Exploring Gut Microbiota and the Influence of Physical Activity Interventions on Overweight and Obese Children and Adolescents: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias
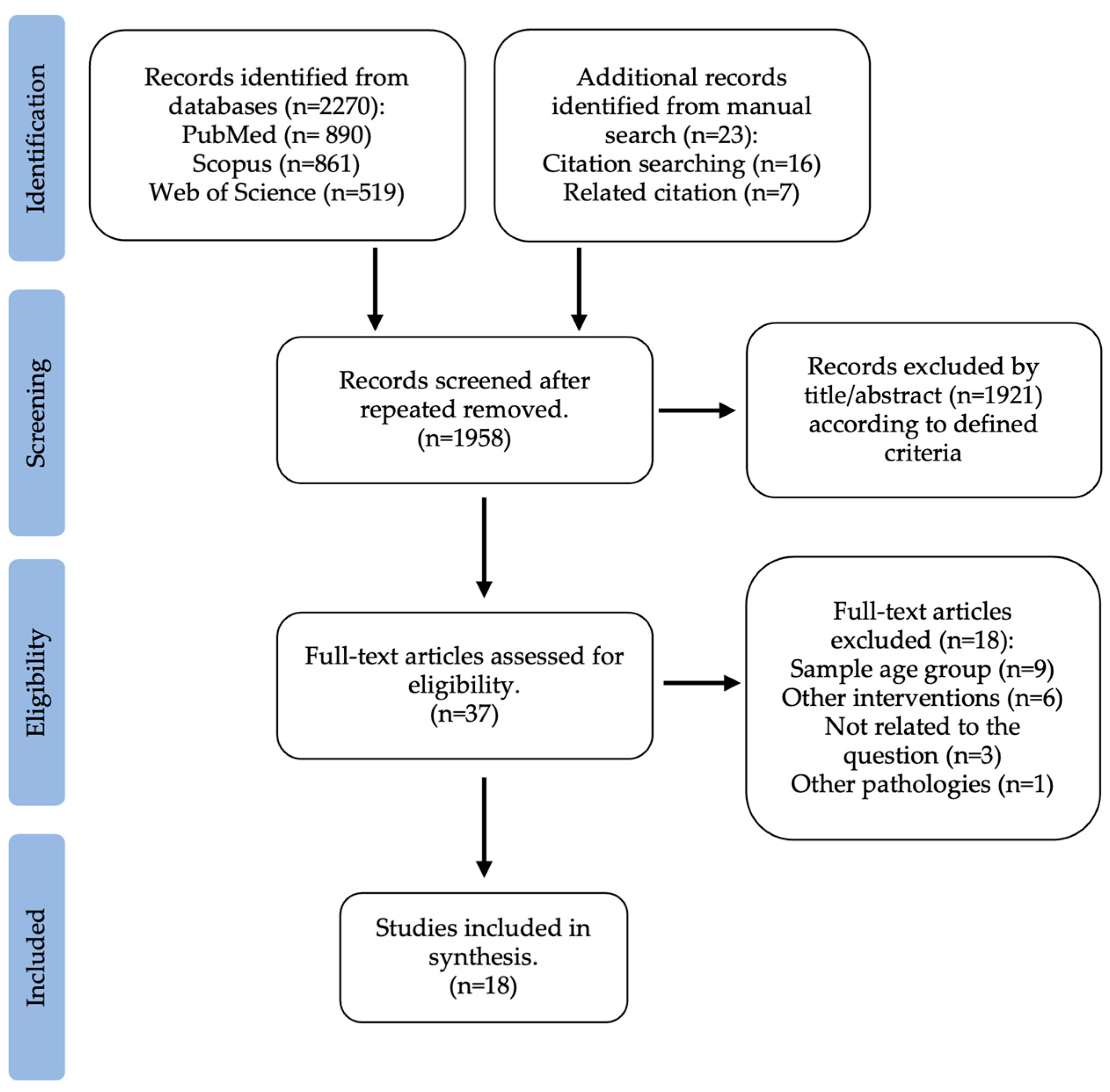
3. Results
3.1. Study Selection and Study Characteristics
3.1.1. Gut Microbiota of Children with Overweight or Obesity
3.1.2. Physical Activity Impact in Children’ and Adolescents Gut Microbiota
3.2. Risk of Bias
3.3. Gut Microbiota Profiles in Overweight and Obese Children
3.4. Physical Activity Interventions on Gut Microbiota in Children and Adolescents with Overweight and Obesity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128.9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. WHO European Regional Obesity Report 2022; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Dietz, W.H. Overweight in Childhood and Adolescence. N. Engl. J. Med. 2004, 350, 855–857. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. WHO European Childhood Obesity Surveillance Initiative (COSI): Report. on the Fourth Round of Data Collection, 2015–2017; WHO Regional Office for Europe: Copenhagen, Denmark, 2021. [Google Scholar]
- Petraroli, M.; Castellone, E.; Patianna, V.; Esposito, S. Gut Microbiota and Obesity in Adults and Children: The State of the Art. Front. Pediatr. 2021, 9, 657020. [Google Scholar] [CrossRef]
- Ash, C.; Mueller, K. Manipulating the Microbiota. Science 2016, 352, 530–531. [Google Scholar] [CrossRef] [PubMed]
- Turroni, S.; Brigidi, P.; Cavalli, A.; Candela, M. Microbiota–Host Transgenomic Metabolism, Bioactive Molecules from the Inside. J. Med. Chem. 2017, 61, 47–61. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human Gut Colonisation May Be Initiated in Utero by Distinct Microbial Communities in the Placenta and Amniotic Fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- de Cuevillas, B.; Milagro, F.I.; Tur, J.A.; Gil-Campos, M.; de Miguel-Etayo, P.; Martínez, J.A.; Navas-Carretero, S. Fecal Microbiota Relationships with Childhood Obesity: A Scoping Comprehensive Review. Obes. Rev. 2022, 23 (Suppl. 1), e13394. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Wang, X.; Zhang, X. Physical Exercise and Diet: Regulation of Gut Microbiota to Prevent and Treat Metabolic Disorders to Maintain Health. Nutrients 2023, 15, 1539. [Google Scholar] [CrossRef] [PubMed]
- Castaner, O.; Goday, A.; Park, Y.M.; Lee, S.H.; Magkos, F.; Shiow, S.A.T.E.; Schröder, H. The Gut Microbiome Profile in Obesity: A Systematic Review. Int. J. Endocrinol. 2018, 2018, 4095789. [Google Scholar] [CrossRef]
- Chen, X.; Sun, H.; Jiang, F.; Shen, Y.; Li, X.; Hu, X.; Shen, X.; Wei, P. Alteration of the Gut Microbiota Associated with Childhood Obesity by 16S RRNA Gene Sequencing. PeerJ 2020, 8, e8317. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Pérez, M.; Pérez-Santiago, J.D.; Tornero-Aguilera, J.F.; González-Soltero, R.; Larrosa, M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Front. Physiol. 2016, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.C.S.; de Silva Coelho, P.M.B.; Estevan, M.d.C.L. Does Microbiota Influence the Risk of Childhood Obesity? Rev. Esp. Nutr. Hum. Diet. 2018, 22, 157–168. [Google Scholar] [CrossRef]
- Cho, K.Y. Lifestyle Modifications Result in Alterations in the Gut Microbiota in Obese Children. BMC Microbiol. 2021, 21, 10. [Google Scholar] [CrossRef]
