Assessing Therapeutic Choices and Adherence to Antidiabetic Therapy in Naïve Patients: A Retrospective Observational Study in a Local Health Authority of the Piedmont Region (Italy)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Analysis
3. Results
3.1. Baseline Characteristics and First-Line Antidiabetic Therapy
3.2. Identification of Comorbidities Based on Dispensed Drugs
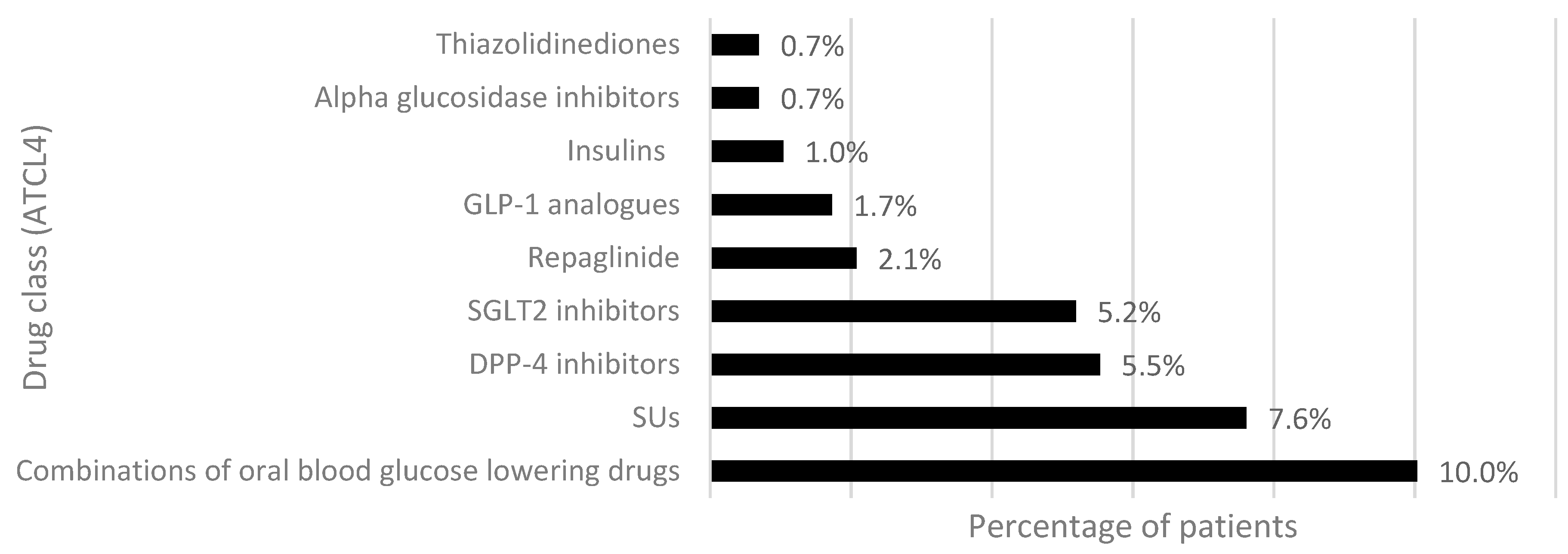
3.3. First Treatment Intensification: Add-On and Switch
3.4. Adherence to Antidiabetic Therapy
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Diabetes. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 1 July 2022).
- Geier, A.S.; Wellmann, I.; Wellmann, J.; Kajüter, H.; Heidinger, O.; Hempel, G.; Hense, H.W. Patterns and determinants of new first-line antihyperglycaemic drug use in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2014, 106, 73–80. [Google Scholar] [CrossRef]
- Ministero della Salute–Direzione Generale della Prevenzione Sanitaria. Stato Delle Conoscenze e Delle Nuove Acquisizioni in Tema di Diabete Mellito; Relazione 2021; Ministero della Salute–Direzione Generale della Prevenzione Sanitaria: Roma, Italy, 2021.
