Role of First Trimester Screening Biochemical Markers to Predict Hypertensive Pregnancy Disorders and SGA Neonates—A Narrative Review
Abstract
:1. Introduction
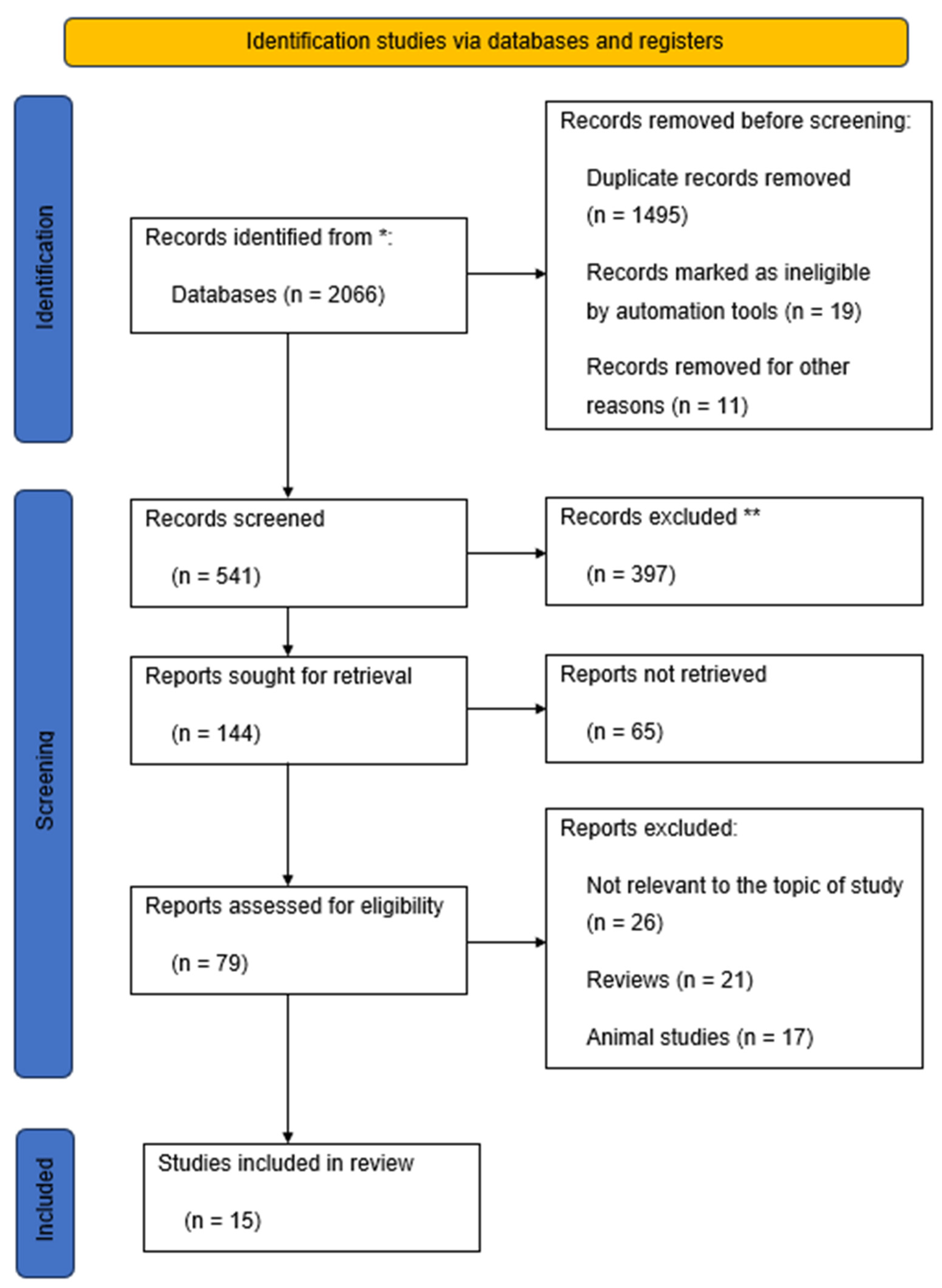
2. Materials and Methods
2.1. Study Design
2.2. Selection Process
2.3. Variables and Measurement
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riise, H.K.R.; Sulo, G.; Tell, G.S.; Igland, J.; Nygård, O.; Iversen, A.; Daltveit, A.K. Association Between Gestational Hypertension and Risk of Cardiovascular Disease Among 617 589 Norwegian Women. J. Am. Heart Assoc. 2018, 7, e008337. [Google Scholar] [CrossRef] [PubMed]
- Berhe, A.K.; Ilesanmi, A.O.; Aimakhu, C.O.; Mulugeta, A. Effect of pregnancy induced hypertension on adverse perinatal outcomes in Tigray regional state, Ethiopia: A prospective cohort study. BMC Pregnancy Childbirth 2019, 20, 7. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B. The Influence of Various Smoking Categories on The Risk of Gestational Hypertension and Pre-Eclampsia. J. Clin. Med. 2020, 9, 1743. [Google Scholar] [CrossRef]
- Bao, X.; Huo, G.; Li, L.; Cao, X.; Liu, Y.; Lakshmipriya, T.; Chen, Y.; Hariri, F.; Gopinath, S.C.B. Coordinated Dispersion and Aggregation of Gold Nanorod in Aptamer-Mediated Gestational Hypertension Analysis. J. Anal. Methods Chem. 2019, 2019, e5676159. [Google Scholar] [CrossRef]
- Naruse, K.; Shigemi, D.; Hashiguchi, M.; Imamura, M.; Yasunaga, H.; Arai, T.; Yasuhi, I.; Ozaki, Y.; Sakajo, A.; Tajima, A.; et al. Placental abruption in each hypertensive disorders of pregnancy phenotype: A retrospective cohort study using a national inpatient database in Japan. Hypertens. Res. 2021, 44, 232–238. [Google Scholar] [CrossRef]
- Shaarawy, M.; Abdel-Magid, A.-M.A. Plasma endothelin-1 and mean arterial pressure in the prediction of pre-eclampsia. Int. J. Gynecol. Obstet. 2020, 68, 105–111. [Google Scholar] [CrossRef]
- Liu, S.; Li, W.; Zhang, J.; Qi, L.; Dong, Y.; Fu, L.; Li, Y. Clinical value of flow-mediated dilatation of brachial artery in hypertensive disorders complicating pregnancy. Clin. Hemorheol. Microcirc. 2022, 82, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Hayward, R.M.; Foster, E.; Tseng, Z.H. Maternal and Fetal Outcomes of Admission for Delivery in Women with Congenital Heart Disease. JAMA Cardiol. 2017, 2, 664–671. [Google Scholar] [CrossRef]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. In Hypertension: From Basic Research to Clinical Practice; Islam, M.S., Ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; Volume 956. [Google Scholar] [CrossRef]
- Liu, Y.; Li, N.; An, H.; Li, Z.; Zhang, L.; Li, H.; Zhang, Y.; Ye, R. Impact of gestational hypertension and preeclampsia on low birthweight and small-for-gestational-age infants in China: A large prospective cohort study. J. Clin. Hypertens. 2021, 23, 835–842. [Google Scholar] [CrossRef]
- Shah, D.A.; Khalil, R.A. Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochem. Pharmacol. 2015, 95, 211–226. [Google Scholar] [CrossRef]