- Pedersini, P.; Turroni, S.; Villafañe, J.H. Gut Microbiota and Physical Activity: Is There an Evidence-Based Link? Sci. Total Environ. 2020, 727, 138648. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise Training Modulates the Gut Microbiota Profile and Impairs Inflammatory Signaling Pathways in Obese Children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Davy, B.M.; Hulver, M.W.; Neilson, A.P.; Bennett, B.J.; Davy, K.P. Does Exercise Alter Gut Microbial Composition? A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 160–167. [Google Scholar] [CrossRef]
- Aragón-Vela, J.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; Álvarez-Mercado, A.I.; Olivares-Arancibia, J.; Plaza-Diaz, J. Impact of Exercise on Gut Microbiota in Obesity. Nutrients 2021, 13, 3999. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Panahi, S.; Tremblay, A. Childhood Obesity: A Role for Gut Microbiota? Int. J. Environ. Res. Public Health 2015, 12, 162. [Google Scholar] [CrossRef]
- Steves, C.J.; Bird, S.; Williams, F.M.; Spector, T.D. The Microbiome and Musculoskeletal Conditions of Aging: A Review of Evidence for Impact and Potential Therapeutics. J. Bone Miner. Res. 2016, 31, 261–269. [Google Scholar] [CrossRef]
- Wijnhoven, T.; van Raaij, J.; Sjöberg, A.; Eldin, N.; Yngve, A.; Kunešová, M.; Starc, G.; Rito, A.; Duleva, V.; Hassapidou, M.; et al. WHO European Childhood Obesity Surveillance Initiative: School Nutrition Environment and Body Mass Index in Primary Schools. Int. J. Environ. Res. Public Health 2014, 11, 11261–11285. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2008, 457, 480–484. [Google Scholar] [CrossRef]
- Khan, M.J.; Gerasimidis, K.; Edwards, C.A.; Shaikh, M.G. Role of Gut Microbiota in the Aetiology of Obesity: Proposed Mechanisms and Review of the Literature. J. Obes. 2016, 2016, 7353642. [Google Scholar] [CrossRef]
- Parekh, P.J.; Balart, L.A.; Johnson, D.A. The Influence of the Gut Microbiome on Obesity, Metabolic Syndrome and Gastrointestinal Disease. Clin. Transl. Gastroenterol. 2015, 6, e91. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ardern, C.L.; Büttner, F.; Andrade, R.; Weir, A.; Ashe, M.C.; Holden, S.; Impellizzeri, F.M.; Delahunt, E.; Dijkstra, H.P.; Mathieson, S.; et al. Implementing the 27 PRISMA 2020 Statement Items for Systematic Reviews in the Sport and Exercise Medicine, Musculoskeletal Rehabilitation and Sports Science Fields: The PERSiST (Implementing Prisma in Exercise, Rehabilitation, Sport Medicine and Sports Science) Guidance. Br. J. Sports Med. 2022, 56, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Kuczmarski, R.J.; Ogden, C.L.; Grummer-Strawn, L.M.; Flegal, K.M.; Guo, S.S.; Wei, R.; Mei, Z.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. CDC Growth Charts: United States. Adv. Data 2000, 314, 1–27. [Google Scholar]
- Higgins, J.; Morgan, R.; Rooney, A.; Taylor, K.; Thayer, K.; Silva, R.; Lemeris, C.; Akl, E.; Arroyave, W.; Bateson, T.; et al. Risk of Bias in Non-Randomized Studies-of Exposure (ROBINS-E); ROBINS-E Development Group. 2023. Available online: https://www.riskofbias.info/welcome/robins-e-tool (accessed on 11 July 2023).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Visuthranukul, C.; Sriswasdi, S.; Tepaamorndech, S.; Joyjinda, Y.; Saengpanit, P.; Kwanbunbumpen, T.; Panichsillaphakit, E.; Uaariyapanichkul, J.; Chomtho, S. Association of Human Intestinal Microbiota with Lifestyle Activity, Adiposity, and Metabolic Profiles in Thai Children with Obesity. J. Nutr. Metab. 2022, 2022, 3029582. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Sun, H.; Jiang, F.; Shen, Y.; Wei, P.; Shen, X. Characterization of the Gut Microbiota in Chinese Children with Overweight and Obesity Using 16S RRNA Gene Sequencing. PeerJ 2021, 9, e11439. [Google Scholar] [CrossRef]
