Abstract
The use of virtual reality (VR) content in neurological disorders with cognitive impairment is increasing. We have developed a device that incorporates virtual drum beating content, designed for digit memorization training. This study aimed to investigate the effects of realistic cognitive training on brain activity using functional near-infrared spectroscopy (fNIRS). Thirty healthy individuals were recruited and randomly assigned into two groups: conventional cognitive exercise (CCE) and a realistic cognitive exergame (RCE). Subjects in the CCE group underwent memory training by memorizing numbers displayed on a computer screen and then writing them on paper. The main outcome measure was the oxyhemoglobin level in the dorsolateral prefrontal cortex (DLPFC). As a result, the average number of digits was 7.86 ± 0.63 for the CCE and 7.6 ± 0.82 for the RCE. The mean difference in ΔHbO was 1.417 ± 0.616 μm (p = 0.029) in channel 2, located in the right DLPFC. Channel 7 and channel 10, which measured activations in the hypothesized medial orbitofrontal cortex (OFC), also showed a significant mean difference of ΔHbO. DLPFC and OFC presented higher activation in the RCE group (p < 0.05), attributable to the simultaneous memory training and virtual drum beating, which provided various sensory inputs (visual, auditory, and vibration). Although DLPFC involvement in cognitive processes remains controversial, our findings suggest that realistic memory training using drumming content can lead to safer activation of the DLPFC compared to conventional cognitive training.
1. Introduction
Cognitive changes are a normal part of the aging process, affecting individuals differently. It is estimated that approximately 15% to 20% of individuals aged 65 and older experience mild cognitive impairment (MCI) [1]. Given the desire to prevent or slow down further cognitive decline in individuals with MCI, there is significant interest in potential treatments [2]. One such treatment approach is cognitive training (CT), which involves structured and repetitive mental exercises aimed at improving specific cognitive processes [3]. CT is a non-pharmacological treatment method that focuses on the guided practice of tasks that target specific cognitive functions, such as memory and attention [4]. A previous review study has shown that CT can lead to significant improvements in memory, executive function, processing speed, attention, fluid intelligence, and the subjective evaluation of cognitive functioning among older adults with MCI [5]. Moreover, several interventions are focused on cognitive training, and many studies have been conducted to investigate their effects [4]. However, it remains unclear whether recently developed CT can effectively assist older adults with MCI in maintaining or improving their cognitive abilities, overall well-being, and general functioning [4].
Memory training content with virtual reality (VR) is one of the most advanced Information Communications Technologies (ICT), offering an immersive, intuitive, motivating, interactive, and multisensory feedback context [6]. VR refers to complete three-dimensional virtual representations of real-world scenarios or objects [7]. The combination of VR with neuroimaging is increasingly being explored in the clinical field due to its potential to enhance neuropsychological treatments [8]. Previous studies have shown the positive effects of VR-based gaming interventions on attention, visual and verbal memory, and executive function in older adults with MCI [9]. A fully immersive VR gaming system can incorporate a wide field of view in high-resolution graphics on a head-mounted display (HMD) with auditory feedback [10]. Moreover, VR applications delivered in an HMD are widely used for rehabilitation, assessment, and even the prediction of cognitive impairments in older adults [11]. However, some reviews have addressed potential adverse effects associated with HMD usage, often referred to as “VR sickness”. This term encompasses a range of symptoms and their severity that are commonly reported by individuals using HMDs [12]. Such symptoms can include feelings of nausea, dizziness, and blurred vision, which have been documented in relation to HMD usage [13].
