Economic Evaluation of Glucosamine in Knee Osteoarthritis Treatments in Vietnam
Abstract
:1. Introduction
2. Materials and Methods
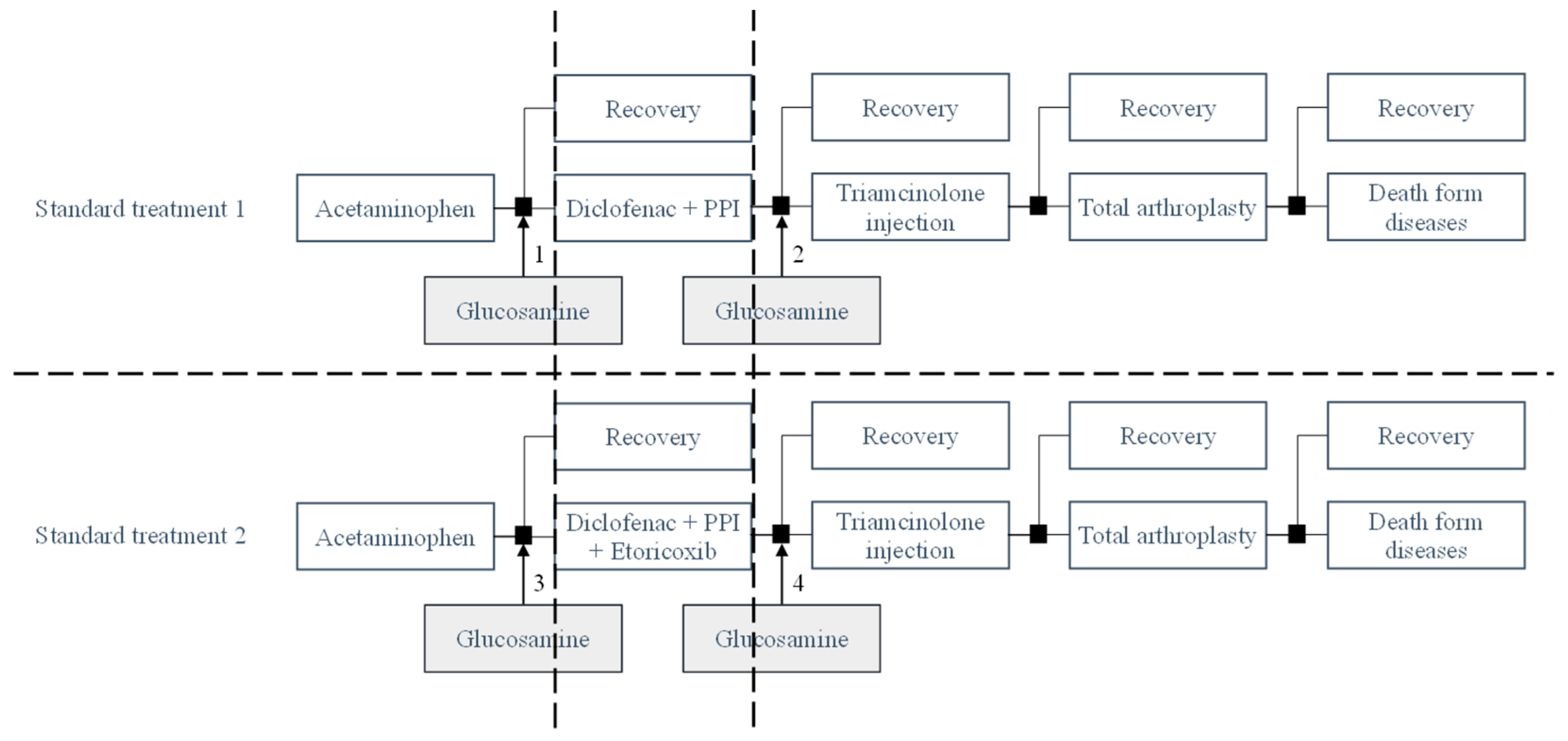
2.1. Model Structure
2.2. Model Assumptions
2.3. Model Input
2.4. Cost Variables
2.5. Efficacy of Pain Relief
2.6. Adverse Events
2.7. Health Outcomes
2.8. Sensitivity Analysis
3. Results
3.1. Base Case
3.1.1. The Base-Case of Standard Treatment 1
3.1.2. The Base Case of Standard Treatment 2
3.2. Sensitivity Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Freitag, J.; Bates, D.; Boyd, R.; Shah, K.; Barnard, A.; Huguenin, L.; Tenen, A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: Reparative pathways, safety and efficacy—A review. BMC Musculoskelet. Disord. 2016, 17, 230. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Deroisy, R.; Rovati, L.C.; Lee, R.L.; Lejeune, E.; Bruyere, O.; Giacovelli, G.; Henrotin, Y.; Dacre, J.E.; Gossett, C. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomised, placebo-controlled clinical trial. Lancet 2001, 357, 251–256. [Google Scholar] [CrossRef]
- Nyvang, J.; Hedstrom, M.; Gleissman, S.A. It’s not just a knee, but a whole life: A qualitative descriptive study on patients’ experiences of living with knee osteoarthritis and their expectations for knee arthroplasty. Int. J. Qual. Stud. Heal. Well-Being 2016, 11, 30193. [Google Scholar] [CrossRef] [PubMed]
- Jinks, C.; Jordan, K.; Croft, P. Measuring the population impact of knee pain and disability with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Pain 2002, 100, 55–64. [Google Scholar] [CrossRef]
- Murphy, L.; Helmick, C.G. The impact of osteoarthritis in the United States: A population-health perspective: A population-based review of the fourth most common cause of hospitalization in U.S. adults. Orthop. Nurs. 2012, 31, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Fibel, K.H.; Hillstrom, H.J.; Halpern, B.C. State-of-the-Art management of knee osteoarthritis. World J. Clin. Cases 2015, 3, 89–101. [Google Scholar] [CrossRef] [PubMed]
- McCorry, M.C.; Puetzer, J.L.; Bonassar, L.J. Characterization of mesenchymal stem cells and fibrochondrocytes in three-dimensional co-culture: Analysis of cell shape, matrix production, and mechanical performance. Stem Cell Res. Ther. 2016, 7, 39. [Google Scholar] [CrossRef]