- Golloso-Gubat, M.J.; Ducarmon, Q.R.; Tan, R.C.A.; Zwittink, R.D.; Kuijper, E.J.; Nacis, J.S.; Santos, N.L.C. Gut Microbiota and Dietary Intake of Normal-Weight and Overweight Filipino Children. Microorganisms 2020, 8, 1015. [Google Scholar] [CrossRef]
- Shin, S.; Cho, K.Y. Altered Gut Microbiota and Shift in Bacteroidetes between Young Obese and Normal-Weight Korean Children: A Cross-Sectional Observational Study. Biomed Res. Int. 2020, 2020, 6587136. [Google Scholar] [CrossRef]
- Gao, X.; Jia, R.; Xie, L.; Kuang, L.; Feng, L.; Wan, C. A Study of the Correlation between Obesity and Intestinal Flora in School-Age Children. Sci. Rep. 2018, 8, 14511. [Google Scholar] [CrossRef]
- Hou, Y.P.; He, Q.Q.; Ouyang, H.M.; Peng, H.S.; Wang, Q.; Li, J.; Lv, X.F.; Zheng, Y.N.; Li, S.C.; Liu, H.L.; et al. Human Gut Microbiota Associated with Obesity in Chinese Children and Adolescents. Biomed Res. Int. 2017, 2017, 7585989. [Google Scholar] [CrossRef]
- Gao, X.; Jia, R.; Xie, L.; Kuang, L.; Feng, L.; Wan, C. Obesity in School-Aged Children and Its Correlation with Gut E. coli and Bifidobacteria: A Case-Control Study. BMC Pediatr. 2015, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Li, M.; Zhang, J.; Zhang, T. Correlation of Intestinal Microbiota with Overweight and Obesity in Kazakh School Children. BMC Microbiol. 2012, 12, 283. [Google Scholar] [CrossRef]
- Murga-Garrido, S.M.; Orbe-Orihuela, Y.C.; Díaz-Benítez, C.E.; Castañeda-Márquez, A.C.; Cornejo-Granados, F.; Ochoa-Leyva, A.; Sanchez-Flores, A.; Cruz, M.; Burguete-García, A.I.; Lagunas-Martínez, A. Alterations of the Gut Microbiome Associated to Methane Metabolism in Mexican Children with Obesity. Children 2022, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- López-Contreras, B.E.; Morán-Ramos, S.; Villarruel-Vázquez, R.; Macías-Kauffer, L.; Villamil-Ramírez, H.; León-Mimila, P.; Vega-Badillo, J.; Sánchez-Muñoz, F.; Llanos-Moreno, L.E.; Canizalez-Román, A.; et al. Composition of Gut Microbiota in Obese and Normal-Weight Mexican School-Age Children and Its Association with Metabolic Traits. Pediatr. Obes. 2018, 13, 381–388. [Google Scholar] [CrossRef]
- Méndez-Salazar, E.O.; Ortiz-López, M.G.; de los Ángeles Granados-Silvestre, M.; Palacios-González, B.; Menjivar, M. Altered Gut Microbiota and Compositional Changes in Firmicutes and Proteobacteria in Mexican Undernourished and Obese Children. Front. Microbiol. 2018, 9, 2494. [Google Scholar] [CrossRef]
- Ignacio, A.; Fernandes, M.R.; Rodrigues, V.A.A.; Groppo, F.C.; Cardoso, A.L.; Avila-Campos, M.J.; Nakano, V. Correlation between Body Mass Index and Faecal Microbiota from Children. Clin. Microbiol. Infect. 2016, 22, 258.e1–258.e8. [Google Scholar] [CrossRef] [PubMed]
- Mbakwa, C.A.; Hermes, G.D.A.; Penders, J.; Savelkoul, P.H.M.; Thijs, C.; Dagnelie, P.C.; Mommers, M.; Zoetendal, E.G.; Smidt, H.; Arts, I.C.W. Gut Microbiota and Body Weight in School-Aged Children: The KOALA Birth Cohort Study. Obesity 2018, 26, 1767–1776. [Google Scholar] [CrossRef]