Recently, realistic cognitive training content involving drumming was developed especially for memory training in older adults. To prevent the risk of VR sickness, this training system deliberately omitted the use of HMD. The focus was to minimize the potential for VR sickness and create a user-friendly experience for older adults and patients with MCI. The content was developed by prioritizing the immersive sensation of playing a musical instrument, replicating the experience of playing a physical drum. To execute the training activities, participants used a handheld controller, which allowed them to interact with the content. The system incorporated tactile feedback through vibrations and auditory cues, simulating the authentic sensory experience of playing a physical drum within the virtual drumming environment.
fMRI is a well-known and the most used tool in neuroimaging studies, providing the most precise test in mapping functional brain activation patterns across the entire brain [14]. During fMRI scans, subjects are generally instructed to stay as motionless as possible, as even minimal body movement can alter or compromise the quality of the data obtained [15]. fMRI is inappropriate to use when subjects are engaged in limb movement, as it cannot effectively mitigate motion-related artifacts [16]. On the other hand, fNIRS is a non-invasive optical neuroimaging method renowned for its ability to provide powerful data even in the presence of movement artifacts. This makes it particularly valuable in studies involving the free movement of limbs [17]. For instance, a 15-channel fNIRS probe configuration can effectively map each channel to different parts of the Brodmann areas when placed on the prefrontal cortex. Among the various neuroanatomical regions of the brain, the dorsolateral prefrontal cortex, the ventrolateral prefrontal cortex, and the frontopolar cortex have been primary subjects of investigation [18]. Current evidence highlights the important role of the frontal lobe, one of the brain’s most significant neuroanatomical structures, particularly in attention processes during cognitive tasks [19].
No prior research has explored the potential effects of instrument playing with fNIRS on brain activity, particularly following hemoglobin pathways. In the present study, we used fNIRS to assess changes in brain activity during virtual drumming exercises. Although the virtual drumming content and equipment were initially designed for older adults’ cognitive training, the present study was conducted on healthy people without MCI as the research was a preliminary study. To our knowledge, this is the first study to investigate differences in brain activity during memory training between conventional methods and a realistic cognitive exergame without causing VR sickness.
2. Materials and Methods
2.1. Study Protocol and Study Design
This study was a randomized controlled trial, single-blinded (assessor), investigating differences in brain activity between conventional cognitive exercise (CCE) and a realistic cognitive exergame (RCE). The research protocol was approved by the Institutional Review Board (IRB) of Dongguk University Ilsan Hospital (IRB No. DUIH 2022-02-005-004), and the study was registered at the Clinical Research Information Service (CRIS, KCT0007463, date of registration: 20 June 2022). Informed consent was obtained from all participants, and all procedures were performed following the Declaration of Helsinki.
2.2. Participants
Thirty healthy individuals were included and randomly assigned to two groups: 15 in the CCE group (control group) and 15 in the RCE group (experimental group). The mean MMSE score was 29.47 ± 0.52 in the RCE group and 29.60 ± 0.51 in the CCE group (Table 1). The average span length of digits was 7.86 ± 0.63 in the CCE group and 7.6 ± 0.82 in the RCE group during the test. No significant differences in memory training levels were observed between the two groups (Table 1).

Table 1.
Baseline characteristic of subjects in the CCE and the RCE group.
2.2.1. Eligibility Criteria
Based on data from subjects who agreed to participate in this study, screening was conducted to select eligible subjects following the inclusion and exclusion criteria. Inclusion criteria were as follows: (1) healthy adults aged between 19 and 40 years, (2) a Mini-Mental Status Examination (MMSE-K) score of 24 or higher. Exclusion criteria were as listed: (1) individuals with cognitive or physical disabilities; (2) those with behavioral disorders that might restrain their ability to perform ‘virtual drum beating content’ and conventional memory training. Table 1 presents the baseline characteristics of the two groups.
2.2.2. Sample Size
The sample size calculation was performed using data from HbO and trunk linear acceleration results obtained from the study of Mori et al. [20] (alpha = 0.05, power = 95%, effect size η2 = 0.34). For the current study, the estimated sample size was 15 participants for each group. We prospectively enrolled 30 suitable subjects from May 2022 to November 2022.
