COVID-19 and Cutaneous Squamous Cell Carcinoma—Impact of the Pandemic on Unequal Access to Healthcare
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Period Definitions
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Patient Demographics
3.2. Lesion Localization and Histological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dimitrijević, M.V.; Brašanac, D.Č.; Todorović, N.R.; Petrović, M.G.; Dimitrijević, A.M. Basal Cell Carcinoma—Principles of Treatment. Srp. Arh. Celok. Lek. 2023, 151, 98–105. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Silvia, C.; Denis, C.; Mario, C.; Luigi, V.; Federico, T.; Marcello, C. Impact of COVID-19 Pandemic on Non-Melanoma Skin Cancer’s Tumor Burden and Care: A Multi-Center Study Based in Northern Italy. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 3616–3621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zeng, W.; Jiang, A.; He, Z.; Shen, X.; Dong, X.; Feng, J.; Lu, H. Global, Regional and National Incidence, Mortality and Disability-Adjusted Life-Years of Skin Cancers and Trend Analysis from 1990 to 2019: An Analysis of the Global Burden of Disease Study 2019. Cancer Med. 2021, 10, 4905–4922. [Google Scholar] [CrossRef]
- Serbian Cancer Society Serbian Cancer Registry. Malignant Tumors in Republic of Serbia for 2018; Serbian Cancer Society Serbian Cancer Registry: Belgrade, Serbia, 2020. [Google Scholar]
- Iorio, M.L.; Ter Louw, R.P.; Kauffman, C.L.; Davison, S.P. Evidence-Based Medicine: Facial Skin Malignancy. Plast. Reconstr. Surg. 2013, 132, 1631–1643. [Google Scholar] [CrossRef]
- Baumann, B.C.; MacArthur, K.M.; Brewer, J.D.; Mendenhall, W.M.; Barker, C.A.; Etzkorn, J.R.; Jellinek, N.J.; Scott, J.F.; Gay, H.A.; Baumann, J.C.; et al. Management of Primary Skin Cancer during a Pandemic: Multidisciplinary Recommendations. Cancer 2020, 126, 3900–3906. [Google Scholar] [CrossRef]
- Thompson, A.K.; Kelley, B.F.; Prokop, L.J.; Murad, M.H.; Baum, C.L. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2016, 152, 419–428. [Google Scholar] [CrossRef]
- Eigentler, T.K.; Leiter, U.; Häfner, H.-M.; Garbe, C.; Röcken, M.; Breuninger, H. Survival of Patients with Cutaneous Squamous Cell Carcinoma: Results of a Prospective Cohort Study. J. Investig. Dermatol. 2017, 137, 2309–2315. [Google Scholar] [CrossRef]
- World Health Organisation Archived: WHO Timeline—COVID-19. Available online: https://www.who.int/news/item/27-04-2020-who-timeline---covid-19 (accessed on 17 May 2023).
- World Health Organisation WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 17 May 2023).
- Ministarstvo Zdravlja Republike Srbije COVID-19-HOMEPAGE. Available online: https://covid19.rs/homepage-english/ (accessed on 17 May 2023).
- Vlada Republike Srbije Odluka o Ukidanju Vanrednog Stanja: 65/2020-4. Available online: https://www.pravno-informacioni-sistem.rs/SlGlasnikPortal/eli/rep/sgrs/skupstina/odluka/2020/65/1/reg (accessed on 17 May 2023).