- Riva, A.; Borgo, F.; Lassandro, C.; Verduci, E.; Morace, G.; Borghi, E.; Berry, D. Pediatric Obesity Is Associated with an Altered Gut Microbiota and Discordant Shifts in Firmicutes Populations. Environ. Microbiol. 2017, 19, 95–105. [Google Scholar] [CrossRef]
- Borgo, F.; Verduci, E.; Riva, A.; Lassandro, C.; Riva, E.; Morace, G.; Borghi, E. Relative Abundance in Bacterial and Fungal Gut Microbes in Obese Children: A Case Control Study. Child. Obes. 2017, 13, 78–84. [Google Scholar] [CrossRef]
- Bervoets, L.; Van Hoorenbeeck, K.; Kortleven, I.; Van Noten, C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in Gut Microbiota Composition between Obese and Lean Children: A Cross-Sectional Study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef]
- Da Silva, C.C.; Monteil, M.A.; Davis, E.M. Overweight and Obesity in Children Are Associated with an Abundance of Firmicutes and Reduction of Bifidobacterium in Their Gastrointestinal Microbiota. Child. Obes. 2020, 16, 204–210. [Google Scholar] [CrossRef]
- Morán-Ramos, S.; Siliceo-Bernardi, M.T.; Villalpando-Carrión, S.; Canizales-Quinteros, S.; Frigolet, M.E.; Gutiérrez-Aguilar, R. Gut Microbiota Composition after a Dietary and Physical Activity Intervention: A Pilot Study in Mexican Children with Obesity. Bol. Med. Hosp. Infant. Mex. 2022, 79, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liao, J.; Fang, Y.; Deng, H.; Yin, H.; Shen, B.; Hu, M. Six-Week Exercise Training With Dietary Restriction Improves Central Hemodynamics Associated With Altered Gut Microbiota in Adolescents With Obesity. Front. Endocrinol. 2020, 11, 569085. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, A.; Marcos, A.; Wärnberg, J.; Martí, A.; Martin-Matillas, M.; Campoy, C.; Moreno, L.A.; Veiga, O.; Redondo-Figuero, C.; Garagorri, J.M.; et al. Interplay between Weight Loss and Gut Microbiota Composition in Overweight Adolescents. Obesity 2009, 17, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Hu, Y.; Bruner, D.W. Composition of Gut Microbiota and Its Association with Body Mass Index and Lifestyle Factors in a Cohort of 7–18 Years Old Children from the American Gut Project. Pediatr. Obes. 2019, 14, e12480. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. In Research Synthesis Methods; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2021; Volume 12, pp. 55–61. [Google Scholar]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO Growth Reference for School-Aged Children and Adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Li, H.; Zong, X.; Ji, C.; Mi, J. Body Mass Index Cut-Offs for Overweight and Obesity in Chinese Children and Adolescents Aged 2–18 Years. Zhonghua Liu Xing Bing Xue Za Zhi 2010, 31, 616–620. [Google Scholar] [PubMed]
- Kim, J.H.; Yun, S.; Hwang, S.S.; Shim, J.O.; Chae, H.W.; Lee, Y.J.; Lee, J.H.; Kim, S.C.; Lim, D.; Yang, S.W.; et al. The 2017 Korean National Growth Charts for Children and Adolescents: Development, Improvement, and Prospects. Korean J. Pediatr. 2018, 61, 135. [Google Scholar] [CrossRef]
- Fredriks, A.M.; Van Buuren, S.; Wit, J.M.; Verloove-Vanhorick, S.P. Body Index Measurements in 1996-7 Compared with 1980. Arch. Dis. Child. 2000, 82, 107–112. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended International (IOTF) Body Mass Index Cut-Offs for Thinness, Overweight and Obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- Meliț, L.E.; Mărginean, C.O.; Săsăran, M.O. The Yin-Yang Concept of Pediatric Obesity and Gut Microbiota. Biomedicines 2022, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Ragab, S.H.; ElBaky, A.A.; Shoeib, A.R.S.; Alhosary, Y.; Fekry, D. Frequency of Firmicutes and Bacteroidetes in Gut Microbiota in Obese and Normal Weight Egyptian Children and Adults. Arch. Med. Sci. 2011, 7, 501. [Google Scholar] [CrossRef]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring Bacterial Community of Human Gut Microbiota Reveals an Increase in Lactobacillus in Obese Patients and Methanogens in Anorexic Patients. PLoS ONE 2009, 4, e7125. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Collado, M.C.; Salminen, S.; Isolauri, E. Early Differences in Fecal Microbiota Composition in Children May Predict Overweight. Am. J. Clin. Nutr. 2008, 87, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Gao, H.; Ren, Q.; He, J. The Abundance of Bifidobacterium in Relation to Visceral Obesity and Serum Uric Acid. Sci. Rep. 2022, 12, 1–7. [Google Scholar] [CrossRef]
- Yan, H.; Qin, Q.; Chen, J.; Yan, S.; Li, T.; Gao, X.; Yang, Y.; Li, A.; Ding, S. Gut Microbiome Alterations in Patients With Visceral Obesity Based on Quantitative Computed Tomography. Front. Cell. Infect. Microbiol. 2022, 11, 823262. [Google Scholar] [CrossRef]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017, 8, 1979. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human Gut Microbiome Viewed across Age and Geography. Nature 2012, 486, 222. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, R.; Zhang, Y.; Lin, X.; Yang, X. Gut Microbiota: Effect of Pubertal Status. BMC Microbiol. 2020, 20, 334. [Google Scholar] [CrossRef] [PubMed]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-Generation Sequencing: Insights to Advance Clinical Investigations of the Microbiome. J. Clin. Investig. 2022, 132, e154944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. eBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome HHS Public Access. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Hughes, R.L. A Review of the Role of the Gut Microbiome in Personalized Sports Nutrition. Front. Nutr. 2019, 6, 191. [Google Scholar] [CrossRef] [PubMed]




| Reference | Country | Sample | Aim | Method of Faecal Microbiota Analysis | Observed Results (Overweight/Obese vs. Normal Weight Children) |
|---|---|---|---|---|---|
| Murga-Garrido et al., 2022 [47] | Mexico | n = 46 Age: 6–12 years Ethnicity: Latino Obese [61]: n = 20 Normal weight: n = 26 | To explore the gut microbiota characteristics, stratified based on their dietary profile and body mass index | Shotgun metagenomics | ↑ Eubacterium sp. and Roseburia sp. ≠ Alpha-diversity ≠ F/B ratio |
| Visuthranukul et al., 2022 [39] | Thailan | n = 164 Age: 7–15 years Ethnicity: Asian Obese [61]: n = 164 Normal weight: n = 45 | To compare the gut microbiota of obese and healthy controls and investigate associations between the microbiome, lifestyle, adiposity, and metabolic profiles | 16S rRNA gene sequencing | ↓ Bacteroidetes and Actinobacteria ↓ Bifidobacterium ↑ Blautia and Lactobacillus ↑ Proteobacteria and Fusobacteria ≠ Alpha-diversity ≠ F/B ratio |
| Chen et al., 2021 [40] | China | n = 100 Age: 6–11 years Ethnicity: Asian Overweight [62]: n = 35 Obese: n = 35 Normal weight: n = 30 | To characterize the gut microbiota in children across different weight categories | 16S rRNA gene sequencing | ↓ species abundance ↓ Bacteroidetes ↓ Actinobacteria and Tenericutes phyla ↑ Aggregatibacter, Fusobacterium, Haemophilus, Megamonas, Prevotella, Sneathia, Sutterella and Veillonella ↑ Proteobacteria and Fusobacteria ≠ F/B ratio |