2.2.3. Randomization Procedure
The randomization process was conducted for subjects who met the inclusion/exclusion criteria and agreed to participate in this study. They were randomly allocated to either the CCE or RCE using the computerized random number generator SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) employing the block randomization method.
2.3. Intervention
The CCE group underwent memory training using conventional paper-and-pencil methods in a dedicated laboratory setting. Digits were visually presented one at a time on a computer screen for five seconds. The memory span length began with three digits and gradually increased to nine. After the numbers disappeared from the screen, participants were asked to write the numbers in the correct sequential order on the response sheet [21].
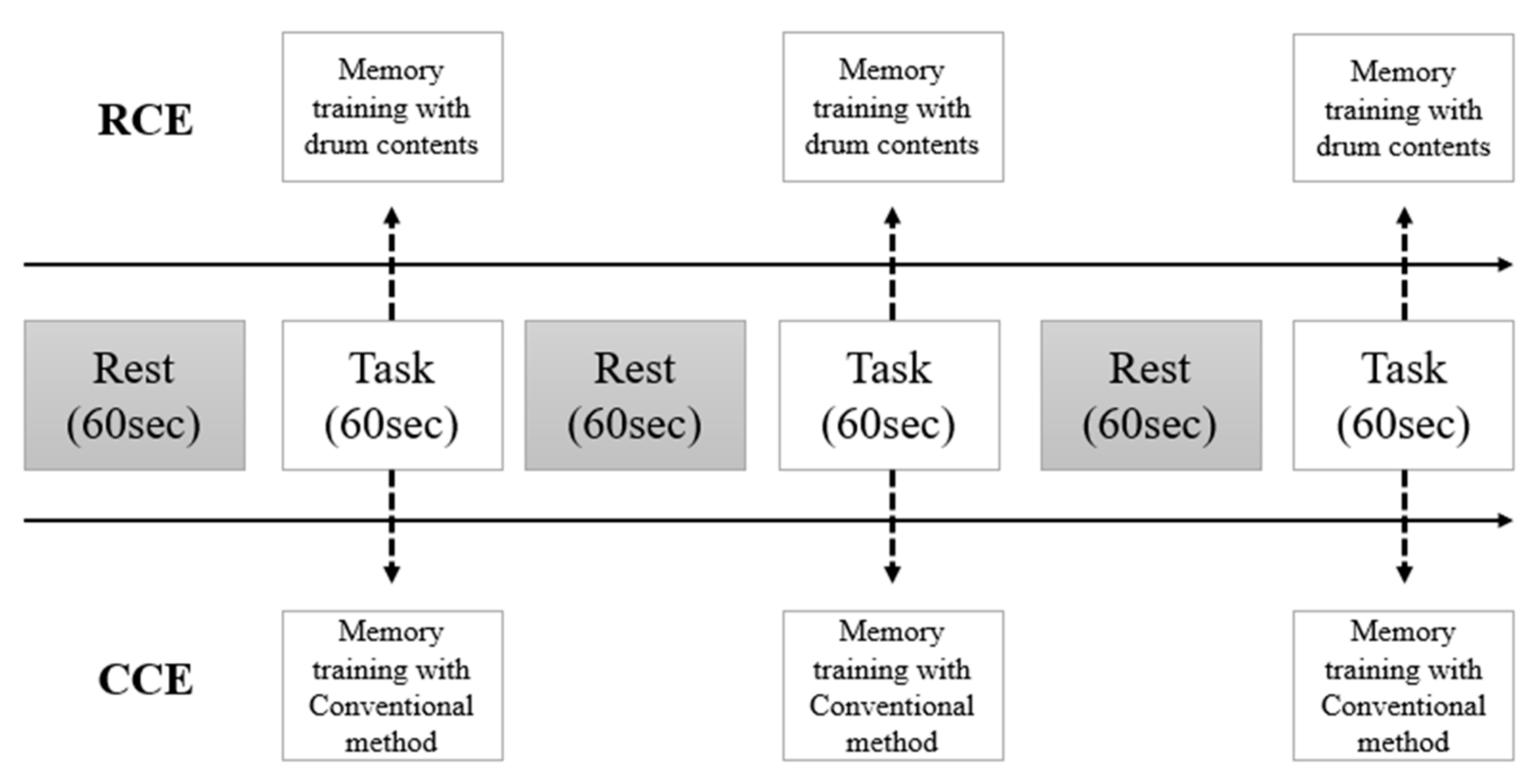
The RCE group performed memory training using realistic drum beating content in an independent laboratory. Participants were asked to stand in front of a 42-inch LED monitor and memorize digits displayed on the screen for five seconds. Thereafter, they were instructed to hit the correct number of virtual drums in sequence using an electronic drumstick held in both hands. This electronic drumstick replicated the vibrations and impact of real drumming, while the monitor reproduced the drumming visual and auditory experience (Figure 1). If the participants successfully struck the correct drum for three consecutive three-digit sequences, the length of the digit span increased to nine digits. This task lasted for 60 s, and the nine-digit span continued until the end of the task. Three tasks were provided after a resting period of 30 s (Figure 2).