- Kornacki, J.; Gutaj, P.; Kalantarova, A.; Sibiak, R.; Jankowski, M.; Wender-Ozegowska, E. Endothelial Dysfunction in Pregnancy Complications. Biomedicines 2021, 9, 1756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, L.; Li, W.; Chen, Q.; Lei, J.; Long, M.; Yang, W.; Li, W.; Zeng, L.; Zeng, S. Early prediction of preeclampsia and small-for-gestational-age via multi-marker model in Chinese pregnancies: A prospective screening study. BMC Pregnancy Childbirth 2019, 19, 304. [Google Scholar] [CrossRef]
- Erkamp, J.S.; Jaddoe, V.W.; Duijts, L.; Reiss, I.K.; Mulders, A.G.; Steegers, E.A.; Gaillard, R. Population screening for gestational hypertensive disorders using maternal, fetal and placental characteristics: A population-based prospective cohort study. Prenat. Diagn. 2020, 40, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Kotlabova, K.; Krofta, L. First-Trimester Screening for Fetal Growth Restriction and Small-for-Gestational-Age Pregnancies without Preeclampsia Using Cardiovascular Disease-Associated MicroRNA Biomarkers. Biomedicines 2022, 10, 718. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Nicolaides, K.H.; Poon, L.C. Prevention of preeclampsia with aspirin. Am. J. Obstet. Gynecol. 2022, 226, S1108–S1119. [Google Scholar] [CrossRef]
- Magee, L.A.; Smith, G.N.; Bloch, C.; Côté, A.-M.; Jain, V.; Nerenberg, K.; von Dadelszen, P.; Helewa, M.; Rey, E. Guideline No. 426: Hypertensive Disorders of Pregnancy: Diagnosis, Prediction, Prevention, and Management. J. Obstet. Gynaecol. Can. 2022, 44, 547–571.e1. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Screening for Preeclampsia: US Preventive Services Task Force Recommendation Statement. JAMA 2017, 317, 1661–1667. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet. Gynecol. 2020, 135, 1492–1495. [Google Scholar] [CrossRef]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynecol. Obstet. 2019, 145, 1–33. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef]
- Lowe, S.A.; Bowyer, L.; Lust, K.; McMahon, L.P.; Morton, M.; North, R.A.; Paech, M.; Said, J.M. SOMANZ guidelines for the management of hypertensive disorders of pregnancy. Aust. N. Z. J. Obstet. Gynaecol. 2014, 55, e1–e29. [Google Scholar] [CrossRef] [PubMed]
- Sotiriadis, A.; Hernandez-Andrade, E.; da Silva Costa, F.; Ghi, T.; Glanc, P.; Khalil, A.; Martins, W.P.; Odibo, A.O.; Papageorghiou, A.T.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Role of ultrasound in screening for and follow-up of pre-eclampsia. Ultrasound Obstet. Gynecol. 2019, 53, 7–22. [Google Scholar] [CrossRef]
- NICE. Hypertension in Pregnancy: Diagnosis and Management. NICE guideline (NG133). Available online: www.nice.org.uk/guidance/ng133 (accessed on 8 June 2023).
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Borowski, D.; Pietryga, M.; Basta, P.; Cnota, W.; Czuba, B.; Dubiel, M.; Fuchs, T.; Huras, H.; Iciek, R.; Jaczynska, R.; et al. Practice guidelines of the Polish Society of Gynecologists and Obstetricians—Ultrasound Section for ultrasound screening in uncomplicated pregnancy. Ginekol. Pol. 2020, 91, 490–501. [Google Scholar] [CrossRef]