| Golloso-Gubat et al., 2020 [41] | Philippines | n = 43 Age: 7–11 years Ethnicity: Asian Overweight [61]: n = 11 Normal weight: n = 32 | To examine the variations in the gut microbiota between normal weight and overweight and determine the associations between dietary intakes and gut microbiota | 16S rRNA gene sequencing | ↓ Bifidobacterium, Turicibacter and Clostridiaceae ↑ Erysipelotrichaceae UCG-003, Lachnospira and Peptostreptococcaceae ≠ Alpha and beta bacterial diversity |
| Da Silva et al., 2020 [55] | Trinidad | n = 51 Age: 6–14 years Ethnicity: Mixed, Africans and Indians Overweight/obese [61]: n = 21; BMI > 85th Normal weight: n = 30 | To describe the composition of the gut microbiota in children with obesity/overweight and children of Normal weight and identify possible associations | 16S rRNA gene sequencing | ↓ Bifidobacterium and Bifidobacteriaceae ↓ alpha diversity ↑ Firmicutes and Lactobacillus |
| Chen et al., 2020 [16] | China | n = 51 Age: 6–11 years Ethnicity: Asian Obese [62]: n = 28 Normal weight: n = 23 | To evaluate gut microbial biodiversity between obese and normal weight children | 16S rRNA gene sequencing | ↓ Bacteroidetes, Oscillospira and Dialister ↓ reduced alpha diversity and observed species ↑ Faecalibacterium, Phascolarctobacterium, Lachnospira, Megamonas, and Haemophilus |
| Shin et al., 2020 [42] | Korea | n = 46 Age: 5–13 years Ethnicity: Asian Obese [63]: n = 22 Normal weight: n = 24 | To compare the gut microbiota composition between obese Korean and normal weight children | 16S rRNA gene sequencing | ↓ Bacteroidetes, Bacteroides ovatus, Porphyromonadaceae, Rikenellaceae, Bacteroidaceae, Devosia_f, Leptotrichiaceae, Odoribacteracea and Staphylococcaceaeat ↑ Actinomyces, GL872355_g, Lachnospiraceae, Weissella and Romboutsia |
| López-Contreras et al., 2018 [48] | Mexico | n = 138 Age: 6–12 years Ethnicity: Latino Obese [36]: n = 71 Normal weight: n = 67; | To analyze the gut microbiota composition between obese and normal weight children | 16S rRNA gene sequencing | ↓ unclassified Christensenellaceae ↑ Bacteroides eggerthii |
| Mbakwa et al., 2018 [51] | Netherlands | n = 295 Age: 6–7 years Ethnicity: Caucasian Overweight/obese [64]: n = 27 Normal weight: n = 268 | To examine the composition of the gut microbiota of school-aged children in association with weight | 16S rRNA gene sequencing | ↓ Akkermansia, Sutterella wadsworthensis, Burkholderia, and Marvinbryantia formatexigens ↑ Streptococcus bovis |
| Méndez-Salazar et al., 2018 [49] | Mexico | n = 36 Age: 9–11 years Ethnicity: Latino Obese [61]: n = 12 Normal weight: n = 24 | To compare bacterial richness and diversity of the gut microbiota in Mexican children according to weight categories | 16S rRNA gene sequencing | ↓ Bacterial richness and diversity ↓ Bacteroidetes ↑ Proteobacteria and Bilophila phylum |
| Gao et al., 2018 [43] | China | n = 77 Age: obese 6.8 ± 1.6 years; control 6.0 ± 2.7 years Ethnicity: Asian Obese [61]: n = 39; Age = 6.8 ± 1.6 Normal weight: n = 38; Age = 6.0 ± 2.7 | To analyse the differences in the structure of intestinal flora between obese and normal weight children | 16S rRNA gene sequencing | ↓ Diversity ↑ Bacteroidetes phylum ↓ Candidatus, Actinobacteria, Firmicutes, and Verrucomicrobia phylum ↑ A. histaminiformans, B. plebeius, B. dorei, B. wadsworthia, C. symbiosum, M. funiformis, P. distasonis, P. excrementihominis, P. stercorea, and O. formigenes |