Figure 1.
Memory training by virtual drum beating.
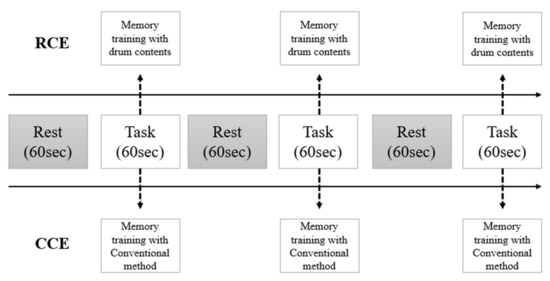
Figure 2.
The protocol of measurement with fNIRS.
2.4. Measurement
During the intervention, we employed fNIRS to monitor changes in brain activity. The fNIRS data from NIRSIT LITE (OBELAB, Inc., Seoul, Republic of Korea) utilized near-infrared light transmission at two distinct wavelengths (780 and 850 nm). The NIRSIT obtained regulatory clearance as a medical device from the Korea Food and Drug Administration in 2017. The fNIRS results were wirelessly collected with time synchronization markers during the trial. The modified Beer–Lambert law was applied to obtain hemodynamic responses, considering the last five seconds of the pre-task as the baseline for each block [22]. We calculated the block-average responses for each channel by subtracting the mean amplitude of the baseline.
