Investigation of Real-Time Diagnostic Ultrasound as a Means of Biofeedback Training in Transversus Abdominus Re-Education of Patients with Non-Specific Low Back Pain: A Prospective Randomized Controlled Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Interventions
2.2.1. Control Group
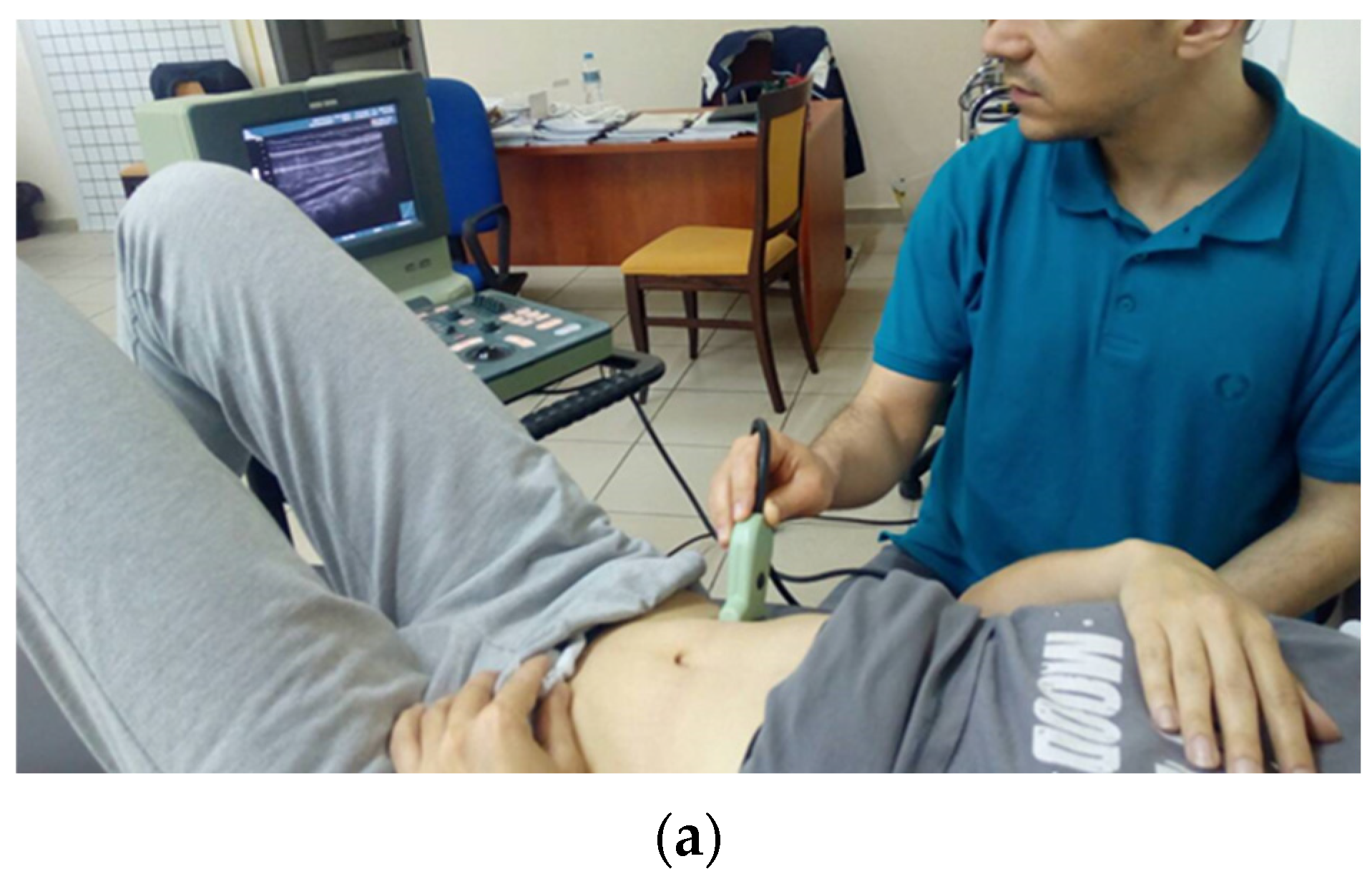
2.2.2. US-Guided Intervention Group
2.3. Outcome Measures
2.3.1. Patient-Reported Measures
2.3.2. Clinician-Reported Measures
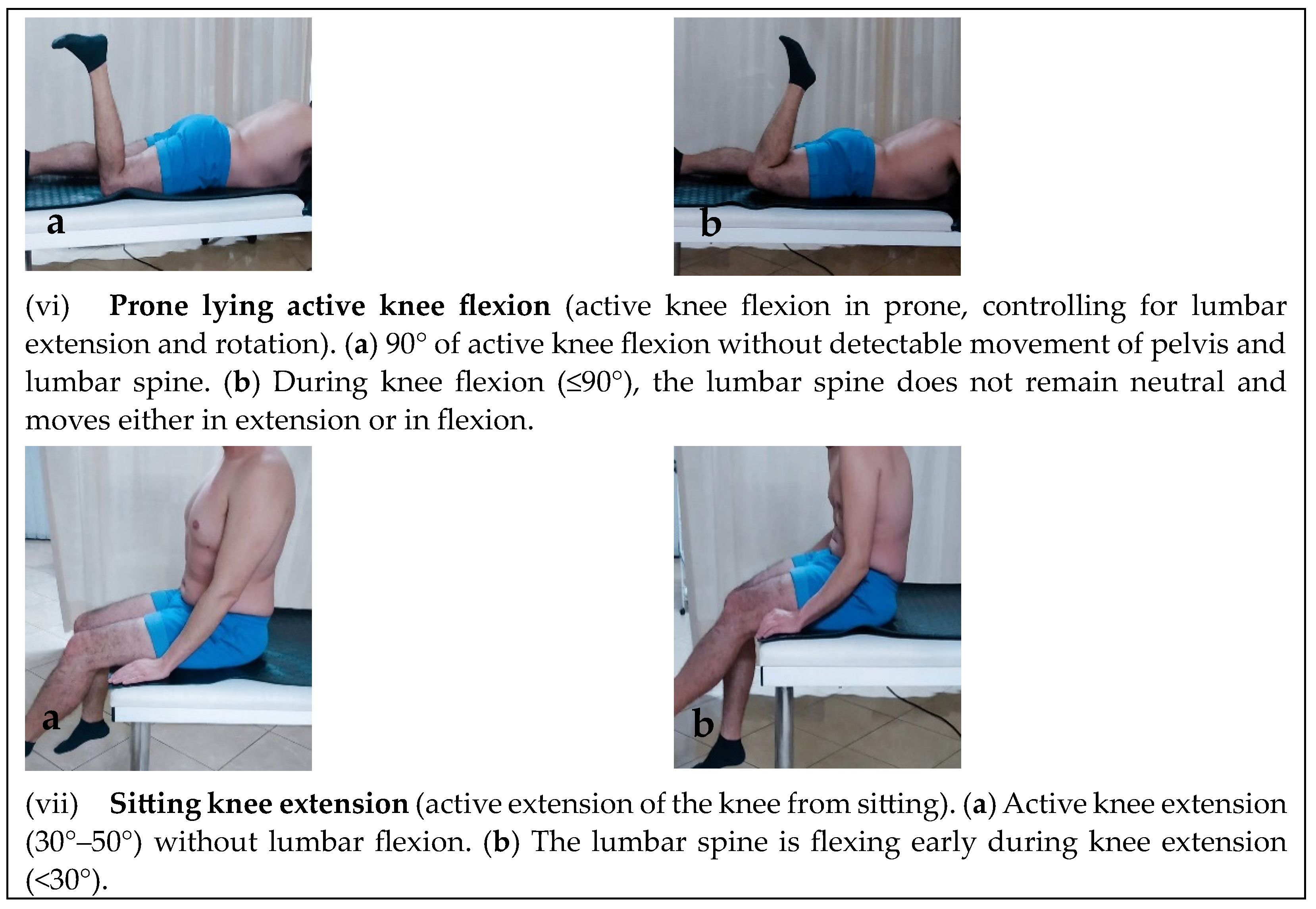
Motor Control Tests
TrA Activation and Standardization of Exercise Progression Level
2.4. Randomization
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Warm-up Phase 3 sets of 10 repetitions | ||
| Intervention/Details | Figures | |
| (1) Patient lies on his back with bent legs and tries to perform a posterior pelvic tilt. |  |  |
| (2) Patient lies on his back with bent legs, and takes each leg with both hands and brings it towards the chest alternately. |  |  |
| (3) Patient lies on his back with bent legs bent and lets them fall left and right together. |  |  |
| (4) From a supine position with the legs loosely stretched the patient tries to lengthen each leg from the pelvis |  |  |
| (5) From a prone position with hands clasped behind the waist, the person lifts the upper body and head slightly from the bed. |  |  |
| (6) From a quadruped position, hands on shoulder height and knees onhip height, patient perform the “cat-camel” exercise. |  |  |
 |  | |
| Main Motor Control Exercise Program 3 sets of 10 repetitions | ||
| Intervention/Details | Figures | |
| (1) From a supine position with bent legs the patient tries to bring the navel towards the spine by tightening lower abdomen (static control). |  |  |
| (2) TrA contraction (from crook linyg) while leting the leg to fallout to the side and back (initial position), where contraction relaxes. Leg bends out as far as patient feels can control TrA contraction, leg and pelvis (roation control). |  |  |
| (3) TrA contraction (from crook linyg) while lifting the leg from the bed and returning it to the initial position (where contraction is relaxed). Leg bends slowly as far as patient feels can control TrA contraction, leg and pelvis (flexion control). |  |  |
| (4) TrA contraction (from crook linyg) while dragging the leg from the bed without losing contact with the ground and back (full knee extension is avoided). Leg is stretched out as far as patient feels can control TrA contraction, leg and pelvis (extension control). |  |  |
| (5) TrA contraction in prone with alternative knee flexion to 90°. Contraction relaxes when leg returns to the starting position (extension/rotation control). |  |  |
| (6) TrA contraction from quadrupedal with hands below shoulder height and knees below hips. Patient brings trunk forward passing over his hands while trying to keep the waist flat. The contraction is relaxed on return to starting position (flexion control). |  |  |
| (7) TrA contraction from quadrupedal (as above). Patient brings the trunk towards his legs while trying to keep the waist flat. The contraction is relaxed on return to starting position (extension control). |  |  |