- Giacalone, S.; Bortoluzzi, P.; Nazzaro, G. Which Are the “Emergent” Dermatologic Practices during COVID-19 Pandemic? Report from the Lockdown in Milan, Italy. Int. J. Dermatol. 2020, 59, e269–e270. [Google Scholar] [CrossRef]
- Aragón-Caqueo, D.; Aedo, G.; Suárez, J.; Toloza, C.; Guglielmetti, A. Impact of the COVID-19 Pandemic on Dermatology Care in the Chilean Public Health Sector. Healthcare 2023, 11, 633. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, J.M.; Yoo, S.H.; Keam, B. Challenges in Care for Non-COVID-19 Patients with Severe Chronic Illnesses during COVID-19 Pandemic: A Qualitative Study of Healthcare Providers Working around Acute Care Hospitals in South Korea. Healthcare 2023, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, G.; Cedrone, F.; Di Giovanni, P.; Romano, F.; Staniscia, T. Impact of COVID-19 Pandemic on Oncological Surgery Activities: A Retrospective Study from a Southern Italian Region. Healthcare 2022, 10, 2329. [Google Scholar] [CrossRef] [PubMed]
- CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)–United States, February 12–March 16, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 343–346. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Khan, M.A.; Amir, M.; Bucheeri, M.; Khan, W.; Khan, I.Z.; Barry, K.M. The Impact of COVID-19 on Emergency General Surgery Admissions and Operative Volumes: A Single Centre Experience. Surgeon 2021, 19, e207–e212. [Google Scholar] [CrossRef]
- Søreide, K.; Hallet, J.; Matthews, J.B.; Schnitzbauer, A.A.; Line, P.D.; Lai, P.B.S.; Otero, J.; Callegaro, D.; Warner, S.G.; Baxter, N.N.; et al. Immediate and Long-Term Impact of the COVID-19 Pandemic on Delivery of Surgical Services: Impact of COVID-19 Pandemic on Delivery of Surgical Services. Br. J. Surg. 2020, 107, 1250–1261. [Google Scholar] [CrossRef]
- de Azambuja, E.; Brandão, M.; Wildiers, H.; Laenen, A.; Aspeslagh, S.; Fontaine, C.; Collignon, J.; Lybaert, W.; Verheezen, J.; Rutten, A.; et al. Impact of Solid Cancer on In-Hospital Mortality Overall and among Different Subgroups of Patients with COVID-19: A Nationwide, Population-Based Analysis. ESMO Open. 2020, 5, e000947. [Google Scholar] [CrossRef] [PubMed]
- Cedrone, F.; Di Martino, G.; Di Giovanni, P.; Greco, E.; Trebbi, E.; Romano, F.; Staniscia, T. Reduction in Hospital Admissions for Cardiovascular Diseases (CVDs) during the Coronavirus Disease 2019 (COVID-19) Pandemic: A Retrospective Study from a Southern Italian Region in the Year 2020. Healthcare 2022, 10, 871. [Google Scholar] [CrossRef]