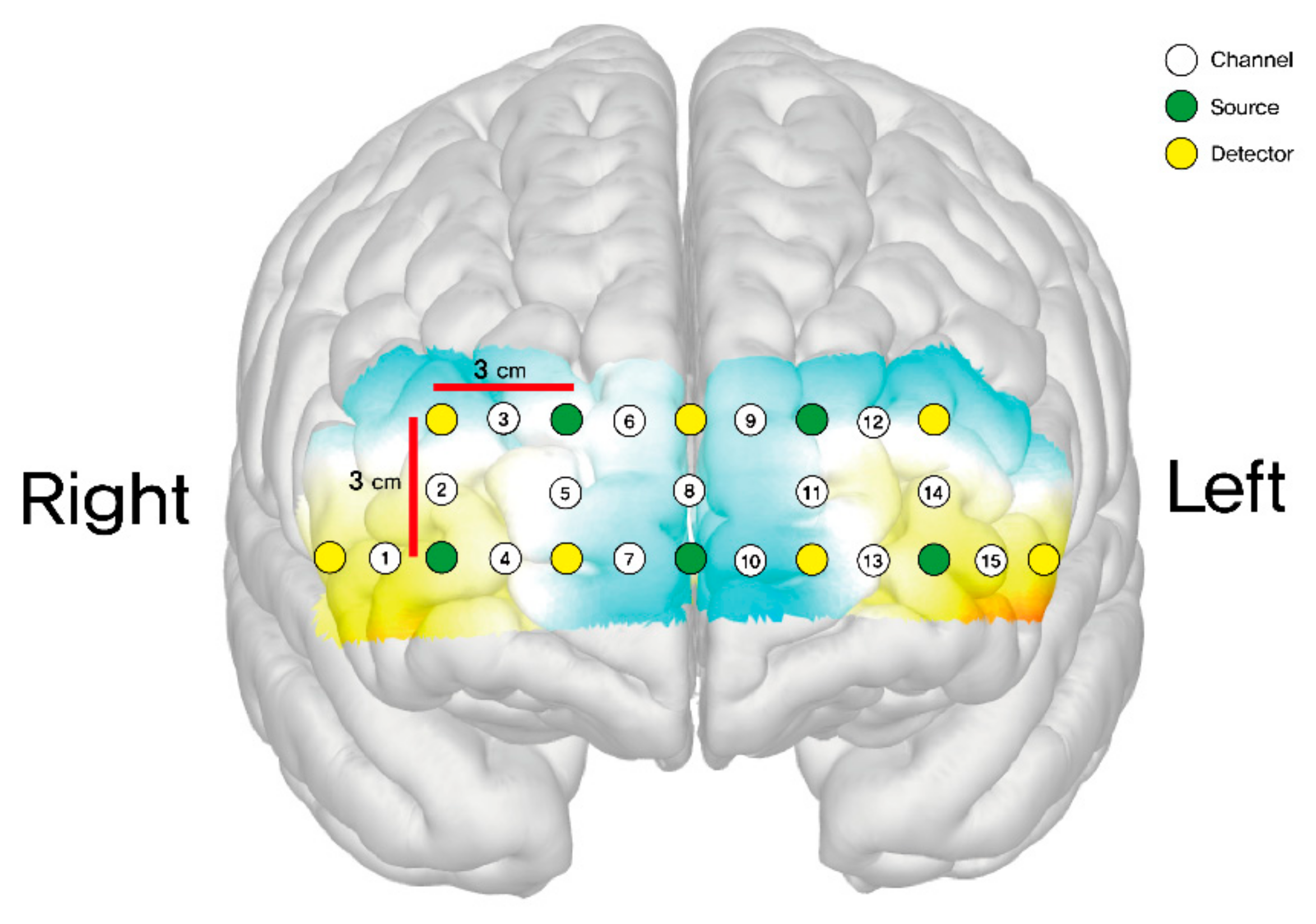
The final output of each optode was subsequently expressed as the mean total oxygenated hemoglobin (HbO μm). A total of 15 channels were positioned on both sides of the dorsolateral prefrontal cortex (DLPFC) (channels 2, 3, 12, 14), orbitofrontal cortex (OFC) (channels 1, 4, 7, 10, 13, 15), and frontopolar prefrontal cortex (FPPFL) (channels 5, 6, 8, 9, 11) [23]. These channels were situated as follows: channel 1 on the right lateral OFC; channels 4 and 7 on the right medial OFC; channels 2 and 3 on the right DLPFC; channel 15 on the left lateral OFC; channels 10 and 13 on the left medial OFC; channels 12 and 14 on the left DLPFC; and channels 5, 6, 8, 9, and 11 on the frontopolar prefrontal cortex. Figure 3 shows the positions of these channels.
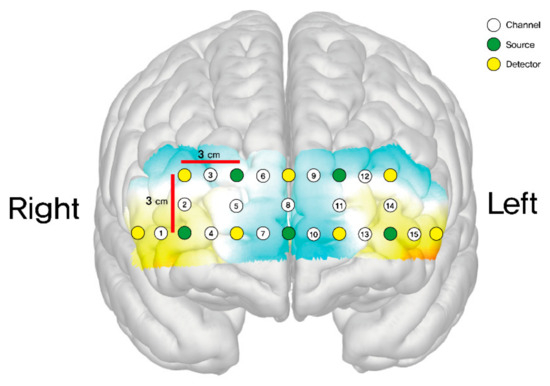
Figure 3.