| (8) TrA contraction in sitting with the body upright and the arms relaxed by the side of the body. Patient extends knee slowly (but not fully) whistle trying th maintain TrA contraction and posture. Contraction is relaxed by returning the leg to the starting position (partial weight-bearing flexion control). |  |  |
| (9) TrA contraction in sitting with the body upright and the arms relaxed by the side of the body. Patient flexes the hip whistle trying th maintain TrA contraction and posture. The contraction is relaxed by returning the leg to the starting position (partial weight-bearing flexion control). |  |  |
| (10) Standing resting comfortable the spine on the wall with knees flexed away from the wall. TrA contraction while the patient performed a posterio pelvic tilt. The contraction is relaxed by returning to the starting position (weight-bearing flexion control). |  |  |
| (11) TrA contraction in standing. Patient tries to bend forwards (bowing) trying to keep the waist straight (flat). Trunk flexion should occur from the hips (not from the waist). Hands can be used for additional support. The contraction is relaxed by returning to the starting position (weight-bearing flexion/extension control). |  |  |
| Stretching Exercises (recovery phase) 60 s/3 repetitions | ||
| Intervention/Details | Figures | |
| (1) Supine position with the legs relaxed, the patient grabs one leg by the knee and brings it to the chest and holds (low lumber muscle stretch). |  |  |
| (2) Supine position with the legs relaxed, the patient bends one leg to touch the opposite side of the bed and lets it drop. At the same time he turns his head to the opposite side (low lumber side muscle stretch). |  |  |
| (3) Supine position with the legs bent, the patient crosses one leg over the other and with both hands grasps the leg resting on the bed and brings it towards the chest (piroformis stretch). |  |  |
| (4) Supine position with the legs relaxed and using a belt the patient brings the leg outstretched into flexion until a pull is felt on the back of the leg and holds it firmly there (hamstring stretch). |  |  |
References
- Jeffries, L.J.; Milanese, S.F.; Grimmer, K.A. Epidemiology of Adolescent Spinal Pain: A systematic overview of the research literature. Spine 2007, 32, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- Balagué, F.; Mannion, A.F.; Pellisé, F.; Cedraschi, C. Non-specific low back pain. Lancet 2012, 379, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Richardson, C.; Jull, G. Evaluation of the relationship between laboratory and clinical tests of transverses abdominus function. Physiother. Res. Int. 1996, 1, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, A. Stability of the lumbar spine: A study in mechanical engineering. Acta Orthop. Scand. Suppl. 1989, 60, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.A.; Hodges, P.W.; Hides, J.A. Therapeutic Exercise for Spinal Segmental Stabilization: A Motor Control Approach for the Treatment and Prevention of Low Back Pain, 2nd ed.; Churchill Livingstone: Edinburgh, UK, 2004. [Google Scholar]
