The Effect of Sexual Intercourse during Pregnancy on Preterm Birth: Prospective Single-Center Cohort Study in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. The Patients
2.2. Questionnaire Survey
2.3. Bacterial Vaginosis Screening
2.4. Data Extraction
2.5. Systematic Review of Worldwide SI Frequency during Pregnancy
2.6. Statistical Analysis
3. Results
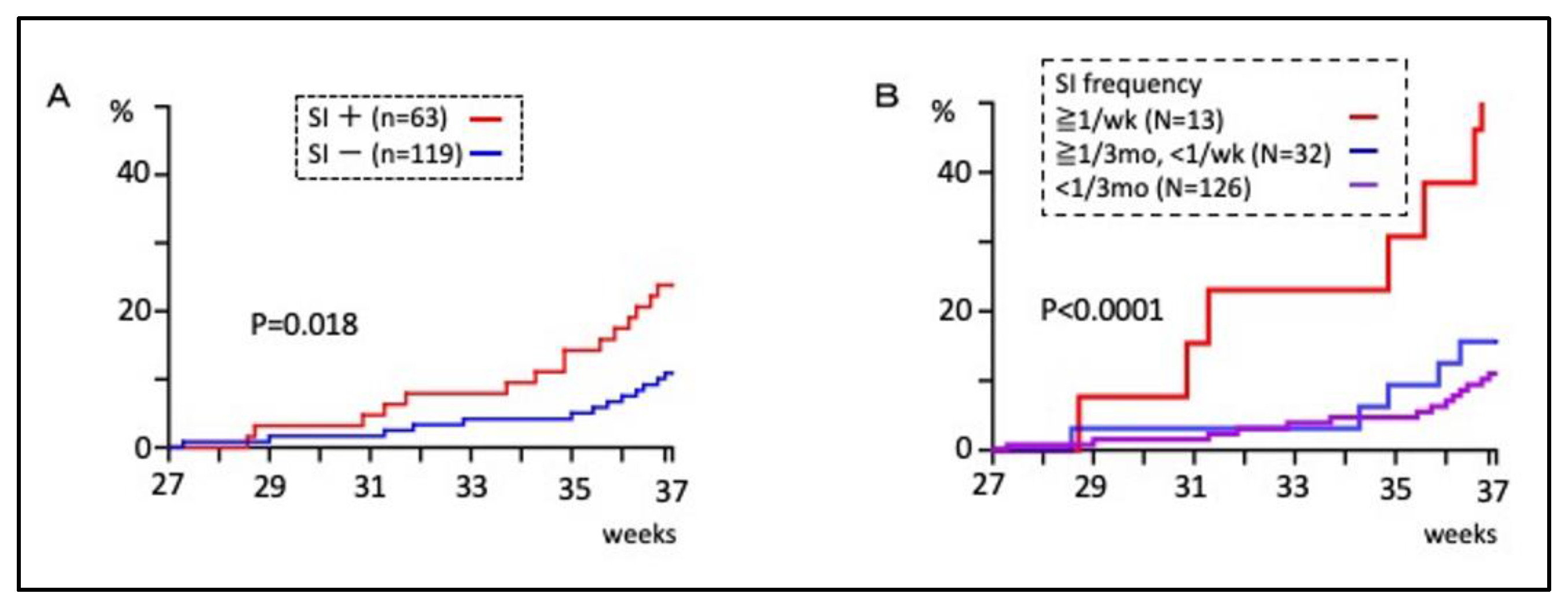
3.1. Sexual Intercourse during Pregnancy and Preterm Birth Rate
3.2. The Risk Factors for Preterm Birth
3.3. Sexual Intercourse, Bacterial Vaginosis, and Preterm Birth
3.4. Systematic Review on Sexual Intercourse during Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walani, S.R. Global burden of preterm birth. Int. J. Gynaecol. Obstet. 2020, 150, 31–33. [Google Scholar] [CrossRef]
- Serenius, F.; Källén, K.; Blennow, M.; Ewald, U.; Fellman, V.; Holmström, G.; Lindberg, E.; Lundqvist, P.; Maršál, K.; Norman, M.; et al. Neurodevelopmental Outcome in Extremely Preterm Infants at 2.5 Years after Active Perinatal Care in Sweden. JAMA 2013, 309, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Gonçalves, L.F.; Kusanovic, J.P.; Friel, L.A.; Nien, J.K. Inflammation in preterm and term labour and delivery. Semin. Fetal Neonatal Med. 2006, 11, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Taylor, R.; Fortunato, S. Chorioamnionitis—A complex pathophysiologic syndrome. Placenta 2010, 31, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Bieda, J.; Chaemsaithong, P.; Miranda, J.; Chaiworapongsa, T.; Ravel, J. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2014, 2, 18. [Google Scholar] [CrossRef]
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.-A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Veyver, I.V.D.; Milosavljevic, A.; et al. A Metagenomic Approach to Characterization of the Vaginal Microbiome Signature in Pregnancy. PLoS ONE 2012, 7, e36466. [Google Scholar] [CrossRef]
- Jung, H.-S.; Ehlers, M.M.; Lombaard, H.; Redelinghuys, M.J.; Kock, M.M. Etiology of bacterial vaginosis and polymicrobial biofilm formation. Crit. Rev. Microbiol. 2017, 43, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Leitich, H.; Bodner-Adler, B.; Brunbauer, M.; Kaider, A.; Egarter, C.; Husslein, P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am. J. Obstet. Gynecol. 2003, 189, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Misra, D.P. Risk factors for bacterial vaginosis during pregnancy among African American women. Am. J. Obstet. Gynecol. 2007, 197, 477.e1–477.e8. [Google Scholar]
- Subtil, D.; Brabant, G.; Tilloy, E.; Devos, P.; Canis, F.; Fruchart, A.; Bissinger, M.-C.; Dugimont, J.-C.; Nolf, C.; Hacot, C.; et al. Early clindamycin for bacterial vaginosis in pregnancy (PREMEVA): A multicentre, double-blind, randomised controlled trial. Lancet 2018, 392, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.C.; Klebanoff, M.A.; Hauth, J.C.; Hillier, S.L.; Thom, E.A.; Ernest, J.M.; Heine, R.P.; Nugent, R.P.; Fischer, M.L.; Leveno, K.J.; et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N. Engl. J. Med. 2000, 342, 534–540. [Google Scholar] [CrossRef] [PubMed]