- Roberge, S.; Bujold, E.; Nicolaides, K.H. Aspirin for the prevention of preterm and term preeclampsia: Systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2018, 218, 287–293.e1. [Google Scholar] [CrossRef]
- Caron, N.; Rivard, G.É.; Michon, N.; Morin, F.; Pilon, D.; Moutquin, J.-M.; Rey, É. Low-dose ASA Response Using the PFA-100 in Women with High-risk Pregnancy. J. Obstet. Gynaecol. Can. 2009, 31, 1022–1027. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Syngelaki, A.; Ashoor, G.; Birdir, C.; Touzet, G. Noninvasive prenatal testing for fetal trisomies in a routinely screened first-trimester population. Am. J. Obstet. Gynecol. 2012, 207, 374.e1–374.e6. [Google Scholar] [CrossRef]
- Belovic, D.K.; Plešinac, S.; Dotlić, J.; Radojević, A.S.; Akšam, S.; Cvjetićanin, M.M.; Kocijančić, A. Biochemical Markers for Prediction of Hypertensive Disorders of Pregnancy. J. Med. Biochem. 2019, 38, 71–82. [Google Scholar] [CrossRef]
- Poon, L.C.; Nicolaides, K.H. First-trimester maternal factors and biomarker screening for preeclampsia. Prenat. Diagn. 2014, 34, 618–627. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the Management of Cardiovascular Diseases during Pregnancy: The Task Force for the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef]
- Villar, J.; Papageorghiou, A.T.; Pang, R.; Ohuma, E.O.; Ismail, L.C.; Barros, F.C.; Lambert, A.; Carvalho, M.; Jaffer, Y.A.; Bertino, E.; et al. The likeness of fetal growth and newborn size across non-isolated populations in the INTERGROWTH-21st Project: The Fetal Growth Longitudinal Study and Newborn Cross-Sectional Study. Lancet Diabetes Endocrinol. 2014, 2, 781–792. [Google Scholar] [CrossRef]
- Victora, C.G.; Villar, J.; Barros, F.C.; Ismail, L.C.; Chumlea, C.; Papageorghiou, A.T.; Bertino, E.; Ohuma, E.O.; Lambert, A.; Carvalho, M.; et al. Anthropometric Characterization of Impaired Fetal Growth: Risk Factors for and Prognosis of Newborns With Stunting or Wasting. JAMA Pediatr. 2015, 169, e151431. [Google Scholar] [CrossRef]
- Meler, E.; Martinez-Portilla, R.J.; Caradeux, J.; Mazarico, E.; Gil-Armas, C.; Boada, D.; Martinez, J.; Carrillo, P.; Camacho, M.; Figueras, F. Severe smallness as predictor of adverse perinatal outcome in suspected late small-for-gestational-age fetuses: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2022, 60, 328–337. [Google Scholar] [CrossRef]
- Lees, C.; Stampalija, T.; Baschat, A.A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Roeckner, J.T.; Pressman, K.; Odibo, L.; Duncan, J.R.; Odibo, A.O. Outcome-based comparison of SMFM and ISUOG definitions of fetal growth restriction. Ultrasound Obstet. Gynecol. 2021, 57, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Honarjoo, M.; Zarean, E.; Tarrahi, M.J.; Kohan, S. Role of pregnancy-associated plasma protein A (PAPP-A) and human-derived chorionic gonadotrophic hormone (free β-hCG) serum levels as a marker in predicting of Small for gestational age (SGA): A cohort study. J. Res. Med. Sci. 2021, 26, 104. [Google Scholar] [CrossRef]