- Hodges, P.W.; Richardson, C.A. Delayed postural contraction of transversus abdominis in low back pain associated with movement of the lower limb. J. Spinal Disord. Tech. 1998, 11, 46–56. [Google Scholar] [CrossRef]
- Panjabi, M.M. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J. Spinal Disord. Tech. 1992, 5, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Khorashadizadeh, S.; Abdi, G. The effect of motor control exercise versus general exercise on lumbar local stabilizing muscles thickness: Randomized controlled trial of patients with chronic low back pain. J. Back Musc. Rehab. 2008, 21, 105–112. [Google Scholar] [CrossRef]
- Mannion, A.F.; Pulkovski, N.; Toma, V.; Sprott, H. Abdominal muscle size and symmetry at rest and during abdominal hollowing exercises in healthy control subjects. J. Anat. 2008, 213, 173–182. [Google Scholar] [CrossRef]
- Hides, J.A.; Richardson, C.A.; Jull, G.A. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine 1996, 21, 2763–2769. [Google Scholar] [CrossRef]
- Hodges, P.W.; Richardson, C.A. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch. Phys. Med. Rehabil. 1999, 80, 1005–1012. [Google Scholar] [CrossRef]
- Hides, J.A.; Jull, G.A.; Richardson, C.A. Long-term effects of specific stabilizing exercises for first-episode low back pain. Spine 2001, 26, E243–E248. [Google Scholar] [CrossRef] [PubMed]
- Crommert, M.E.; Ekblom, M.M.; Thorstensson, A. Activation of transversus abdominis varies with postural demand in standing. Gait Posture 2011, 33, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, C.J.C.; Meijer, O.G.; Daffertshofer, A.; Wuisman, P.I.J.M.; Beek, P.J. Effects of chronic low back pain on trunk coordination and back muscle activity during walking: Changes in motor control. Eur.Spine J. 2006, 15, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, M.G.; Rasmussen-Barr, E.; Grooten, W.J.A. Motor control exercises reduces pain and disability in chronic and recurrent low back pain. Spine 2013, 38, E350–E358. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, P.B.; Phyty, G.D.; Twomey, L.T.; Allison, G.T. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine 1997, 22, 2959–2967. [Google Scholar] [CrossRef]
- Hides, J.; Richardson, C.A.; Jull, G.A.; Davies, S. Ultrasound imaging in rehabilitation. Aust. J. Physiother. 1995, 41, 187193. [Google Scholar] [CrossRef]
- Henry, S.M.; Westervelt, K.C. The use of real-time ultrasound feedback in teaching abdominal hollowing exercises to healthy subjects. J. Orthop. Sports Phys. Ther. 2005, 35, 338–345. [Google Scholar] [CrossRef]
- Weiser, S.; Rossignol, M. Triage for Nonspecific Lower-back Pain. Clin. Orthop. Relat. Res. 2006, 443, 147–155. [Google Scholar] [CrossRef]
- Lizier, D.T.; Perez, M.V.; Sakata, R.K. Exercises for Treatment of Nonspecific Low Back Pain. Rev. Bras. Anestesiol. 2012, 62, 838–846. [Google Scholar] [CrossRef]
- Joaquim, A. Initial approach to patients with acute lower back pain. Rev. Assoc. Méd. Bras. 2016, 62, 186–191. [Google Scholar] [CrossRef]