- McDonald, H.M.; O’Loughlin, J.A.; Vigneswaran, R.; Jolley, P.T.; Harvey, J.A.; Bof, A.; McDonald, P.J. Impact of metronidazole therapy on preterm birth in women with bacterial vaginosis flora (Gardnerella vaginalis): A randomised, placebo controlled trial. Br. J. Obs. Gynaecol. 1997, 104, 1391–1397. [Google Scholar] [CrossRef]
- Brocklehurst, P.; Gordon, A.; Heatley, E.; Milan, S.J. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst. Rev. 2013, CD000262. [Google Scholar] [CrossRef]
- Goodlin, R.C.; Schmidt, W.; Creevy, D.C. Uterine tension and fetal heart rate during maternal orgasm. Obstet. Gynecol. 1972, 39, 125–127. [Google Scholar] [PubMed]
- Brustman, L.E.; Raptoulis, M.; Langer, O.; Anyaegbunam, A.; Merkatz, I.R. Changes in the pattern of uterine contractility in relationship to coitus during pregnancies at low and high risk for preterm labor. Obstet. Gynecol. 1989, 73, 166–168. [Google Scholar] [PubMed]
- Read, J.S.; Klebanoff, M.A. Sexual intercourse during pregnancy and preterm delivery: Effects of vaginal microorganisms. Am. J. Obstet. Gynecol. 1993, 168, 514–519. [Google Scholar] [CrossRef]
- Mills, J.L.; Harlap, S.; Harley, E.E. Should coitus late in pregnancy be discouraged? Lancet 1981, 2, 136–138. [Google Scholar] [CrossRef]
- Klebanoff, M.A.; Nugent, R.P.; Rhoads, G.G. Coitus during pregnancy: Is it safe? Lancet 1984, 2, 914–917. [Google Scholar] [CrossRef]
- Kurki, T.; Ylikorkala, O. Coitus during pregnancy is not related to bacterial vaginosis or preterm birth. Am. J. Obstet. Gynecol. 1993, 169, 1130–1134. [Google Scholar] [CrossRef]
- National Collaborating Centre for Women’s and Children’s Health (UK). Antenatal Care: Routine Care for the Healthy Pregnant Woman; RCOG Press: London, UK, 2008. [Google Scholar]
- Wylie, K. A Global Survey of Sexual Behaviours. J. Fam. Reprod. Health 2009, 3, 39–49. [Google Scholar]
- Laghi, L.; Zagonari, S.; Patuelli, G.; Zhu, C.; Foschi, C.; Morselli, S.; Pedna, M.F.; Sambri, V.; Marangoni, A. Vaginal metabolic profiles during pregnancy: Changes between first and second trimester. PLoS ONE 2021, 16, e0249925. [Google Scholar] [CrossRef] [PubMed]
- Nugent, R.P.; A Krohn, M.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Shimaoka, M.; Yo, Y.; Doh, K.; Kotani, Y.; Suzuki, A.; Tsuji, I.; Mandai, M.; Matsumura, N. Association between preterm delivery and bacterial vaginosis with or without treatment. Sci. Rep. 2019, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Jawed-Wessel, S.; Sevick, E. The Impact of Pregnancy and Childbirth on Sexual Behaviors: A Systematic Review. J. Sex Res. 2017, 54, 411–423. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Staruch, M.; Kucharczyk, A.; Zawadzka, K.; Wielgos, M.; Szymusik, I. Sexual activity during pregnancy. Neuro Endocrinol. Lett. 2016, 37, 53–58. [Google Scholar]
- Kulhawik, R.; Zborowska, K.; Grabarek, B.O.; Boroń, D.; Skrzypulec-Plinta, V.; Drosdzol-Cop, A. Changes in the Sexual Behavior of Partners in Each Trimester of Pregnancy in Otwock in Polish Couples. Int. J. Environ. Res. Public Health 2022, 19, 2921. [Google Scholar] [CrossRef]