- De Filippo, O.; D’Ascenzo, F.; Angelini, F.; Bocchino, P.P.; Conrotto, F.; Saglietto, A.; Secco, G.G.; Campo, G.; Gallone, G.; Verardi, R.; et al. Reduced Rate of Hospital Admissions for ACS during COVID-19 Outbreak in Northern Italy. N. Engl. J. Med. 2020, 383, 88–89. [Google Scholar] [CrossRef]
- De Rosa, S.; Spaccarotella, C.; Basso, C.; Calabrò, M.P.; Curcio, A.; Filardi, P.P.; Mancone, M.; Mercuro, G.; Muscoli, S.; Nodari, S.; et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur. Heart J. 2020, 41, 2083–2088. [Google Scholar] [CrossRef]
- Kiss, P.; Carcel, C.; Hockham, C.; Peters, S.A.E. The impact of the COVID-19 pandemic on the care and management of patients with acute cardiovascular disease: A systematic review. Eur. Heart J. Qual. Care Clin. Outcomes 2020, 7, 18–27. [Google Scholar] [CrossRef]
- Jeremić, J.; Suđecki, B.; Radenović, K.; Mihaljević, J.; Radosavljević, I.; Jovanović, M.; Milić, N.; Pavlović, V.; Brašanac, D.; Jović, M. Impact of the COVID-19 Pandemic on Melanoma Diagnosis: Increased Breslow Thickness in Primary Melanomas—A Single Center Experience. Int. J. Environ. Res. Public Health 2022, 19, 16806. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.-J.; Xi, Y.; Sun, C.-Z.; Lei, Q.; Li, L. Effects of the COVID-19 Pandemic on Elderly Patients with Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 966011. [Google Scholar] [CrossRef] [PubMed]
- Sangers, T.E.; Wakkee, M.; Kramer-Noels, E.C.; Nijsten, T.; Louwman, M.W.J.; Jaspars, E.H.; Hollestein, L.M. Limited Impact of COVID-19-Related Diagnostic Delay on Cutaneous Melanoma and Squamous Cell Carcinoma Tumour Characteristics: A Nationwide Pathology Registry Analysis. Br. J. Dermatol. 2022, 187, 196–202. [Google Scholar] [CrossRef]
- Alam, M.; Ratner, D. Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Venables, Z.C.; Autier, P.; Nijsten, T.; Wong, K.F.; Langan, S.M.; Rous, B.; Broggio, J.; Harwood, C.; Henson, K.; Proby, C.M.; et al. Nationwide Incidence of Metastatic Cutaneous Squamous Cell Carcinoma in England. JAMA Dermatol. 2019, 155, 298–306. [Google Scholar] [CrossRef]
- Dash, S.; Saha, S.; Gupta, S.; Singhal, M. Approach to a Patient with Cutaneous Malignancy in the Time of COVID-19 Pandemic. J. Cutan. Aesthetic Surg. 2020, 13, 349–352. [Google Scholar]