- Peterfy, C.G.; Gold, G.; Eckstein, F.; Cicuttini, F.; Dardzinski, B.; Stevens, R. MRI protocols for whole-organ assessment of the knee in osteoarthritis. Osteoarthr. Cartil. 2006, 14 (Suppl. A), A95–A111. [Google Scholar] [CrossRef]
- Edd, S.N.; Giori, N.J.; Andriacchi, T.P. The role of inflammation in the initiation of osteoarthritis after meniscal damage. J. Biomech. 2015, 48, 1420–1426. [Google Scholar] [CrossRef]
- Waddell, D.D.; Bert, J.M. The use of hyaluronan after arthroscopic surgery of the knee. Arthroscopy 2010, 26, 105–111. [Google Scholar] [CrossRef]
- Uth, K.; Trifonov, D. Stem cell application for osteoarthritis in the knee joint: A minireview. World J. Stem. Cells 2014, 6, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.A.; Little, D.; Toth, A.P.; Moorman, C.T., 3rd; Tucker, B.S.; Ciccotti, M.G.; Guilak, F. Stem cell therapies for knee cartilage repair: The current status of preclinical and clinical studies. Am. J. Sports Med. 2014, 42, 2253–2261. [Google Scholar] [CrossRef]
- Kane, P.; Frederick, R.; Tucker, B.; Dodson, C.C.; Anderson, J.A.; Ciccotti, M.G.; Freedman, K.B. Surgical restoration/repair of articular cartilage injuries in athletes. Phys. Sportsmed. 2013, 41, 75–86. [Google Scholar] [CrossRef]
- Gupta, P.K.; Das, A.K.; Chullikana, A.; Majumdar, A.S. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res. Ther. 2012, 3, 25. [Google Scholar] [CrossRef]
- Smith, B.; Sigal, I.R.; Grande, D.A. Immunology and cartilage regeneration. Immunol. Res. 2015, 63, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.A.; Loeser, R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Lo, W.C.; Hsu, W.C.; Wei, H.J.; Liu, H.Y.; Lee, C.H.; Tina Chen, S.Y.; Shieh, Y.H.; Williams, D.F.; Deng, W.P. Synergistic anabolic actions of hyaluronic acid and platelet-rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials 2014, 35, 9599–9607. [Google Scholar] [CrossRef]
- March, L.M.; Schwarz, J.M.; Carfrae, B.H.; Bagge, E. Clinical validation of self-reported osteoarthritis. Osteoarthr. Cartil. 1998, 6, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef]
- Ho-Pham, L.T.; Lai, T.Q.; Mai, L.D.; Doan, M.C.; Pham, H.N.; Nguyen, T.V. Prevalence of radiographic osteoarthritis of the knee and its relationship to self-reported pain. PLoS ONE 2014, 9, e94563. [Google Scholar] [CrossRef]
- National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions—United States, 2003. MMWR Morb. Mortal. Wkly. Rep. 2007, 56, 4–7.