- Yanikkerem, E.; Goker, A.; Ustgorul, S.; Karakus, A. Evaluation of sexual functions and marital adjustment of pregnant women in Turkey. Int. J. Impot. Res. 2016, 28, 176–183. [Google Scholar] [CrossRef]
- Erenel, A.S.; Eroglu, K.; Vural, G.; Dilbaz, B. A pilot study: In what ways do women in Turkey experience a change in their sexuality during pregnancy? Sex Disabil. 2011, 29, 207–216. [Google Scholar] [CrossRef]
- Kumar, R.; Brant, H.; Robson, K.M. Childbearing and maternal sexuality: A prospective survey of 119 primiparae. J. Psychosom. Res. 1981, 25, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Bartellas, E.; Crane, J.M.G.; Daley, M.; Bennett, K.A.; Hutchens, D. Sexuality and sexual activity in pregnancy. BJOG: Int. J. Obstet. Gynaecol. 2000, 107, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Iliyasu, Z.; Galadanci, H.S.; Ahmed, Z.; Gajida, A.U.; Aliyu, M.H. Prevalence and Patterns of Sexual Activity during Pregnancy in Kano, Northern Nigeria. Afr. J. Reprod. Health 2016, 20, 99–107. [Google Scholar] [CrossRef]
- Naim, M.; Bhutto, E. Sexuality during pregnancy in Pakistani women. J. Pak. Med Assoc. 2000, 50, 38–44. [Google Scholar] [PubMed]
- Phan, T.C.; Hoang, L.B.; Tran, T.K.; Pham, T.T.; Bui, A.V.; Dao, H.T.; Ngo, T.V.; Tran, C.D. Fear-Related Reasons for Avoiding Sexual Intercourse in Early Pregnancy: A Cross-Sectional Study. Sex Med. 2021, 9, 100430. [Google Scholar] [CrossRef] [PubMed]
- Fok, W.Y.; Chan, L.Y.; Yuen, P.M. Sexual behavior and activity in Chinese pregnant women. Acta Obs. Gynecol Scand. 2005, 84, 934–938. [Google Scholar] [CrossRef]
- Chen, L.; Jin, M.; Luo, D.; Chen, X.; Huang, S.; Cai, W. Association between sexual intercourse frequency and pelvic floor muscle morphology in pregnant women. Int. Urogynecol. J. 2019, 31, 1933–1941. [Google Scholar] [CrossRef]
- Kong, L.; Li, T.; Li, L. The impact of sexual intercourse during pregnancy on obstetric and neonatal outcomes: A cohort study in China. J. Obstet. Gynaecol. 2019, 39, 455–460. [Google Scholar] [CrossRef]
- Adeyemi, A.B.; Fatusi, A.O.; Makinde, O.N.; Omojuwa, I.; Asa, S.; Onwudiegwu, U. Changes in sexual practices and responses among ante-natal clinic attendees in a Nigerian teaching hospital. J. Obs. Gynaecol. 2005, 25, 796–802. [Google Scholar] [CrossRef]
- Guendler, J.A.; Katz, L.; Flamini, M.E.D.M.; Lemos, A.; Amorim, M.M. Prevalence of Sexual Dysfunctions and their Associated Factors in Pregnant Women in an Outpatient Prenatal Care Clinic. Rev. Bras. Ginecol Obstet. 2019, 41, 555–563. [Google Scholar] [CrossRef]
- Radoš, S.N.; Vraneš, H.S.; Šunjić, M. Limited Role of Body Satisfaction and Body Image Self-Consciousness in Sexual Frequency and Satisfaction in Pregnant Women. J. Sex Res. 2014, 51, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Branecka-Woźniak, D.; Wójcik, A.; Błażejewska-Jaśkowiak, J.; Kurzawa, R. Sexual and Life Satisfaction of Pregnant Women. Int. J. Environ. Res. Public Health 2020, 17, 5894. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos, P.A.; Dodos, D.; Mechleris, D. Sexuality in pregnancy and premature labour. BJOG Int. J. Obstet. Gynaecol. 1984, 91, 891–893. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.L.; Nugent, R.P.; Eschenbach, D.A.; Krohn, M.A.; Gibbs, R.S.; Martin, D.H.; Cotch, M.F.; Edelman, R.; Pastorek, J.G., 2nd; Rao, A.V.; et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N. Engl. J. Med. 1995, 333, 1737–1742. [Google Scholar] [CrossRef]