- Trakatelli, M.; Barkitzi, K.; Apap, C.; Majewski, S.; De Vries, E.; EPIDERM Group. Skin Cancer Risk in Outdoor Workers: A European Multicenter Case-Control Study. J. Eur. Acad. Dermatol. Venereol. JEADV 2016, 30 (Suppl. S3), 5–11. [Google Scholar] [CrossRef]
- Kang, S.Y.; Toland, A.E. High Risk Cutaneous Squamous Cell Carcinoma of the Head and Neck. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 136–140. [Google Scholar] [CrossRef]
- Fania, L.; Didona, D.; Di Pietro, F.R.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 171. [Google Scholar] [CrossRef]
- Valenti, M.; Pavia, G.; Gargiulo, L.; Facheris, P.; Nucca, O.; Mancini, L.; Sacrini, F.; Borroni, R.G.; Narcisi, A.; Costanzo, A. Impact of Delay in Follow-up Due to COVID-19 Pandemic on Skin Cancer Progression: A Real-Life Experience from an Italian Hub Hospital. Int. J. Dermatol. 2021, 60, 860–863. [Google Scholar] [CrossRef]
- Yélamos, O.; Geller, S.; Tokez, S. Skin Cancer Special Issue in Skin Health and Disease. Skin Health Dis. 2023, 3, e224. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Anker, J.; Ribero, S.; Yélamos, O.; García-Herrera, A.; Alos, L.; Alejo, B.; Combalia, M.; Moreno-Ramírez, D.; Malvehy, J.; Puig, S. Basal Cell Carcinoma Characterization Using Fusion Ex Vivo Confocal Microscopy: A Promising Change in Conventional Skin Histopathology. Br. J. Dermatol. 2020, 182, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Renzi, C.; Mastroeni, S.; Passarelli, F.; Mannooranparampil, T.J.; Caggiati, A.; Potenza, C.; Pasquini, P. Factors Associated with Large Cutaneous Squamous Cell Carcinomas. J. Am. Acad. Dermatol. 2010, 63, 404–411. [Google Scholar] [CrossRef]
- Perez, M.; Abisaad, J.A.; Rojas, K.D.; Marchetti, M.A.; Jaimes, N. Skin Cancer: Primary, Secondary, and Tertiary Prevention. Part I. J. Am. Acad. Dermatol. 2022, 87, 255–268. [Google Scholar] [CrossRef]
- Tejera-Vaquerizo, A.; Paradela, S.; Toll, A.; Santos-Juanes, J.; Jaka, A.; López, A.; Cañueto, J.; Bernal, À.; Villegas-Romero, I.; Ferrándiz-Pulido, C.; et al. Effects of COVID-19 Lockdown on Tumour Burden of Melanoma and Cutaneous Squamous Cell Carcinoma. Acta Derm. Venereol. 2021, 101, adv00525. [Google Scholar] [CrossRef]
- Rashid, S.; Tsao, H. Effect of the COVID-19 Pandemic on Delayed Skin Cancer Services. Dermatol. Clin. 2021, 39, 627–637. [Google Scholar] [CrossRef]
- Nolan, G.S.; Dunne, J.A.; Kiely, A.L.; Pritchard Jones, R.O.; Gardiner, M.; Jain, A. The Effect of the COVID-19 Pandemic on Skin Cancer Surgery in the United Kingdom: A National, Multi-Centre, Prospective Cohort Study and Survey of Plastic Surgeons. Br. J. Surg. 2020, 107, e598–e600. [Google Scholar]