- Allen, R.; Aquilina, J. Prospective observational study to determine the accuracy of first-trimester serum biomarkers and uterine artery Dopplers in combination with maternal characteristics and arteriography for the prediction of women at risk of preeclampsia and other adverse pregnancy outcomes. J. Matern. Neonatal Med. 2018, 31, 2789–2806. [Google Scholar] [CrossRef]
- Hendrix, M.L.E.; Bons, J.A.P.; Snellings, R.R.G.; Bekers, O.; van Kuijk, S.M.J.; Spaanderman, M.E.A.; Al-Nasiry, S. Can Fetal Growth Velocity and First Trimester Maternal Biomarkers Improve the Prediction of Small-for-Gestational Age and Adverse Neonatal Outcome? Fetal Diagn. Ther. 2019, 46, 274–284. [Google Scholar] [CrossRef]
- Papastefanou, I.; Wright, D.; Lolos, M.; Anampousi, K.; Mamalis, M.; Nicolaides, K.H. Competing-risks model for prediction of small-for-gestational-age neonate from maternal characteristics, serum pregnancy-associated plasma protein-A and placental growth factor at 11–13 weeks’ gestation. Ultrasound Obstet. Gynecol. 2021, 57, 392–400. [Google Scholar] [CrossRef]
- Boutin, A.; Gasse, C.; Demers, S.; Blanchet, G.; Giguère, Y.; Bujold, E. Does Low PAPP-A Predict Adverse Placenta-Mediated Outcomes in a Low-Risk Nulliparous Population? the Great Obstetrical Syndromes (GOS) Study. J. Obstet. Gynaecol. Can. 2018, 40, 663–668. [Google Scholar] [CrossRef]
- Sharma, K.; Singh, R.; Kumar, M.; Gupta, U.; Rohil, V.; Bhattacharjee, J. First-Trimester Inflammatory Markers for Risk Evaluation of Pregnancy Hypertension. J. Obstet. Gynecol. India 2018, 68, 27–32. [Google Scholar] [CrossRef]
- Genc, S.; Ozer, H.; Emeklioglu, C.N.; Cingillioglu, B.; Sahin, O.; Akturk, E.; Sirinoglu, H.A.; Basaran, N.; Mihmanli, V. Relationship between extreme values of first trimester maternal pregnancy associated plasma Protein-A, free-β-human chorionic gonadotropin, nuchal translucency and adverse pregnancy outcomes. Taiwan. J. Obstet. Gynecol. 2022, 61, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, L.; Wang, X.; Liu, Y. Association between serum beta-human chorionic gonadotropin and inflammation, oxidative stress in pregnancy-induced hypertension. Microvasc. Res. 2021, 135, 104130. [Google Scholar] [CrossRef]
- Elazab, R.; Alkhiary, M.; Bedairi, M.; Wageh, A. Simultaneous use of Tumor Necrosis Factor, Lipid Profile, and β-hCG As Markers of Severity of Preeclampsia. J. Obstet. Gynecol. India 2022, 72, 83–88. [Google Scholar] [CrossRef]
- Khanam, Z.; Mittal, P.; Suri, J. Does the Addition of Serum PAPP-A and β-hCG Improve the Predictive Value of Uterine Artery Pulsatility Index for Preeclampsia at 11–14 Weeks of Gestation? A Prospective Observational Study. J. Obstet. Gynecol. India 2021, 71, 226–234. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Sarno, M.; Wright, A. Ophthalmic artery Doppler in the prediction of preeclampsia. Am. J. Obstet. Gynecol. 2021, 226, S1098–S1101. [Google Scholar] [CrossRef]