- Donders, G.G.; Van Bulck, B.; Caudron, J.; Londers, L.; Vereecken, A.; Spitz, B. Relationship of bacterial vaginosis and mycoplasmas to the risk of spontaneous abortion. Am. J. Obs. Gynecol. 2000, 183, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, X.; Guo, X.; Liang, H.; Cao, D.; Shi, J. Maternal preterm birth prediction in the United States: A case-control database study. BMC Pediatr. 2022, 22, 547. [Google Scholar] [CrossRef]
- Dongarwar, D.; Tahseen, D.; Aliyu, M.H.; Salihu, H.M. Pregnancy outcomes among Asian Americans of advanced maternal age, 1992–2018. J. Obstet. Gynaecol. Res. 2021, 47, 2117–2125. [Google Scholar] [CrossRef]
- Saccone, G.; Gragnano, E.; Ilardi, B.; Marrone, V.; Strina, I.; Venturella, R.; Berghella, V.; Zullo, F. Maternal and perinatal complications according to maternal age: A systematic review and meta-analysis. Int. J. Gynecol. Obstet. 2022, 159, 43–55. [Google Scholar] [CrossRef]
- Platz-Christensen, J.; Brandberg, A.; Wiqvist, N. Increased prostaglandin concentrations in the cervical mucus of pregnant women with bacterial vaginosis. Prostaglandins 1992, 43, 133–141. [Google Scholar] [CrossRef]
- Challis, J.R.; Sloboda, D.M.; Alfaidy, N.; Lye, S.J.; Gibb, W.; Patel, F.A.; Whittle, W.L.; Newnham, J.P. Prostaglandins and mechanisms of preterm birth. Reproduction 2002, 124, 1–17. [Google Scholar] [CrossRef]
- Grisaru-Granovsky, S.; Altarescu, G.; Finci, S.; Weintraub, A.; Tevet, A.; Samueloff, A.; Schimmel, M.S. Prostanoid DP receptor (PTGDR) variants in mothers with post-coital associated preterm births: Preliminary observations. J. Perinatol. 2010, 30, 33–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bygdeman, M.; Samuelsson, B. Prostaglandins in human seminal plasma and their effects on human myometrium. Int. J. Fertil. 1967, 12, 17–20. [Google Scholar] [PubMed]
- Ratten, L.K.; Plummer, E.L.; Murray, G.L.; Danielewski, J.; Fairley, C.K.; Garland, S.M.; Hocking, J.S.; Tachedjian, G.; Chow, E.P.F.; Bradshaw, C.S.; et al. Sex is associated with the persistence of non-optimal vaginal microbiota following treatment for bacterial vaginosis: A prospective cohort study. BJOG 2021, 128, 756–767. [Google Scholar] [CrossRef] [PubMed]


| Univariate Analysis | p Value | Multivariate Analysis | p Value | |
|---|---|---|---|---|
| Age | 1.01 (0.94–1.08) | 0.80 | N/A | |
| Primipara | 1.30 (0.63–2.66) | 0.48 | N/A | |
| Previous PTB | 7.99 (3.04–20.99) | 0.00002 | 6.02 (1.68–21.57) | 0.006 |
| Smoking | 7.82 (2.72–22.49) | 0.0001 | 4.54 (1.01–20.46) | 0.049 |
| BV in the 1st trimester | 1.46 (0.18–12.15) | 0.73 | N/A | |
| BV in the 2nd trimester | 4.00 (1.71–9.36) | 0.001 | 3.53 (1.42–8.79) | 0.007 |