- McClean, A.; Matteucci, P.; Totty, J. The Impact of COVID19 on the Presentation, Diagnosis and Management of Cutaneous Melanoma and Squamous Cell Carcinoma in a Single Tertiary Referral Centre. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2022, 75, 2831–2870. [Google Scholar] [CrossRef]
- Dawkins, B.; Renwick, C.; Ensor, T.; Shinkins, B.; Jayne, D.; Meads, D. What Factors Affect Patients’ Ability to Access Healthcare? An Overview of Systematic Reviews. Trop. Med. Int. Health TM IH 2021, 26, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
| Total | Pre-Pandemic n = 219 | Pandemic n = 306 | Post-Pandemic n = 176 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Capital n = 176 | Non-Capital n = 43 | p Value | Capital n = 254 | Non-Capital n = 52 | p Value | Capital n = 145 | Non-Capital n = 31 | p Value | ||
| Age, mean ± SD | 75.9 ± 10.2 | 76.2 ± 10.8 | 75.16 ± 8.8 | 0.533 | 76.0 ± 9.6 | 76.2 ± 8.7 | 0.933 | 76.6 ± 10.6 | 70.5 ± 12.3 | 0.005 |
| Sex, n* (%) | 0.006 | 0.007 | 0.935 | |||||||
| Male | 428 (61.1%) | 106 (60.2%) | 16 (37.2%) | 172 (67.7%) | 25 (48.1%) | 90 (62.1%) | 19 (61.3%) | |||
| Female | 273 (38.9%) | 70 (39.8%) | 27 (62.8%) | 82 (32.3%) | 27 (51.9%) | 55 (37.9%) | 12 (38.7%) | |||
| First procedure on lesion, n (%) | 0.586 | 1.000 | 0.660 | |||||||
| Yes | 677(96.6%) | 173 (98.3%) | 42 (97.7%) | 245 (96.5%) | 50 (96.2%) | 138 (95.2%) | 29 (93.5%) | |||
| No | 24(3.4%) | 3 (1.7%) | 1 (2.3%) | 9 (3.5%) | 2 (3.8%) | 7 (4.8%) | 2 (6.5%) | |||
| Lesions per patient, n* (%) | 0.016 | 0.485 | 0.316 | |||||||
| One | 519 (86.9%) | 147 (90.7%) | 23 (74.2%) | 184 (85.2%) | 41 (89.1%) | 104 (88.9%) | 20 (80.0%) | |||
| More | 78 (13.1%) | 15 (9.3%) | 3 (25.8%) | 32 (14.8%) | 5 (10.9%) | 13 (11.1%) | 5 (20.0%) | |||
| Body localization, n (%) | 0.010 | 0.399 | 0.184 | |||||||
| Head and Neck | 517 (73.7%) | 121 (68.8%) | 38 (88.4%) | 186 (73.2%) | 41 (78.8%) | 105 (72.4%) | 26 (83.9%) | |||
| Other regions | 184 (26.3%) | 55 (31.3%) | 5 (11.6%) | 68 (26.8%) | 11 (21.2%) | 40 (27.6%) | 5 (16.1%) | |||
| Tumor thickness, median (range) | 4.0 (2–41) | 3 (0.2–25) | 6 (0.5–20) | 0.002 | 4 (0.5–40) | 3.5 (0.5–25) | 0.362 | 3 (0.5–41) | 3 (0.5–24) | 0.314 |
| Disease form, n (%) | 0.084 | 0.176 | 0.022 | |||||||
| Morbus Bowen | 198 (28.2%) | 61 (34.7%) | 9 (20.9%) | 72 (28.3%) | 10 (19.2%) | 43 (29.7%) | 3 (9.7%) | |||