- Focht, B.C. Move to Improve: How Knee Osteoarthritis Patients Can Use Exercise to Enhance Quality of Life. ACSMs Health Fit J. 2012, 16, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.T. Guidelines for the Diagnosis and Treatment of Musculoskeletal Disorders; Decision 361/QĐ-BYT; Ministry of Health, Vietnam: Hanoi, Vietnam, 2014; p. 124.
- Perry, A.R.; Mosler, C.; Atkins, A.; Minehart, M. Cardiovascular Risk Associated With NSAIDs and COX-2 Inhibitors. US Pharm. 2014, 39, 35–38. [Google Scholar]
- Zhao, N.; Chung, M.; Wong, J.B.; Wang, C. Economic burden of medications and nutraceuticals in knee osteoarthritis. Osteoarthr. Cartil. 2015, 23, A198–A199. [Google Scholar] [CrossRef]
- Zhang, B.-D.; Liang, Z.J.; Zhang, H.-T.; He, M.-T.; Li, D.-S. Cost-effectiveness analysis on the treatment of knee osteoarthritis by glucosamine hydrochloride and glucosamine sulfate. Chin. J. Tissue Eng. Res. 2012, 16, 9867–9872. [Google Scholar]
- Cohen, M.; Wolfe, R.; Mai, T.; Lewis, D. A randomized, double blind, placebo controlled trial of a topical cream containing glucosamine sulfate, chondroitin sulfate, and camphor for osteoarthritis of the knee. J. Rheumatol. 2003, 30, 523–528. [Google Scholar]
- Pavelká, K.; Gatterová, J.; Olejarová, M.; Machacek, S.; Giacovelli, G.; Rovati, L.C. Glucosamine sulfate use and delay of progression of knee osteoarthritis: A 3-year, randomized, placebo-controlled, double-blind study. Arch. Intern. Med. 2002, 162, 2113–2123. [Google Scholar] [CrossRef]
- Runhaar, J.; Rozendaal, R.M.; Middelkoop, M.v.; Bijlsma, H.J.W.; Doherty, M.; Dziedzic, K.S.; Lohmander, L.S.; McAlindon, T.; Zhang, W.; Zeinstra, S.B. Subgroup analyses of the effectiveness of oral glucosamine for knee and hip osteoarthritis: A systematic review and individual patient data meta-analysis from the OA trial bank. Ann. Rheum. Dis. 2017, 76, 1862–1869. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Sang, L.; Wu, D.; Rong, J.; Jiang, L. Effectiveness and safety of glucosamine and chondroitin for the treatment of osteoarthritis: A meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2018, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.; Agaliotis, M.; Nairn, L.; Votrubec, M.; Bridgett, L.; Su, S.; Jan, S.; March, L.; Edmonds, J.; Norton, R.; et al. Glucosamine and chondroitin for knee osteoarthritis: A double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann. Rheum. Dis. 2015, 74, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Rozendaal, R.M.; Koes, B.W.; Osch, G.J.V.M.; Uitterlinden, E.J.; Garling, E.H.; Willemsen, S.P.; Ginai, A.Z.; Verhaar, J.A.N.; Weinans, H.; Bierma-Zeinstra, S.M.A. Effect of glucosamine sulfate on hip osteoarthritis: A randomized trial. Ann. Intern. Med. 2008, 148, 268–277. [Google Scholar] [CrossRef]
- Sawitzke, A.D.; Shi, H.; Finco, M.F.; Dunlop, D.D.; Harris, C.L.; Singer, N.G.; Bradley, J.D.; Silver, D.; Jackson, C.G.; Lane, N.E.; et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann. Rheum. Dis. 2010, 69, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- World Bank. Life Expectancy at Birth, Total (Years)—Vietnam. Available online: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=VN (accessed on 12 May 2023).