- Falla, D.; Bilenkij, G.; Jull, G. Patients with chronic neck pain demonstrate altered patterns of muscle activation during performance of a functional upper limb task. Spine 2004, 29, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Moseley, L.G. Pain and motor control of the lumbopelvic region: Effect and possible mechanisms. J. Electromyogr. Kinesiol. 2003, 13, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Woollacott, M.H. Motor Control: Translating Research into Clinical Practice, 5th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2016. [Google Scholar]
- Ferreira, M.L.; Ferreira, P.H.; Latimer, J.; Herbert, R.D.; Hodges, P.W.; Jennings, M.D.; Maher, C.G.; Refshauge, K.M. Comparison of general exercise, motor control exercise and spinal manipulative therapy for chronic low back pain: A randomized trial. Pain 2007, 131, 31–37. [Google Scholar] [CrossRef]
- Costa, L.O.; Maher, C.G.; Latimer, J.; Hodges, P.W.; Herbert, R.D.; Refshauge, K.M.; McAuley, J.H.; Jennings, M.D. Motor control exercise for chronic low back pain: A randomized placebo-controlled trial. Phys. Ther. 2009, 89, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Franca, F.R.; Burke, T.N.; Hanada, E.S.; Marques, A.P. Segmental stabilization and muscular strengthening in chronic low back pain—A comparative study. Clinics 2010, 65, 1013–1017. [Google Scholar] [CrossRef]
- Fradkin, A.J.; Zazryn, T.R.; Smoliga, J.M. Effects of warming-up on physical performance: A systematic review with meta-analysis. J. Strength Cond. Res. 2010, 24, 140–148. [Google Scholar] [CrossRef]
- Sahrmann, S.A. Diagnosis and Treatment of Movement Impairment Syndromes, 1st ed.; Mosby: St. Louis, MO, USA, 2002. [Google Scholar]
- O’Sullivan, P.B. Diagnosis and classification of chronic low back pain disorders: Maladaptive movement and motor control impairments as underlying mechanism. Man. Ther. 2005, 10, 242–255. [Google Scholar] [CrossRef]
- Okragly, R. Static Stretching. In Encyclopedia of Sports Medicine; Micheli, L.J., Ed.; Sage Publications: Thousand Oaks, CA, USA, 2011; pp. 1397–1398. [Google Scholar] [CrossRef]
- McHugh, M.; Cosgrave, C. To stretch or not to stretch: The role of stretching in injury prevention and performance. Scand. J. Med. Sci. Sports 2010, 20, 169–181. [Google Scholar] [CrossRef]
- Costa, L.O.P.; da Cunha Menezes Costa, L.; Cançado, R.L.; De Melo Oliveira, W.; Ferreira, P.H. Intra-tester reliability of two clinical tests of transversus abdominis muscle recruitment. Physiother. Res. Int. 2006, 11, 48–50. [Google Scholar] [CrossRef]
- Whittaker, J.L. Ultrasound Imaging for Rehabilitation of the Lumbopelvic Region: A Clinical Approach, 1st ed.; Churchill Livingstone: New York, NY, USA, 2007. [Google Scholar]
- McMeeken, J.M.; Beith, I.D.; Newham, D.J.; Milligan, P.; Critchley, D.J. The relationship between EMG and change in thickness of transversus abdominis. Clin. Biomech. 2004, 19, 337–342. [Google Scholar] [CrossRef]
- Teyhen, D.S.; Miltenberger, C.E.; Deiters, H.M.; del Toro, Y.M.; Pulliam, J.N.; Childs, J.D.; Boyles, R.E.; Flynn, T.W. The use of ultrasound imaging of the abdominal drawing-in maneuver in subjects with low back pain. J. Orthop. Sports Phys. Ther. 2005, 35, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.D.; Piva, S.R.; Fritz, J.M. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine 2005, 30, 1331–1334. [Google Scholar] [CrossRef]