- Istat. Rapporto Annuale 2020; La situazione del Paese; Istat: Chicago, IL, USA, 2020. [Google Scholar]
- Piette, J.D.; Kerr, E.A. The Impact of Comorbid Chronic Conditions on Diabetes Care. Diabetes Care 2006, 29, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Eilat-Tsanani, S.; Margalit, A.; Golan, L.N. Occurence of comorbidities in newly diagnosed type 2 diabetes patients and their impact after 11 years’ follow-up. Sci. Rep. 2021, 11, 11071. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, M.; Zghebi, S.S.; Ashcroft, D.M.; Buchan, I.; Chew-Graham, C.; Holt, T.; Mallen, C.; Marwijk, H.V.; Perera-Salazar, R.; Reeves, D.; et al. The comorbidity burden of type 2 diabetes mellitus: Patterns, clusters and predictions from a large English primary care cohort. BMC Med. 2019, 17, 145. [Google Scholar] [CrossRef] [Green Version]
- Putignano, D.; Guerriero, F.; Orlando, V.; Fiorentino, D.; Menditto, E. La prescrizione di antidiabetici nel paziente anziano: Trend prescrittivi. G. Ital. Farm. E Farm. 2016, 8, 17–23. [Google Scholar]
- Linee Guida della Società Italiana di Diabetologia (SID) e dell’Associazione dei Medici Diabetologi (AMD). Standard Italiani per la Cura del Diabete Mellito 2018; Linee Guida della Società Italiana di Diabetologia (SID) e dell’Associazione dei Medici Diabetologi (AMD): Roma, Italy, 2018. [Google Scholar]
- Linee Guida della Società Italiana di Diabetologia (SID) e dell’Associazione dei Medici Diabetologi (AMD). La Terapia del Diabete Mellito di Tipo 2; Linee Guida della Società Italiana di Diabetologia (SID) e dell’Associazione dei Medici Diabetologi (AMD): Roma, Italy, 2021. [Google Scholar]
- Miglio, G.; Basso, L.; Armando, L.G.; Traina, S.; Benetti, E.; Diarassouba, A.; Baroetto Parisi, R.; Esiliato, M.; Rolando, C.; Remani, E.; et al. A Network Approach for the Study of Drug Prescriptions: Analysis of Administrative Records from a Local Health Unit (ASL TO4, Regione Piemonte, Italy). Int. J. Environ. Res. Public Health 2021, 18, 4859. [Google Scholar] [CrossRef] [PubMed]
- Armando, L.G.; Baroetto Parisi, R.; Remani, E.; Esiliato, M.; Rolando, C.; Vinciguerra, V.; Diarassouba, A.; Cena, C.; Miglio, G. Persistence to Medications for Benign Prostatic Hyperplasia/Benign Prostatic Obstruction-Associated Lower Urinary Tract Symptoms in the ASL TO4 Regione Piemonte (Italy). Healthcare 2022, 10, 2567. [Google Scholar] [CrossRef] [PubMed]
- Orlando, V.; Guerriero, F.; Putignano, D.; Monetti, V.M.; Tari, D.U.; Farina, G.; Illario, M.; Iccarino, G.; Menditto, E. Prescription Patterns of Antidiabetic Treatment in the Elderly. Results from Southern Italy. Curr. Diabetes Rev. 2016, 12, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Baviera, M.; Cortesi, L.; Tettamanti, M.; Avanzini, F.; Marelli, G.; Marzona, I.; Nobili, A.; Riva, E.; Fortino, I.; Bortolotti, A.; et al. Changes in prescribing patterns and clinical outcomes in elderly diabetic patients in 2000 and 2010: Analysis of a large Italian population-based study. Eur. J. Clin. Pharmacol. 2014, 70, 965–974. [Google Scholar] [CrossRef]
- Murray, P.; Norris, H.; Metcalfe, S.; Betty, B.; Young, V.; Locke, B. Dispensing patterns for antidiabetic agents in New Zealand: Are the guidelines being followed? NZMA 2017, 130, 1175–8716. [Google Scholar]