- Herrero-Beaumont, G.; Ivorra, J.A.; Del Carmen Trabado, M.; Blanco, F.J.; Benito, P.; Martín-Mola, E.; Paulino, J.; Marenco, J.L.; Porto, A.; Laffon, A.; et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: A randomized, double-blind, placebo-controlled study using acetaminophen as a side comparator. Arthritis Rheum. 2007, 56, 555–567. [Google Scholar] [CrossRef]
- Zacher, J.; Feldman, D.; Gerli, R.; Scott, D.; Hou, S.M.; Uebelhart, D.; Rodger, I.W.; Ozturk, Z.E. A comparison of the therapeutic efficacy and tolerability of etoricoxib and diclofenac in patients with osteoarthritis. Curr. Med. Res. Opin. 2003, 19, 725–736. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2017, 317, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Fortin, P.R.; Clarke, A.E.; Joseph, L.; Liang, M.H.; Tanzer, M.; Ferland, D.; Phillips, C.; Partridge, A.J.; Bélisle, P.; Fossel, A.H.; et al. Outcomes of total hip and knee replacement: Preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999, 42, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Curtis, S.P.; FitzGerald, G.A.; Krum, H.; Kaur, A.; Bolognese, J.A.; Reicin, A.S.; Bombardier, C.; Weinblatt, M.E.; van der Heijde, D.; et al. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: A randomised comparison. Lancet 2006, 368, 1771–1781. [Google Scholar] [CrossRef]
- MOH_Vietnam. Drug Prices. Available online: https://dichvucong.dav.gov.vn/congbogiathuoc/index (accessed on 12 May 2023).
- Ministry of Health, Vietnam. Determining the Price of Medical Examination and Treatment Services Covered by Health Insurance among Patients of the Same Class Nationwide and Guiding the Application of Prices and Payment of Medical Examination and Treatment Expenses in Some Cases; Document 39/2018/TT-BYT; Ministry of Health, Vietnam: Hanoi, Vietnam, 2018.
- Research Center for Employment Relations (ERC). Living Wage Report, Rural Vietnam, Soc Trang to Thai Binh. Available online: https://www.isealalliance.org/sites/default/files/resource/2017-12/Rural_Vietnam_Living_Wage_Benchmark_Report.pdf (accessed on 16 May 2023).
- van den Hout, W.B.; de Jong, Z.; Munneke, M.; Hazes, J.M.; Breedveld, F.C.; Vliet Vlieland, T.P. Cost-utility and cost-effectiveness analyses of a long-term, high-intensity exercise program compared with conventional physical therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2005, 53, 39–47. [Google Scholar] [CrossRef]
- Hien, T.T.; Lien, T.K.N. Evaluation of the rehabilitation results of patients with knee osteoarthritis after general knee replacement operation. Vietnam. Med. J. 2022, 519, 368–372. [Google Scholar]
- Latimer, N.; Lord, J.; Grant, R.L.; O’Mahony, R.; Dickson, J.; Conaghan, P.G.; National Institute for Health; Clinical Excellence Osteoarthritis Guideline Development Group. Cost effectiveness of COX 2 selective inhibitors and traditional NSAIDs alone or in combination with a proton pump inhibitor for people with osteoarthritis. BMJ 2009, 339, b2538. [Google Scholar] [CrossRef]
- Bruyere, O.; Reginster, J.Y.; Honvo, G.; Detilleux, J. Cost-effectiveness evaluation of glucosamine for osteoarthritis based on simulation of individual patient data obtained from aggregated data in published studies. Aging Clin. Exp. Res. 2019, 31, 881–887. [Google Scholar] [CrossRef]
- Losina, E.; Daigle, M.E.; Suter, L.G.; Hunter, D.J.; Solomon, D.H.; Walensky, R.P.; Jordan, J.M.; Burbine, S.A.; Paltiel, A.D.; Katz, J.N. Disease-modifying drugs for knee osteoarthritis: Can they be cost-effective? Osteoarthr. Cartil. 2013, 21, 655–667. [Google Scholar] [CrossRef]
- Feeny, D.; Wu, L.; Eng, K. Comparing short form 6D, standard gamble, and Health Utilities Index Mark 2 and Mark 3 utility scores: Results from total hip arthroplasty patients. Qual. Life Res. 2004, 13, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- World Bank. Consumer Price Index in Vietnam. Available online: https://data.worldbank.org/indicator/FP.CPI.TOTL?locations=VN (accessed on 16 May 2023).