| Riva et al., 2017 [52] | Italy | n = 78 Age: 6–16 years Ethnicity: Caucasian Obese [61]: n = 42 Normal weight: n = 36 | To describe the gut microbiota composition in children with and without obesity | 16S rRNA gene sequencing | ↓ Bacteroidetes and Bacteroidaceae ↑ Firmicutes and Ruminococcaceae |
| Hou et al., 2017 [44] | China | n = 143 Age: 3–18 years Ethnicity: Asian Obese: n = 87 Normal weight: n = 56 | To examine differences in gut microbiota between obese and healthy children | 16S rRNA gene sequencing | ↓ Gram-negative bacteria: Verrucomicrobia and Lentisphaerae ↑ F/B ratio ↑ Firmicutes, Enterococcus and Blautia ↑ Proteobacteria, Sutterella and Klebsiella ↑ Actinobacteria and Collinsella |
| Borgo et al., 2017 [53] | Italy | n = 61 Age: 10.03–0.68 years Ethnicity: Caucasian Obese [61]: n = 28 Normal weight: n = 33 | To assess the biodiversity of gut microbiota in obese and non-obese children | 16S rRNA gene sequencing | ↓ Akkermansia muciniphyla ↓Bacteroides/Prevotella ↓ Candida spp., F. prausnitzii, and Saccharomyces spp. |
| Ignacio et al., 2016 [50] | Brazil | n = 84 Age: 3–11 years Ethnicity: Latino Overweight/Obese [61]: n = 54 Normal weight: n = 30 | Analyse faecal samples for bacteria composition and sought a correlation between the body mass index and these bacteria | 16S rRNA gene sequencing | ↑ B. fragilis, Lactobacillus spp. ↓ Bifidobacterium spp. |
| Gao et al., 2015 [45] | China | n = 126 Age: obese 6.8 ± 2.1 years; control 6.8 ± 2.4 years Ethnicity: Asian Obese [61]: n = 64 Normal weight: n = 62 | To investigate the correlation between obesity and imbalance of gut microbes in children | 16S rRNA-based qPCR | ↓ Bifidobacteria ↓ B/E ratio ↑ E. coli |
| Bervoets et al., 2013 [54] | Belgium | n = 53 Age: 6–16 years Ethnicity: Caucasian Obese [65]: n = 26 Normal weight: n = 27 | To examine the composition of the gut microbiota composition in obese and non-obese children | 16S rRNA gene sequencing | ↓ B. vulgatus ↑ F/B ratio ↑ Lactobacillus spp. |
| Xu et al., 2012 [46] | China | n = 175 Age: 7–13 years Ethnicity: Asian Overweight/obese [62]: n = 84 Normal weight: n = 91 | To explore correlations between the composition of the gut microbiota and obesity in children from Kazakh. | 16S rRNA gene sequencing | ↓ Bacteroidetes ↓ F/B ratio |
| Reference | Country | Sample | Aim | Methodology | Baseline Analysis | Post Intervention Analysis |
|---|---|---|---|---|---|---|
| Morán-Ramos et al., 2022 [56] | Mexico | Obese [36] male children aged 11–14 years old (n = 6) | To analyze the composition of the gut microbiota in obese Mexican children before and after a 6-week intervention |
| Bacteroidetes (46.5%), Firmicutes (45.7%), and Proteobacteria (3.7%) | ↑ Odoribacter (Bacteroidetes) ≠ Gut microbiota composition ≠ Gut microbiota diversity Significant proportion of gut microbial variation (individual divergence) |
| Cho 2021 [19] | Korea | Children (n = 60): Obese children [63] (n = 36)
| To evaluate variations in the gut microbiota after an 8-week program |
| Dysbiotic features of obese children compared with control group: ↑ Blautia ↑ Dorea ↑ E. hallii ↑ Fusicatenibacter ↓ Bacteroidetes ↓ Oscillibacter ↓ Parabacteroides | After intervention, fat loss group showed: ↑ Firmicutes ↑ Clostridiales orde r↓ Bacteroidetes phylum ↓ Bacteroides genus ↓ Microbial richness ≠ beta diversity |