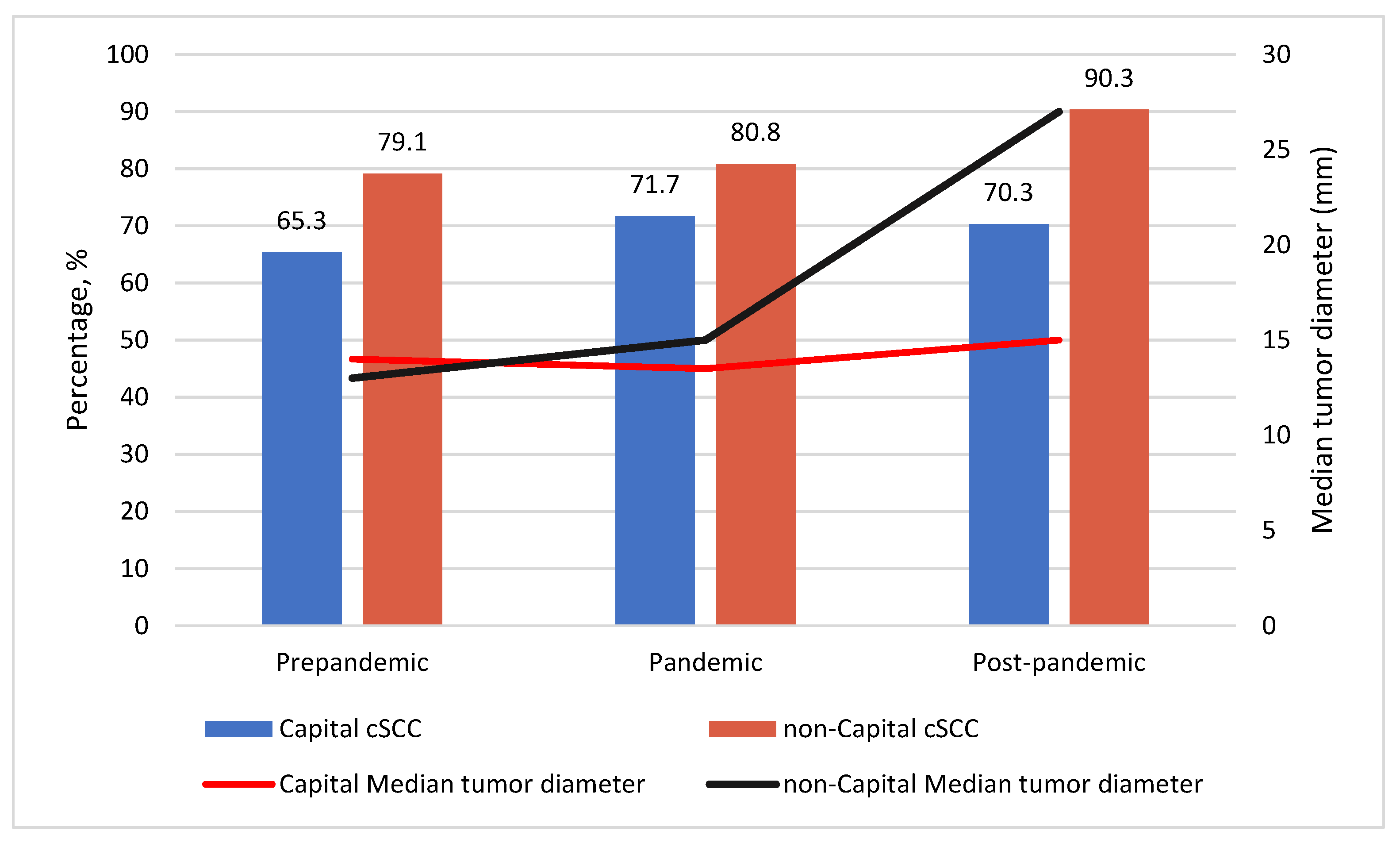
| Invasive | 503 (71.8%) | 115 (35.3%) | 34 (79.1%) | 182 (71.7%) | 42 (80.8%) | 102 (70.3%) | 28 (90.3%) | |||
| Histology grade, n** (%) | 1.000 | 0.528 | 0.418 | |||||||
| G1 | 229 (46.5%) | 52 (45.2%) | 14 (45.2%) | 84 (46.2%) | 21 (50.0%) | 45 (46.9%) | 13 (50.0%) | |||
| G2 | 185 (37.6%) | 38 (33.0%) | 11 (35.3%) | 68 (37.4%) | 16 (38.1%) | 39 (40.6%) | 13 (50.0%) | |||
| G3 | 48 (9.8%) | 20 (17.4%) | 3 (9.7%) | 17 (9.3%) | 3 (7.1%) | 5 (5.2%) | 0 (0.0%) | |||
| G4 | 30 (6.1%) | 5 (4.3%) | 3 (9.7%) | 13 (7.1%) | 2 (4.8%) | 7 (7.3%) | 0 (0.0%) | |||
| Invasion depth layer, n*** (%) | 0.097 | 0.183 | 0.103 | |||||||
| In situ | 200 (29.3%) | 62 (36.5%) | 10 (23.8%) | 71 (28.2%) | 11 (21.6%) | 43 (31.2%) | 3 (10.3%) | |||
| Papillary dermis | 58 (8.5%) | 16 (9.4%) | 4 (9.5%) | 24 (9.5%) | 4 (7.8%) | 6 (4.3%) | 4 (13.8%) | |||
| Reticular dermis | 274 (40.2%) | 59 (34.7%) | 17 (40.5%) | 101 (40.1%) | 22 (43.1%) | 62 (44.9%) | 13 (44.8%) | |||
| Subcutaneous tissue | 86 (12.6%) | 20 (11.8%) | 5 (11.9%) | 37 (14.7%) | 6 (11.8%) | 11 (8.0%) | 7 (24.1%) | |||
| Muscles | 64 (9.4%) | 13 (7.6%) | 6 (14.3%) | 19 (7.5%) | 8 (15.7%) | 16 (11.6%) | 2 (6.9%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jović, M.; Marinković, M.; Suđecki, B.; Jurišić, M.; Bukumirić, Z.; Jovanović, M.; Stojičić, M.; Jeremić, J. COVID-19 and Cutaneous Squamous Cell Carcinoma—Impact of the Pandemic on Unequal Access to Healthcare. Healthcare 2023, 11, 1994. https://doi.org/10.3390/healthcare11141994
Jović M, Marinković M, Suđecki B, Jurišić M, Bukumirić Z, Jovanović M, Stojičić M, Jeremić J. COVID-19 and Cutaneous Squamous Cell Carcinoma—Impact of the Pandemic on Unequal Access to Healthcare. Healthcare. 2023; 11(14):1994. https://doi.org/10.3390/healthcare11141994
Chicago/Turabian StyleJović, Marko, Milana Marinković, Branko Suđecki, Milana Jurišić, Zoran Bukumirić, Milan Jovanović, Milan Stojičić, and Jelena Jeremić. 2023. "COVID-19 and Cutaneous Squamous Cell Carcinoma—Impact of the Pandemic on Unequal Access to Healthcare" Healthcare 11, no. 14: 1994. https://doi.org/10.3390/healthcare11141994
APA StyleJović, M., Marinković, M., Suđecki, B., Jurišić, M., Bukumirić, Z., Jovanović, M., Stojičić, M., & Jeremić, J. (2023). COVID-19 and Cutaneous Squamous Cell Carcinoma—Impact of the Pandemic on Unequal Access to Healthcare. Healthcare, 11(14), 1994. https://doi.org/10.3390/healthcare11141994