- Roland, M.; Morris, R. A study of the natural history of back pain. Part I: Development of a reliable and sensitive measure of disability in low-back pain. Spine 1983, 8, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Luomajoki, H.; Kool, J.; de Bruin, E.D.; Airaksinen, O. Reliability of movement control tests in the lumbar spine. BMC Musculosk. Disord. 2007, 8, 90. [Google Scholar] [CrossRef]
- Cole, B.; Finch, E.; Gowland, C.; Mayo, N. Physical Rehabilitation Outcome Measures; Basmajian, J., Ed.; The Canadian Physiotherapy Association in Cooperation with Health and Welfare Canada and the Canada Communications Group—Publishing, Supply and Services: Toronto, ON, Canada, 1994; pp. 106–122. [Google Scholar]
- Richardson, C.; Jull, G.; Hodges, P.; Hides, J. Therapeutic Exercise for Spinal Segmental Stabilisation in Low Back Pain, Scientific Basis and Clinical Approach, 1st ed.; Churchill Livingstone: London, UK, 1999. [Google Scholar]
- Cairns, M.C.; Foster, N.E.; Wright, C. Randomized controlled trial of specific spinal stabilization exercises and conventional physiotherapy for recurrent low back pain. Spine 2006, 31, E670–E681. [Google Scholar] [CrossRef]
- Costa, L.O.P.; Costa, L.C.M.; Cançado, R.L.; Oliveira, W.M.; Ferreira, P.H. Confiabilidade do teste palpatório e da unidade de biofeedback pressórico na ativação do músculo transverso abdominal em indivíduos normais. Acta Fisiátrica 2004, 11, 101–105. [Google Scholar] [CrossRef]
- Chattanooga, G. Stabilizer Pressure Bio-Feedback. Operating Instructions; Chattanooga Group Inc.: Hixson, TN, USA, 2005. [Google Scholar]
- Lima, P.; Oliveira, R.; Moura Filho, A.; Raposo, M.; Costa, L.; Laurentino, G. Concurrent validity of the pressure biofeedback unit and surface electromyography in measuring transversus abdominis muscle activity in patients with chronic nonspecific low back pain. Rev. Bras. Fisioter. 2012, 16, 389–395. [Google Scholar] [CrossRef]
- Chon, S.C.; Chang, K.Y.; You, J.S. Effect of the abdominal drawin manoeuvre in combination with ankle dorsiflexion in strengthening the transverse abdominal muscle in healthy young adults: A preliminary, randomised, controlled study. Physiotherapy 2010, 96, 130–136. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, T.H.; Lee, B.H. The effect of abdominal bracing in combination with low extremity movement son changes in thickness of abdominal muscles and lumbar strength for low back pain. J. Phys. Ther. Sci. 2014, 26, 157–160. [Google Scholar] [CrossRef]
- Schneider, S.; Randoll, D.; Buchner, M. Why do women have back pain more than men? A representative prevalence study in the federal republic of Germany. Clin. J. Pain 2006, 22, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Van, K.; Hides, J.A.; Richardson, C.A. The use of real-time ultrasound imaging for biofeedback of lumbar multifidus muscle contraction in healthy subjects. J. Orthop. Sports Phys. Ther. 2006, 36, 920–925. [Google Scholar] [CrossRef]
