |
| Whole Sample (n = 30) | Localisation Group (n = 15) | Manual Therapy Group (n = 15) | Significance (p-Value) | |
|---|---|---|---|---|
| Mean ± SD | ||||
| NDI score (%) | 21 ± 0.9 | 22 ± 1 | 19 ± 0.8 | 0.359 |
| HADs (Total) | 11.83 ± 5.91 | 12.07 ± 6.11 | 11.60 ± 5.91 | 0.838 |
| HADS-Anxiety | 7.97 ± 3.92 | 8.07 ± 4.15 | 7.87 ± 3.81 | 0.902 |
| HADS-Depression | 3.87 ± 2.67 | 4 ± 2.62 | 3.73 ± 2.81 | 0.624 |
| BASELINE | POST-INTERVENTION | ||||||
|---|---|---|---|---|---|---|---|
| Localisation Group | Manual Therapy Group | t-Test | Localisation Group | Manual Therapy Group | t-Test | ||
| Mean (±SD) | p Value | Mean (±SD) | p Value | ||||
| Pain intensity (NPRS) | 4.93 (±1.33) | 4.33 (±1.45) | 0.345 | 3.73 (±1.58) | 3.20 (±1.26) | 0.389 | |
| PPT levels | |||||||
| Central (5th square) | 4.42 (±1.28) | 3.77 (±1.17) | 0.161 | 4.06 (±1.47) | 3.80(±1.24) | 0.605 | |
| Right (6th square) | 4.09 (±1.51) | 3.79 (±1.18) | 0.542 | 4.07 (±1.47) | 3.71 (±1.24) | 0.367 | |
| Left (4th square) | 4.17 (±1.59) | 3.68 (±1.08) | 0.337 | 4.10 (±1.45) | 3.87 (±1.06) | 0.629 | |
| ROM (°) | |||||||
| Right Rotation | 75 (±5.70) | 75 (±7.25) | 0.824 | 78 (±4.44) | 79 (±5.42) | 0.384 | |
| Left Rotation | 71 (±10.45 | 73 (±7.58) | 0.478 | 75 (±9.25) | 77 (±7.97) | 0.616 | |
| Right Lateral Flexion | 42 (±5.55) | 40 (±8.59) | 0.270 | 47 (±5.88) | 45 (±8.95) | 0.379 | |
| Left Lateral Flexion | 39 (±6.97) | 37 (±9.39) | 0.620 | 41 (±6.60) | 42 (±9.11) | 0.612 | |
| Flexion | 45 (±9.29) | 51 (±8.59) | 0.108 | 47 (±8.06) | 54 (±8.53) | 0.030 * | |
| Extension | 52 (±9.62) | 47(±10.79) | 0.154 | 53 (±9.17) | 51 (±9.04) | 0.480 | |
| Interaction Time * Group F (p Values) | |
|---|---|
| NPRS now | 0.02 (p = 0.892) |
| PPT central | 3.21 (p = 0.084) |
| PPT right | 0.07 (p = 0.793) |
| PPT left | 1.89 (p = 0.180) |
| ROM Rotation R | 0.48 (p = 0.495) |
| ROM Rotation L | 0.29 (p = 0.595) |
| ROM Lateral Flexion R | 0.07 (p = 0.794) |
| ROM Lateral Flexion L | 5.721 (p = 0.024 *) |
| ROM Flexion | 1.34 (p = 0.256) |
| ROM Extension | 4.24 (p = 0.049 *) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomaidou, E.; McCarthy, C.J.; Tsepis, E.; Fousekis, K.; Billis, E. Manual Therapy versus Localisation (Tactile, Sensory Training) in Patients with Non-Specific Neck Pain: A Randomised Clinical Pilot Trial. Healthcare 2023, 11, 1385. https://doi.org/10.3390/healthcare11101385
Thomaidou E, McCarthy CJ, Tsepis E, Fousekis K, Billis E. Manual Therapy versus Localisation (Tactile, Sensory Training) in Patients with Non-Specific Neck Pain: A Randomised Clinical Pilot Trial. Healthcare. 2023; 11(10):1385. https://doi.org/10.3390/healthcare11101385
Chicago/Turabian StyleThomaidou, Eleftheria, Christopher James McCarthy, Elias Tsepis, Konstantinos Fousekis, and Evdokia Billis. 2023. "Manual Therapy versus Localisation (Tactile, Sensory Training) in Patients with Non-Specific Neck Pain: A Randomised Clinical Pilot Trial" Healthcare 11, no. 10: 1385. https://doi.org/10.3390/healthcare11101385