- Zumaeta, A.M.; Wright, A.; Syngelaki, A.; Maritsa, V.A.; Da Silva, A.B.; Nicolaides, K.H. Screening for pre-eclampsia at 11–13 weeks’ gestation: Use of pregnancy-associated plasma protein-A, placental growth factor or both. Ultrasound Obstet. Gynecol. 2020, 56, 400–407. [Google Scholar] [CrossRef]
- Tang, Z.; Ji, Y.; Zhou, S.; Su, T.; Yuan, Z.; Han, N.; Jia, J.; Wang, H. Development and Validation of Multi-Stage Prediction Models for Pre-eclampsia: A Retrospective Cohort Study on Chinese Women. Front. Public Health 2022, 10, 911975. [Google Scholar] [CrossRef]
- Duan, Z.; Li, C.; Leung, W.T.; Wu, J.; Wang, M.; Ying, C.; Wang, L. Alterations of Several Serum Parameters Are Associated with Preeclampsia and May Be Potential Markers for the Assessment of PE Severity. Dis. Markers 2020, 2020, e7815214. [Google Scholar] [CrossRef]
- ACOG Committee on Obstetric Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int. J. Gynaecol. Obstet. 2002, 77, 67–75. [Google Scholar]
- Piani, F.; Agnoletti, D.; Baracchi, A.; Scarduelli, S.; Verde, C.; Tossetta, G.; Montaguti, E.; Simonazzi, G.; Degli Esposti, D.; Borghi, C.; et al. Serum uric acid to creatinine ratio and risk of preeclampsia and adverse pregnancy outcomes. J. Hypertens. 2023, 41, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, A.; Gaedechens, D.; Ramírez, V.; Zuñiga, E.; Kusanovic, J.P.; Inostroza, C.; Varas-Godoy, M.; Silva, K.; Salomon, C.; Rice, G.; et al. Placental biomarkers and angiogenic factors in oral fluids of patients with preeclampsia. Prenat. Diagn. 2016, 36, 476–482. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górczewski, W.; Górecka, J.; Massalska-Wolska, M.; Staśkiewicz, M.; Borowski, D.; Huras, H.; Rybak-Krzyszkowska, M. Role of First Trimester Screening Biochemical Markers to Predict Hypertensive Pregnancy Disorders and SGA Neonates—A Narrative Review. Healthcare 2023, 11, 2454. https://doi.org/10.3390/healthcare11172454
Górczewski W, Górecka J, Massalska-Wolska M, Staśkiewicz M, Borowski D, Huras H, Rybak-Krzyszkowska M. Role of First Trimester Screening Biochemical Markers to Predict Hypertensive Pregnancy Disorders and SGA Neonates—A Narrative Review. Healthcare. 2023; 11(17):2454. https://doi.org/10.3390/healthcare11172454
Chicago/Turabian StyleGórczewski, Wojciech, Joanna Górecka, Magdalena Massalska-Wolska, Magdalena Staśkiewicz, Dariusz Borowski, Hubert Huras, and Magda Rybak-Krzyszkowska. 2023. "Role of First Trimester Screening Biochemical Markers to Predict Hypertensive Pregnancy Disorders and SGA Neonates—A Narrative Review" Healthcare 11, no. 17: 2454. https://doi.org/10.3390/healthcare11172454
APA StyleGórczewski, W., Górecka, J., Massalska-Wolska, M., Staśkiewicz, M., Borowski, D., Huras, H., & Rybak-Krzyszkowska, M. (2023). Role of First Trimester Screening Biochemical Markers to Predict Hypertensive Pregnancy Disorders and SGA Neonates—A Narrative Review. Healthcare, 11(17), 2454. https://doi.org/10.3390/healthcare11172454