- Kermode, F. Benefits of utilizing real-time ultrasound imaging in the rehabilitation of the lumbar spine stabilizing muscles following low back injury in the elite athlete—A single case study. Phys. Ther. Sport 2004, 5, 13–16. [Google Scholar] [CrossRef]
- Worth, S.; Henry, S.M.; Bunn, Y. Real-time ultrasound feedback and abdominal hollowing exercises for people with back pain. N. Z. J. Physiother. 2007, 35, 4–11. [Google Scholar]
- Herbert, W.J.; Heiss, D.G.; Basso, D.M. Influence of feedback schedule in motor performance and learning of a lumbar multifidus muscle task using rehabilitative ultrasound imaging: A randomized clinical trial. Phys. Ther. 2008, 88, 261–269. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.L.; Watson, T. Training of transversus abdominis activation in the supine position with ultrasound biofeedback translated to increased transversus abdominis activation during upright loaded functional tasks. PM&R 2014, 6, 612–623. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chai, H.M.; Yang, J.L.; Lin, Y.J.; Wang, S.F. Reliability and validity of Transversus Abdominis measurement at the posterior muscle-fascia junction with ultrasonography in asymptomatic participants. J. Manip. Physiol. Ther. 2015, 38, 581–586. [Google Scholar] [CrossRef]
- Moseley, G.L.; Hodges, P.W.; Gandevia, S.C. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine 2002, 27, E29–E36. [Google Scholar] [CrossRef]
- Goldby, L.J.; Moore, A.P.; Doust, J.; Trew, M.E. A randomized controlled trial investigating the efficiency of musculoskeletal physiotherapy on chronic low back disorder. Spine 2006, 31, 1083–1093. [Google Scholar] [CrossRef]
- Macedo, L.G.; Maher, C.G.; Latimer, J.; McAuley, J.H. Motor control exercise for persistent, nonspecific low back pain: A systematic review. Phys. Ther. 2009, 89, 9–25. [Google Scholar] [CrossRef]
- Ferreira, P.H.; Ferreira, M.L.; Maher, C.G.; Refshauge, K.; Herbert, R.D.; Hodges, P.W. Changes in recruitment of transversus abdominis correlate with disability in people with chronic low back pain. Br. J. Sports Med. 2010, 44, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- McGill, S. Low Back Disorders: Evidence-Based Prevention and Rehabilitation; Human Kinetics: Champaign, IL, USA, 2007. [Google Scholar]
- Rasouli, O.; Arab, A.M.; Jaberzadeh, S. Ultrasound measurement of deep abdominal muscle activity in sitting positions with different stability levels in subjects with and without chronic low back pain. Man. Ther. 2011, 16, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.F.; Ferreira, P.H.; Franco, M.R.; Ferreira, M.C.; Ferreira, M.L.; Teixeira-Salmela, L.F.; Oliveira, V.C.; Maher, C. The effect of lumbar posture on abdominal muscle thickness during an isometric leg task in people with and without non-specific low back pain. Man. Ther. 2011, 16, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.; Hodges, P.W. Immediate changes in feed forward postural adjustments following voluntary motor training. Exp. Brain Res. 2007, 181, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W. Pain and motor control: From the laboratory to rehabilitation. J. Electromyogr. Kinesiol. 2011, 21, 220–228. [Google Scholar] [CrossRef]