| Huang et al., 2020 [57] | China | Obese [62] adolescents aged 9–16 years old (n = 24) | To investigate the impact of an exercise program and dietary restrictions over 6 weeks on gut microbiome and central hemodynamics in obese adolescents |
| ↑ Lactobacillales, Bacilli, Streptococcaceae, Streptococcus, and Veillonellawere (members of Firmicutes phylum) | ↓ Firmicutes ↑ Bacteroidetes ↓ F/B ratio ↑ Alpha diversity ↑ Lentisphaeria ↓ Lactobacillales, Bacilli, Streptococcaceae and Veillonella (members of Firmicutes phylum) |
| Quiroga et al., 2020 [23] | Spain | Children aged 7–12 years old (n = 53):
| To study the influence of a 12-week training program on gut microbiota and inflammation in children with obesity |
| Obese group: ↑ Bacteroidetes ↑ Proteobacteria ↓ Firmicutes ↓ Actinobacteria | After intervention, the Oe group showed: ↑ Firmicutes phylum (↑ beneficial bacterial genera: Blautia, Dialister, and Roseburia) ↓ Proteobacteria phylum (↓ Gammaproteobacteria class) |
| Bai et al., 2018 [59] | USA | Children aged 7–18 years old (n = 267) 62.9%—normal BMI level 21.0%—underweight 16.1%—overweight and obese [36] | To investigate the relationships between gut microbiota, body mass index and lifestyles (exercise and diet) |
| Overall analysis: ↑ Firmicutes ↑ Bacteroidetes ↑ Proteobacteria | Children who Daily Exercise: ↑ Firmicutes phylum ↑ Alpha-diversity ↑ Clostridiales ↑ Lachnospiraceae ↑ Erysipelotrichaceae |
| Santacruz et al., 2009 [58] | Spain | Overweight [65] adolescents aged 13–15 years (n = 36): High weight–loss (>4.0 kg of weight loss, n = 23) Low weight–loss (<2.0 kg of weight loss, n = 13) | To evaluate the effect of a weight reduction program on the body weight and gut microbiota in overweight adolescents. |
| High weight–loss group: ↑ B. fragilis ↑ C. leptum ↓ C. coccoides ↓ Lactobacillus ↓ Bifidobacterium | Both groups: ↓ Clostridium coccoides ↓ B. longum ↓ B. adolescentes ↑ Bacteroides fragilis ↑ Lactobacillus High weight–loss group: ↓ C. coccoides ↓ B. longum ↑ Bacteroides fragilis ↑ Lactobacillus High weight–loss vs. Low weight–loss: ↓ C. coccoides ↓ Lactobacillus ↓ Bifidobacterium (B. breve and B. bifidum) ↑ B. fragilis ↑ C. leptum ↑ B. catenulatum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgado, M.C.; Sousa, M.; Coelho, A.B.; Costa, J.A.; Seabra, A. Exploring Gut Microbiota and the Influence of Physical Activity Interventions on Overweight and Obese Children and Adolescents: A Systematic Review. Healthcare 2023, 11, 2459. https://doi.org/10.3390/healthcare11172459
Morgado MC, Sousa M, Coelho AB, Costa JA, Seabra A. Exploring Gut Microbiota and the Influence of Physical Activity Interventions on Overweight and Obese Children and Adolescents: A Systematic Review. Healthcare. 2023; 11(17):2459. https://doi.org/10.3390/healthcare11172459
Chicago/Turabian StyleMorgado, Micaela C., Mónica Sousa, André B. Coelho, Júlio A. Costa, and André Seabra. 2023. "Exploring Gut Microbiota and the Influence of Physical Activity Interventions on Overweight and Obese Children and Adolescents: A Systematic Review" Healthcare 11, no. 17: 2459. https://doi.org/10.3390/healthcare11172459
APA StyleMorgado, M. C., Sousa, M., Coelho, A. B., Costa, J. A., & Seabra, A. (2023). Exploring Gut Microbiota and the Influence of Physical Activity Interventions on Overweight and Obese Children and Adolescents: A Systematic Review. Healthcare, 11(17), 2459. https://doi.org/10.3390/healthcare11172459