Schematic positions of fNIRS channels.
The statistical analysis was performed using the NIRSIT Lite Analysis Tool v3.1.0. HbO is widely recognized for its robust and reproducible indication of changes in regional cerebral blood flow. Numerous studies have demonstrated a stronger correlation between the fMRI BOLD response and HbO as opposed to deoxyhemoglobin (deoxyHb) [24]. This is possibly attributable to the higher signal-to-noise ratio in HbO [25]. During task performance, we employed topographical maps to illustrate various regions of prefrontal cortex activity and highlight their differences. The intensity of the red color indicated an augmentation in the observed quantity of oxygenated hemoglobin within that specific prefrontal cortex area, signifying enhanced activation and cognitive engagement. On the other hand, the dark blue color represented a reduction in oxygenated hemoglobin levels and hypo-activation within the area.
2.5. Statistical Analysis
For demographic and clinical characteristics, categorical variables such as gender are presented as a frequency and percentage, and we assessed the pre-homogeneity through chi-square tests. Continuous variables are represented as the mean and standard deviation (SD). We analyzed subjects’ height and weight pre-homogeneity using a Student’s t-test, satisfying normality. For variables that did not satisfy normality, we used a Wilcoxon rank sum test. A two-sample t-test was used to compare group-level differences in HbO-response-accompanying events between the control and experimental groups. All values are presented as mean and SD (mean ± SD). Statistical analyses were conducted using IBM SPSS Statistics 21 (SPSS Inc., Chicago, IL, USA), and the statistical significance level was set at p < 0.05.
3. Results
All 30 participants completed the tasks without experiencing any adverse events, such as VR sickness (nausea, dizziness, or blurred vision).
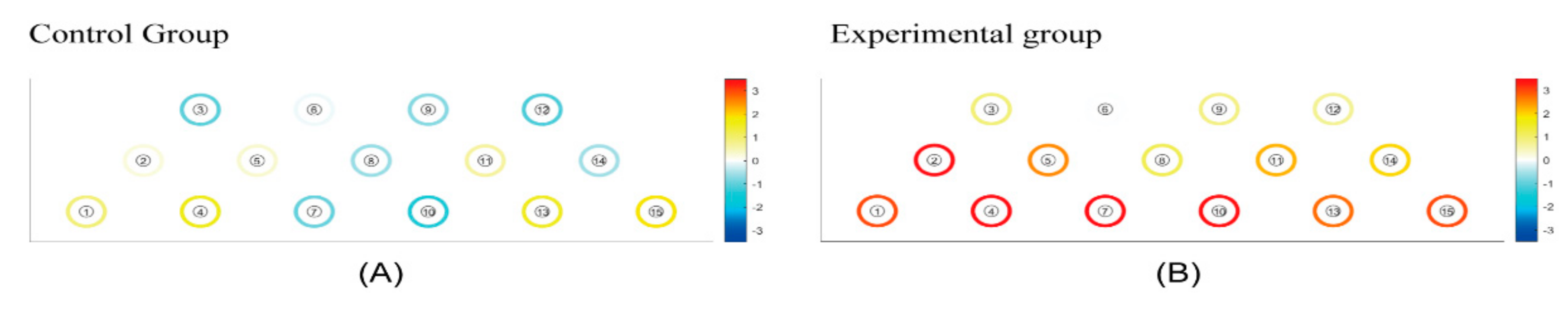
Figure 4 visually presents the regions of HbO (μm) responses, highlighting increases in HbO levels (red color) and decreases (blue color). White indicates the initial state of the brain (baseline) in both the CCE and RCE groups. The statistical difference in ΔHbO (μm) of each channel is presented in Table 2. Considering all regions measured, the RCE group showed more robust HbO responses compared to the CCE group during all tasks (Figure 4). In particular, ΔHbO (μm) was statistically significant in channel 2, located on the right DLPFC, showing a mean difference of ΔHbO (μm) equal to 1.417 ± 0.616 (p = 0.029). In channel 7, the ΔHbO (μm) was −0.304 ± 1.234 μm for the CCE group and 0.790 ± 0.842 for the RCE group. The mean difference in ΔHbO (μm) between the CCE and RCE groups showed a significant value of 1.904 ± 0.386 (p = 0.008). For channel 10, the mean difference in ΔHbO (μm) was 0.592 ± 0.661 μm in the RCE group, indicating greater activation compared to the CCE group (−0.480 ± 1.229 μm). The mean difference in ΔHbO was 1.073 ± 0.360 μm (p = 0.006). Channels 7 and 10, which measured activations in the hypothesized medial OFC, showed significant differences between the two groups (Table 2).