- Nishimura, R.; Kato, H.; Kisanuki, K.; Oh, A.; Hiroi, A.; Onishi, Y.; Guelfucci, F.; Shimasaki, Y. Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: A claims-based cohort study. BMJ Open 2019, 9, e025806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flory, J.; Gerhard, T.; Stempniewicz, N.; Keating, S.; Rowan, C.G. Comparative adherence to diabetes drugs: An analysis of electronic health records and claims data. Diabetes Obes. Metab. 2017, 19, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Yuen-Sodihardjo, F.; van Dijk, L.; Wensing, M.; De Smet, P.A.G.M.; Teichert, M. Use of pharmacy dispensing data to measure adherence and identify nonadherence with oral hypoglycemic agents. Eur. J. Clin. Pharmacol. 2017, 73, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caio, L.; Casula, M.; Tragni, E. Aspetti farmacoeconomici del trattamento del diabete mellito di tipo 2. G. Ital. Farm. Farm. 2016, 8, 30–36. [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC Classification Index with DDDs, 2023; Norwegian Institute of Public Health: Oslo, Norway, 2022. [Google Scholar]
- Pratt, N.L.; Kerr, M.; Barratt, J.D.; Kemp-Casey, A.; Kalisch, L.M.; Ramsay, E.; Roughead, E.E. The validity of the Rx-Risk Comorbidity Index using medicines mapped to the Anatomical Therapeutic Chemical (ATC) Classification System. BMJ Open 2018, 8, e021122. [Google Scholar] [CrossRef]
- O’Shea, M.; Teeling, M.; Bennett, K. The prevalence and ingredient cost of chronic comorbidity in the Irish elderly population with medication treated type 2 diabetes: A retrospective cross-sectional study using a national pharmacy claims database. BMC Health Serv. Res. 2013, 13, 23. [Google Scholar] [CrossRef] [Green Version]
- Engler, C.; Leo, M.; Pfeifer, B.; Juchum, M.; Chen-Koenig, D.; Poelzi, K.; Schoenherr, H.; Vill, D.; Oberdanner, J.; Eisendle, E.; et al. Long-term trends in the prescription of antidiabetic drugs: Real-world evidence from the Diabetes Registry Tyrol 2012–2018. BMJ Open Diab. Res. Care 2020, 8, e001279. [Google Scholar] [CrossRef]
- Evans, M.; Engberg, S.; Faurby, M.; Da Rocha Fernandes, J.D.; Hudson, P.; Polonsky, W. Adherence to and persistence with antidiabetic medications and associations with clinical and economic outcomes in people with type 2 diabetes mellitus: A systematic literature review. Diabetes Obes. Metab. 2022, 24, 377–390. [Google Scholar] [CrossRef]
- Heintjes, E.M.; Overbeek, J.A.; Hall, G.C.; Prieto-Alhambra, D.; Lapi, F.; Hammar, N.; Bezemer, I.D. Factors associated with type 2 diabetes mellitus treatment choice across four European countries. Clin. Ther. 2017, 39, 2296–2310.e14. [Google Scholar] [CrossRef]
- Desai, N.R.; Shrank, W.H.; Fischer, M.; Avorn, J.; Liberman, J.N.; Scheeweiss, S.; Pakes, J.; Brennan, T.A.; Choudhry, N.K. Patterns of medication initiation in newly diagnosed diabetes mellitus: Quality and cost implications. Am. J. Med. 2012, 125, 302.e1–302.e7. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Fernández de Alba, I.; Orlando, V.; Monetti, V.M.; Mucherino, S.; Gimeno-Miguel, A.; Vaccaro, O.; João Forjaz, M.; Poblador Plou, B.; Prados-Torres, A.; Riccardi, G.; et al. Comorbidity in an Older Population with Type-2 Diabetes Mellitus: Identification of the Characteristics and Healthcare Utilization of High-Cost Patients. Front. Pharmacol. 2020, 11, 586187. [Google Scholar] [CrossRef] [PubMed]
- AIFA. Istituzione Della Nota AIFA 100 Relativa Alla Prescrizione Degli Inibitori SGLT2, Degli Agonisti Recettoriali del GLP1, Degli Inibitori del DPP4 e Loro Associazioni nel Trattamento del Diabete Mellito Tipo 2. Available online: https://www.aifa.gov.it/web/guest/nota-100 (accessed on 1 August 2022).