- World Bank. GDP per Capita (Current US$)—Vietnam. Available online: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?end=2022&locations=VN&start=1985 (accessed on 12 May 2023).
- Moreno, A.; Vargas, E.; Soto, J.; Rejas, J. Cost-effectiveness analysis of the use of celecoxib for the treatment of osteoarthritis. Gac Sanit 2003, 17, 27–36. [Google Scholar] [CrossRef]
- Bruyère, O.; Altman, R.D.; Reginster, J.Y. Efficacy and safety of glucosamine sulfate in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016, 45, S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Scholtissen, S.; Bruyère, O.; Neuprez, A.; Severens, J.L.; Herrero-Beaumont, G.; Rovati, L.; Hiligsmann, M.; Reginster, J.Y. Glucosamine sulphate in the treatment of knee osteoarthritis: Cost-effectiveness comparison with paracetamol. Int. J. Clin. Pract. 2010, 64, 756–762. [Google Scholar] [CrossRef]
- Bruyère, O.; Detilleux, J.; Reginster, J.Y. Cost-Effectiveness Assessment of Different Glucosamines in Patients with Knee Osteoarthritis: A Simulation Model Adapted to Germany. Curr. Aging Sci. 2021, 14, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Day, S.E.; Chapman, A.B.; Osborne, R.H. Can we reduce disease burden from osteoarthritis? Med. J. Aust. 2004, 180, S11–S17. [Google Scholar] [CrossRef]
- Luksameesate, P.; Tanavalee, A.; Taychakhoonavudh, S. An economic evaluation of knee osteoarthritis treatments in Thailand. Front. Pharmacol. 2022, 13, 926431. [Google Scholar] [CrossRef] [PubMed]
- Black, C.; Clar, C.; Henderson, R.; MacEachern, C.; McNamee, P.; Quayyum, Z.; Royle, P.; Thomas, S. The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: A systematic review and economic evaluation. Health Technol. Assess. 2009, 13, 1–148. [Google Scholar] [CrossRef]
- Chaiyakunapruk, N.; Saokaew, S.; Pansang, S. Cost-effectiveness analysis of glucosamine sulphate for the treatment of osteoarthritis in Thailand. Value Health 2010, 13, A502. [Google Scholar] [CrossRef]



| Component | Estimate | Sources |
|---|---|---|
| 1. Transition probabilities | ||
| Acetaminophen | 0.3380 | Herrero-Beaumont et al. (2007) [35] |
| Diclofenac + PPI | 0.8647 | Zacher et al. (2003) [36] |
| Triamcinolone injection | 0.0392 | McAlindon et al. (2017) [37] |
| Total arthroplasty | 0.5122 | Fortin et al. (1999) [38] |
| Etoricoxib | 0.5657 | Cannon et al. (2006) [39] |
| Crystalline glucosamine sulfate | 0.3857 | Herrero-Beaumont et al. (2007) [35] |
| 2. Costs—VND (USD) | ||
| Costs of treatment | ||
| Acetaminophen (3000 mg/day) 500 mg—6 times/day | 588,600 | Vietnam Drug Administration, Ministry of Health, Vietnam [40] |
| (25.06) | ||
| Diclofenac (150 mg/day) 50 mg—3 times/day | 60,210 | Vietnam Drug Administration, Ministry of Health, Vietnam [40] |
| (2.56) | ||
| Omeprazole (20 mg/day) 20 mg—1 times/day | 344,700 | Vietnam Drug Administration, Ministry of Health, Vietnam [40] |
| (14.68) | ||
| Etoricoxib (60 mg/day) 30–60 mg—1–2 times/day | 785,700 | Vietnam Drug Administration, Ministry of Health, Vietnam [40] |
| (33.45) | ||