- Hides, J.A.; Richardson, C.A.; Jull, G.A. Use of real-time ultrasound imaging for feedback in rehabilitation. Man. Ther. 1998, 3, 125–131. [Google Scholar] [CrossRef]
- Hides, J.A.; Stokes, M.J.; Saide, M.; Jull, G.A.; Cooper, D.H. Evidence of lumbar multifidus muscles wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine 1994, 19, 165–177. [Google Scholar] [CrossRef]
- Parkhurst, T.M.; Burnett, C.N. Injury and proprioception in the lower back. J. Orthop. Sports Phys. Ther. 1994, 19, 282–295. [Google Scholar] [CrossRef]
- Jarus, T.; Ratzon, N.Z. The implementation of motor learning principles in designing prevention programs at work. Work 2005, 24, 171–182. [Google Scholar] [CrossRef]



| US Group (n = 12) | Control Group (n = 11) | Between Group Differences | |||
|---|---|---|---|---|---|
| Mean (SD) | 95% CI (Lower–Upper Bound) | Mean (SD) | 95% CI (Lower-Upper Bound) | p-Value | |
| Age (years) | 47.67 (8.86) | 42.04–53.29 | 47.18 (14.56) | 37.40–56.96 | 0.923 |
| Height (cm) | 168.83 (9.49) | 162.81–174.86 | 166.73 (8.95) | 160.72–172.74 | 0.590 |
| Weight (kg) | 4.08 (3.15) | 2.08–6.08 | 3.73 (3.29) | 1.52–5.94 | 0.793 |
| BMI (kg/m2) | 2.75 (0.45) | 2.46–3.04 | 2.82 (0.75) | 2.31–3.32 | 0.915 |
| Gender | Frequency (Percentage) | ||||
| Male | 4 (33.3%) | 2 (18.2%) | |||
| Female | 8 (66.7%) | 9 (81.8%) | 0.408 | ||
| Baseline | Post Intervention | Independent t | |
|---|---|---|---|
| Mean Value (SD) | p-Value | ||
| NPRS (at worst) | 7.87 (1.74) | 2.61 (1.75) | <0.001 ** |
| NPRS (at best) | 1.87 (1.60) | 0.13 (0.46) | <0.001 ** |
| NPRS (leg pain) | 4.04 (4.09) | 1.00 (1.54) | <0.001 ** |
| RMDQ | 9.91 (5.06) | 2.43 (2.43) | <0.001 ** |
| HADS-Anxiety | 7.52 (3.85) | 7.04 (3.90) | 0.460 |
| HADS-Depression | 5.39 (3.04) | 3.91 (3.15) | 0.023 * |
| Frequency (Percentage) | X2 | ||
| TrA biofeedback level | |||
| Level 1 | 0 (0%) | 0 (0%) | 0.017 * |
| Level 2 | 1 (4.3%) | 0 (0%) | |
| Level 3 | 12 (52.2%) | 7 (30.4%) | |
| Level 4 | 9 (39.1%) | 8 (34.8%) | |
| Level 5 | 0 (0%) | 5 (21.7%) | |
| Level 6 | 1 (4.3%) | 3 (13%) | |
| Motor Control tests | |||
| Waiter’s bow | 12 (52.2%) | 21 (91.3%) | 0.004 * |
| Pelvic tilt | 15 (65.2%) | 22 (95.7%) | 0.010 * |
| Hook lying | 3 (13%) | 21 (91.3%) | <0.001 ** |
| Quadruped (flexion-control) | 9 (39.1%) | 19 (82.6%) | 0.003 * |
| Quadruped (extension-control) | 5 (21.7%) | 17 (73.9%) | <0.001 ** |
| Active knee flexion in (prone) | 15 (65.2%) | 23 (100%) | 0.002 * |
| Sitting knee extension | 18 (78.3%) | 23 (100%) | 0.019 * |
| US-Guided Group (n = 12) | Control Group (n = 11) | ||||
|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | Two-Way Anova | |
| Mean Value (SD) | p-Value | ||||
| NPRS (worst) | 7.33 (1.97) | 2.83 (1.70) | 8.45 (1.29) | 2.36 (1.86) | 0.593 |
| NPRS (best) | 1.75 (1.71) | 0.08 (0.30) | 2 (1.55) | 0.18 (0.60) | 0.655 |
| NPRS (leg pain) | 4.42 (4.70) | 1.33 (1.83) | 3.64 (3.50) | 0.64 (1.12) | 0.425 |
| RMDQ | 8.75 (4.52) | 2.83 (2.33) | 11.18 (5.53) | 2 (2.57) | 0.529 |
| HADS-Anxiety | 6.58 (4.21) | 6.75 (3.96) | 8.55 (3.30) | 7.36 (4.01) | 0.329 |
| HADS-Depression | 5.92 (2.97) | 4.08 (3.29) | 4.82 (3.16) | 3.73 (3.29) | 0.539 |