- Simard, P.; Presse, N.; Roy, L.; Dorais, M.; White-Guay, B.; Räkel, A.; Perreault, S. Persistence and adherence to oral antidiabetics: A population-based cohort study. Acta Diabetol. 2015, 52, 547–556. [Google Scholar] [CrossRef] [PubMed]

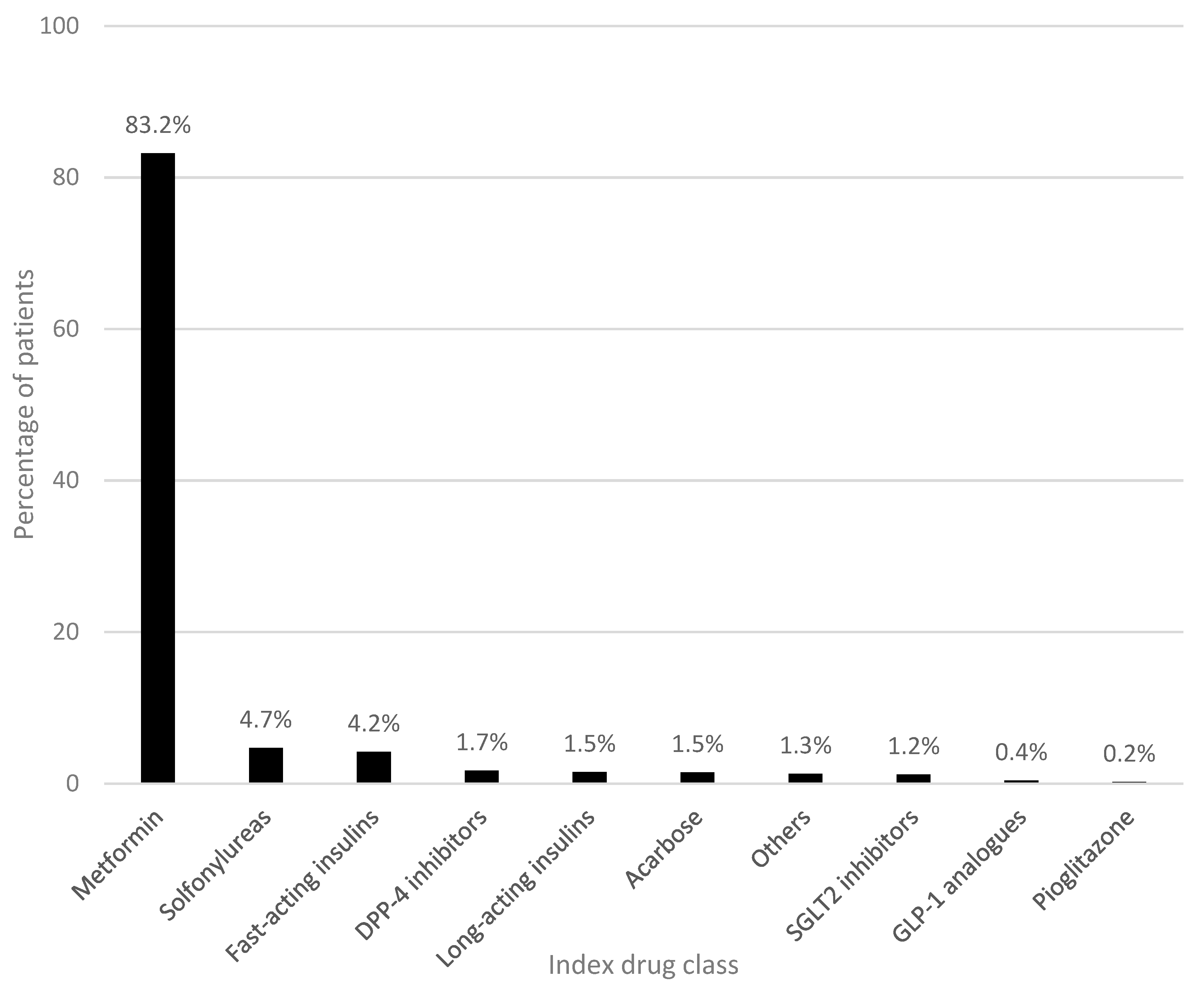



| Study population, N | 1927 |
| Males, N (%) | 1090 (56.6) |
| Age, median [IQR] | 67.0 [58.0–75.0] |
| Males | 66.0 [57.0–73.0] |
| Females | 70.0 [60.0–78.0] |
| Number of index drugs, N (%) | |
| 1 index drug | 1759 (91.3) |
| 2 index drugs | 152 (7.9) |
| 3 index drugs | 13 (0.7) |
| ≥4 index drugs | 3 (0.2) |
| Characteristics N = 1361 | 1 AD | 2 ADs | 3 ADs | 4 ADs | ≥5 ADs | p-Value |
|---|---|---|---|---|---|---|
| Patients, N (%) | 931 (68.4) | 289 (21.1) | 92 (6.8) | 31 (2.3) | 18 (1.3) | |
| Gender, N (%) | 0.14 | |||||
| Males | 488 (52.4) | 176 (60.9) | 54 (58.7) | 18 (58.1) | 10 (55.6) | |
| Age, median [IQR] | 71.0 [62.0–78.0] | 65.0 [57.0–74.0] | 64.5 [54.2–73.0] | 64.5 [54.2–73.0] | 62.0 [53.0–72.0] | <0.01 |
| Males | 69.0 [62.0–75.0] | 65.0 [56.0–73.0] | 62.0 [53.0–70.0] | 62.0 [53.0–70.0] | 58.0 [53.0–73.0] | |
| Females | 72.0 [62.0–80.0] | 67.0 [60.0–75.2] | 66.0 [60.0–74.0] | 66.0 [60.0–74.0] | 64.0 [59.0–69.0] | |
| Drugs of other classes used during the study period, median [IQR] | 10.0 [6.0–15.0] | 11.0 [7.0–17.0] | 9.0 [6.0–15.0] | 12.0 [6.5–18.5] | 14.0 [7.7–17.5] | 0.13 |
| Males | 9.0 [5.0–15.0] | 10.0 [5.7–15.0] | 9.0 [5.0–10.0] | 9.5 [7.0–17.0] | 15.0 [13.0–22.5] | |
| Females | 12.0 [8.0–17.0] | 13.0 [8.0–19.0] | 9.5 [7.0–16.7] | 16.0 [6.0–22.0] | 9.0 [6.0–16.0] |
| Drug Classes (ATCL2) | Related Medical Conditions | Patients with Prescriptions in the Drug Class during the Study Period (%) | ||||