| SI during pregnancy | 2.36 (1.12–4.97) | 0.023 | 2.97 (1.24–7.16) | 0.015 |
| First Author | Reference | Year | Country | Region | Number | Overall (%) | 1st Trimester (%) | 2nd Trimester (%) | 3rd Trimester (%) |
|---|---|---|---|---|---|---|---|---|---|
| Staruch M | [28] | 2016 | Poland | Eastern Europe | 149 | 87 | NA | NA | NA |
| Kulhawik R | [29] | 2022 | 100 | 86 | 86 | 60 | 56 | ||
| Yanikkerem E | [30] | 2016 | Turkey | 298 | 84 | 79 | 84 | 65 | |
| Erene AS | [31] | 2011 | 336 | 95 | 95 | 87 | 41 | ||
| Kumar R | [32] | 1981 | England | Western Europe | 119 | 90 | 90 | 65 | |
| Bartellas E | [33] | 2000 | Canada | North America | 139 | 96 | 96 | 89 | 67 |
| Iliyasu Z | [34] | 2016 | Nigeria | Western Africa | 336 | 97 | 88 | 91 | 97 |
| Naim M | [35] | 2000 | Pakistan | Southern Asia | 150 | 89 | 89 | 82 | 74 |
| Phan TC | [36] | 2021 | Vietnam | South-Eastern Asia | 250 | 71 | 71 | NA | |
| Fok WY | [37] | 2005 | China | Eastern Asia | 298 | 66 | 57 | 66 | 50 |
| Chen L | [38] | 2020 | 323 | 51 | NA | NA | NA | ||
| Kong L | [39] | 2019 | 406 | 51 | 12 | 49 | 15 | ||
| Yo Y | This study | Japan | 182 | 35 | NA | 31 | 19 | ||
| First Author | Reference | Year | Country | Number | Increased (%) | Decreased (%) | No change (%) | NA (%) |
|---|---|---|---|---|---|---|---|---|
| Adeyemi AB | [40] | 2005 | Nigelia | 134 | 16.5 | 37.4 | 46.1 | 0 |
| Iliyasu Z | [34] | 2016 | Nigelia | 336 | 16.3 | 55.4 | 23.8 | 4.5 |
| Guendler JA | [41] | 2019 | Brasil | 262 | 7.6 | 64.9 | 27.5 | 0 |
| Bartellas E | [33] | 2000 | Canada | 139 | 5.8 | 71.2 | 23 | 0 |
| Radoš SN | [42] | 2014 | Croatia | 150 | 1.4 | 79.3 | 19.3 | 0 |
| Branecka-Woźniak D | [43] | 2020 | Poland | 181 | 10.5 | 89.5 | 0 | 0 |
| Fok WY | [37] | 2005 | China | 298 | 1.3 | 91.6 | 7 | 0 |
| Naim M | [35] | 2000 | Pakistan | 150 | 0.7 | 99.3 | 0 | 0 |
| Yoshie Y | This study | Japan | 182 | 0.5 | 85.7 | 6.6 | 7.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yo, Y.; Kawasaki, K.; Moriuchi, K.; Shiro, R.; Shimaoka, M.; Matsumura, N. The Effect of Sexual Intercourse during Pregnancy on Preterm Birth: Prospective Single-Center Cohort Study in Japan. Healthcare 2023, 11, 1657. https://doi.org/10.3390/healthcare11111657
Yo Y, Kawasaki K, Moriuchi K, Shiro R, Shimaoka M, Matsumura N. The Effect of Sexual Intercourse during Pregnancy on Preterm Birth: Prospective Single-Center Cohort Study in Japan. Healthcare. 2023; 11(11):1657. https://doi.org/10.3390/healthcare11111657
Chicago/Turabian StyleYo, Yoshie, Kaoru Kawasaki, Kaori Moriuchi, Reona Shiro, Masao Shimaoka, and Noriomi Matsumura. 2023. "The Effect of Sexual Intercourse during Pregnancy on Preterm Birth: Prospective Single-Center Cohort Study in Japan" Healthcare 11, no. 11: 1657. https://doi.org/10.3390/healthcare11111657
APA StyleYo, Y., Kawasaki, K., Moriuchi, K., Shiro, R., Shimaoka, M., & Matsumura, N. (2023). The Effect of Sexual Intercourse during Pregnancy on Preterm Birth: Prospective Single-Center Cohort Study in Japan. Healthcare, 11(11), 1657. https://doi.org/10.3390/healthcare11111657