| Baseline | Post-Intervention | ||||
|---|---|---|---|---|---|
| Clinical Tests | US-Guided | Control Group | US-Guided | Control Group | |
| TrA Biofeedback Level | Frequency (Percentage) | Frequency (Percentage) | p-Value | ||
| Level 1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.898 |
| Level 2 | 0 (0%) | 1 (9.1%) | 0 (0%) | 0 (0%) | |
| Level 3 | 4 (33.3%) | 8 (72.7%) | 4 (33.3%) | 3 (27.3%) | |
| Level 4 | 8 (66.7%) | 1 (9.1%) | 4 (33.3%) | 4 (36.4%) | |
| Level 5 | 0 (0%) | 0 (0%) | 2 (16.7%) | 3 (27.3%) | |
| Level 6 | 0 (0%) | 1 (9.1%) | 2 (16.7%) | 1 (9.1%) | |
| Motor Control Tests | |||||
| Waiters bow | 6 (50.0%) | 6 (54.5%) | 11 (91.7%) | 10 (90.9%) | 0.950 |
| Pelvic tilt | 8 (66.7%) | 7 (63.6%) | 12 (100%) | 10 (90.9%) | 0.296 |
| Hook lying | 3 (25.0%) | 0 (0%) | 12 (100%) | 9 (81.8%) | 0.131 |
| Quadruped (flexion-control) | 5 (41.7%) | 4 (36.4%) | 11 (91.7%) | 9 (75.0%) | 0.242 |
| Quadruped (extension-control) | 4 (33.3%) | 1 (9.1%) | 9 (75.0%) | 9 (75.0%) | 0.903 |
| Active knee flexion (prone) | 9 (75.0%) | 6 (54.5%) | 12 (100%) | 11 (100%) | 1.000 |
| Sitting knee extension | 9 (75.0%) | 9 (81.8%) | 12 (100%) | 11 (100%) | 1.000 |
| ICC1,2 Value | |
|---|---|
| Biofeedback level assessment procedure | 0.86 |
| Motor Control Tests | |
| Waiters bow | 0.75 |
| Pelvic tilt | 0.65 |
| Hook lying position | 0.39 |
| Quadruped (flexion control) | 0.55 |
| Quadruped (extension control) | 0.72 |
| Active knee flexion (in prone) | 0.66 |
| Sitting knee extension | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taxiarchopoulos, N.; Drakonaki, E.; Gianniotis, M.; Matzaroglou, C.; Tsepis, E.; Billis, E. Investigation of Real-Time Diagnostic Ultrasound as a Means of Biofeedback Training in Transversus Abdominus Re-Education of Patients with Non-Specific Low Back Pain: A Prospective Randomized Controlled Pilot Study. Healthcare 2023, 11, 1396. https://doi.org/10.3390/healthcare11101396
Taxiarchopoulos N, Drakonaki E, Gianniotis M, Matzaroglou C, Tsepis E, Billis E. Investigation of Real-Time Diagnostic Ultrasound as a Means of Biofeedback Training in Transversus Abdominus Re-Education of Patients with Non-Specific Low Back Pain: A Prospective Randomized Controlled Pilot Study. Healthcare. 2023; 11(10):1396. https://doi.org/10.3390/healthcare11101396
Chicago/Turabian StyleTaxiarchopoulos, Nikolaos, Elena Drakonaki, Maria Gianniotis, Charalampos Matzaroglou, Elias Tsepis, and Evdokia Billis. 2023. "Investigation of Real-Time Diagnostic Ultrasound as a Means of Biofeedback Training in Transversus Abdominus Re-Education of Patients with Non-Specific Low Back Pain: A Prospective Randomized Controlled Pilot Study" Healthcare 11, no. 10: 1396. https://doi.org/10.3390/healthcare11101396
APA StyleTaxiarchopoulos, N., Drakonaki, E., Gianniotis, M., Matzaroglou, C., Tsepis, E., & Billis, E. (2023). Investigation of Real-Time Diagnostic Ultrasound as a Means of Biofeedback Training in Transversus Abdominus Re-Education of Patients with Non-Specific Low Back Pain: A Prospective Randomized Controlled Pilot Study. Healthcare, 11(10), 1396. https://doi.org/10.3390/healthcare11101396