|---|---|---|---|---|---|---|
| 1 AD | 2 ADs | 3 ADs | 4 ADs | ≥5 ADs | ||
| DM-concordant conditions | ||||||
| C01, C04, C05, C07, C08, B01 | Cardiovascular/cerebrovascular diseases and heart diseases | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| C02, C03, C09 | Hypertension | 100.0 | 100.0 | 94.6 | 100.0 | 77.8 |
| C10 | Hyperlipidemia | 62.5 | 69.6 | 80.4 | 64.5 | 61.1 |
| M04 | Hyperuricemia/Gout | 18.0 | 20.4 | 13.0 | 16.1 | 11.1 |
| DM-discordant conditions | ||||||
| J01–J07 | Infectious diseases | 83.8 | 87.5 | 88.0 | 100.0 | 100.0 |
| A02 | Acid related disorders | 57.1 | 60.2 | 54.3 | 67.7 | 77.8 |
| M01–M03, M09 | Inflammatory/Rheumatic disorders | 56.6 | 52.6 | 43.5 | 48.4 | 55.6 |
| H02 | Corticosteroid-responsive diseases | 40.7 | 45.7 | 33.7 | 32.3 | 44.4 |
| A08–A09, A11–A16 | Nutrition-related diseases | 36.5 | 32.9 | 29.3 | 29.0 | 33.3 |
| N02 | Pain, including migraine | 29.9 | 29.4 | 23.9 | 41.9 | 33.3 |
| N06, N07 | Depression and other mental disorders | 21.4 | 21.1 | 20.7 | 19.4 | 27.8 |
| R03 | Chronic obstructive airways diseases | 19.9 | 20.8 | 23.9 | 22.6 | 61.1 |
| A03–A04, A06–A07 | Gastrointestinal disorders and nausea | 19.9 | 19.4 | 18.5 | 22.6 | 50.0 |
| B03 | Anemia | 17.7 | 17.3 | 9.8 | 6.5 | 11.1 |
| G01–G04 | Diseases of the genito-urinary system, including benign prostatic hypertrophy | 17.4 | 19.0 | 16.3 | 6.5 | 11.1 |
| H03 | Thyroid disorders | 12.5 | 13.5 | 7.6 | 9.7 | 11.1 |
| R01–R02, R05–R07 | Respiratory diseases | 10.2 | 9.7 | 10.9 | 3.2 | 11.1 |
| N03 | Epilepsy | 9.7 | 13.5 | 5.4 | 29.0 | 27.8 |
| D01–D11 | Dermatological diseases, including psoriasis | 7.3 | 8.3 | 9.8 | 0.0 | 22.2 |
| S01, S03 | Eye disorders, including glaucoma | 7.1 | 6.2 | 6.5 | 12.9 | 11.1 |