| Crystalline glucosamine sulfate (1500 mg/day) 500 mg—3 times/day | 186,300 | Vietnam Drug Administration, Ministry of Health, Vietnam [40] |
| (7.93) | ||
| Triamcinolone injection (40 mg every 3 months)—80 mg/2 mL | 19,400 | Vietnam Drug Administration, Ministry of Health, Vietnam [40] |
| (0.83) | ||
| Total Knee Arthroplasty (TKA) | 88,712,500 | Decree 39/2018/TT-BYT, Ministry of Health, Vietnam [41] |
| (3777.25) | ||
| Direct non-medical costs (VND) | ||
| Food costs per person (1 month) | 2,700,000 | Research Center for Employment Relations (ERC) [42] |
| (114.96) | ||
| Transportation costs per person (1 month) | 2,953,620 | Wilbert B. et al. (2005) [43] |
| (125.76) | ||
| Indirect costs | ||
| Cost of absenteeism (1 month-absent for Knee Joint Recovery Post-Surgery) | 7,342,373 | Hien Thu Trinh et al. (2022) [44] |
| (312.63) | ||
| 3. Utilities | ||
| Utility Acetaminophen | 0.7010 | Latimer et al. (2009) [45] |
| Utility Diclofenac + PPI | 0.7230 | Latimer et al. (2009) [45] |
| Utility Etoricoxib | 0.7230 | Latimer et al. (2009) [45] |
| Utility Glucosamine | 0.6760 | Olivier Bruyère et al. (2019) [46] |
| Utility Triamcinolone injection | 0.6400 | Losina et al. (2013) [47] |
| Utility total knee arthroplasty | 0.7600 | David Feeny et al. (2004) [48] |
| COST VND (USD) | QALYs | |
|---|---|---|
| Standard treatment 1 (PD) | 314,758,471 (13,401.96) | 56.9374 |
| Standard treatment 1 + glucosamine before NSAIDs (PGD) | 348,133,295 (14,823.01) | 100.7445 |
| Standard treatment 1 + glucosamine after NSAIDs (PDG) | 339,027,398 (14,435.30) | 92.7119 |
| ICERPGD/PD VND (USD) per QALYs | 761,858 (32.44) | |
| ICERPDG/PD VND (USD) per QALYs | 678,386 (28.88) | |
| ICERPGD/PDG VND (USD) per QALYs | 1,133,615 (48.27) | |
| Standard treatment 2 (PDE) | 367,478,671 (15,646.71) | 129.8038 |
| Standard treatment 2 + glucosamine before NSAIDs (PGDE) | 443,969,715 (18,903.59) | 230.6720 |
| Standard treatment 2 + glucosamine after NSAIDs (PDEG) | 425,013,733 (18,096.47) | 211.3288 |
| ICERPDE/PD VND (USD) per QALYs | 723,519 (30.81) | |
| ICERPGDE/PDE VND (USD) per QALYs | 758,326 (32.29) | |
| ICERPDEG/PDE VND (USD) per QALYs | 705,735 (30.05) | |
| ICERPGDE/PDEG VND (USD) per QALYs | 979,981 (41.73) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, N.X.; Che, U.T.T.; Ngo, T.T.T.; Bui, T.T. Economic Evaluation of Glucosamine in Knee Osteoarthritis Treatments in Vietnam. Healthcare 2023, 11, 2502. https://doi.org/10.3390/healthcare11182502
Vo NX, Che UTT, Ngo TTT, Bui TT. Economic Evaluation of Glucosamine in Knee Osteoarthritis Treatments in Vietnam. Healthcare. 2023; 11(18):2502. https://doi.org/10.3390/healthcare11182502
Chicago/Turabian StyleVo, Nam Xuan, Uyen Thi Thuc Che, Thanh Thi Thanh Ngo, and Tien Thuy Bui. 2023. "Economic Evaluation of Glucosamine in Knee Osteoarthritis Treatments in Vietnam" Healthcare 11, no. 18: 2502. https://doi.org/10.3390/healthcare11182502
APA StyleVo, N. X., Che, U. T. T., Ngo, T. T. T., & Bui, T. T. (2023). Economic Evaluation of Glucosamine in Knee Osteoarthritis Treatments in Vietnam. Healthcare, 11(18), 2502. https://doi.org/10.3390/healthcare11182502