| N05 | Psychotic illnesses | 3.3 | 5.5 | 3.3 | 6.5 | 11.1 |
| 1 AD | 2 ADs | 3 ADs | 4 ADs | ≥5 ADs | |
|---|---|---|---|---|---|
| Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | |
| Adherence to ADs | 46.0 [31.0–64.0] | 60.0 [41.0–84.0] | 62.0 [41.0–82.0] | 63.0 [52.0–83.0] | 63.0 [49.0–75.0] |
| Males | 47.0 [33.0–66.0] | 60.0 [41.0–84.0] | 64.0 [42.0–82.0] | 61.0 [47.0–81.0] | 61.0 [49.0–71.0] |
| Females | 43.0 [29.0–61.0] | 60.0 [44.0–84.0] | 58.0 [36.0–76.0] | 66.0 [54.0–85.0] | 64.0 [51.0–79.0] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armando, L.G.; Miglio, G.; Baroetto Parisi, R.; Esiliato, M.; Rolando, C.; Vinciguerra, V.; Diarassouba, A.; Cena, C. Assessing Therapeutic Choices and Adherence to Antidiabetic Therapy in Naïve Patients: A Retrospective Observational Study in a Local Health Authority of the Piedmont Region (Italy). Healthcare 2023, 11, 1655. https://doi.org/10.3390/healthcare11111655
Armando LG, Miglio G, Baroetto Parisi R, Esiliato M, Rolando C, Vinciguerra V, Diarassouba A, Cena C. Assessing Therapeutic Choices and Adherence to Antidiabetic Therapy in Naïve Patients: A Retrospective Observational Study in a Local Health Authority of the Piedmont Region (Italy). Healthcare. 2023; 11(11):1655. https://doi.org/10.3390/healthcare11111655
Chicago/Turabian StyleArmando, Lucrezia Greta, Gianluca Miglio, Raffaella Baroetto Parisi, Mariangela Esiliato, Cristina Rolando, Valeria Vinciguerra, Abdoulaye Diarassouba, and Clara Cena. 2023. "Assessing Therapeutic Choices and Adherence to Antidiabetic Therapy in Naïve Patients: A Retrospective Observational Study in a Local Health Authority of the Piedmont Region (Italy)" Healthcare 11, no. 11: 1655. https://doi.org/10.3390/healthcare11111655