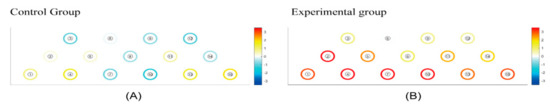
Figure 4.
(A) The mean hemodynamic changes (ΔHbO) when performing three instances of conventional cognition training. (B) The mean hemodynamic changes (ΔHbO) when taking three periods of rest between pre and post experimental cognition training. White: initial state of the brain (baseline). White < yellow < red: increase in HbO level. White > light Blue > blue: decrease in HbO level.

Table 2.
Differences in HbO when undergoing cognitive memory training in both groups (μm).
4. Discussion
A realistic memory training exercise involving drumming was newly developed for cognitive training among older adults. We aimed to investigate prefrontal cortex activation during memory training that combines cognitive and exergame elements (dual activity). We recruited thirty healthy individuals who were randomly assigned to two groups (CCE and RCE groups). The CCE group underwent memory training using a paper-and-pencil method, a conventional approach often used in rehabilitation. In contrast, the RCE engaged in memory training by memorizing numbers displayed on a mirror-like LED screen and then matching them by beating the corresponding virtual drums. Although both interventions provided an equal level of working memory training (3–9 digits or colors), the RCE group exhibited a greater increase in brain activity compared to the CCE group.
Interestingly, our findings contrast those of Vermeij et al. [26], who observed no changes in prefrontal activation related to working memory training. Meanwhile, Belleville et al. [27], using fMRI, reported activation in frontal, temporal, and parietal areas following memory training. Additionally, they showed higher activation in the dorsolateral prefrontal cortex during memory tasks when compared to their cognitively preserved counterparts [27].
4.1. Different Prefrontal Cortex Activation with DLPFC
In the results, the mean ΔHbO μm in channel 2, located on the right DLPFC of the RCE group, was statistically significant when compared to the CCE group (Table 2). Activation of the DLPFC is observed when participants learn unfamiliar motor skills. The level of activation diminishes as the learning process advances [19]. Previous neuroimaging studies have provided insights into the multifaceted role of the DLPFC, which encompasses top-down attentional control [28] and dispute management [29]. However, no consensus has been found regarding the specific part within the DLPFC that acts in the above-mentioned cognitive processes. This variability in findings can be attributed to variations in control processes inherent to different task paradigms employed across prior studies [30]. Therefore, right–left differences in DLPFC activation during motor learning, including visuomotor transformation learning, may also depend on the cognitive processes involved in each motor task [28]. The DLPFC was found to be involved in the higher-order cognitive control of reaching movements, which is only needed during the early stages of visuomotor transformation learning [31]. This finding aligns with prior neuroimaging research focusing on different types of motor learning, where the author observed the activation of the DLPFC during the early stages of explicit motor learning by trial and error [32]. These studies reported differences in the right–left DLPFC activation among their participants, but the involved DLPFC side varied across studies. For instance, Sakai et al. [33] observed noticeable activation in the DLPFC starting on the left side, while continuous activation was observed in the right DLPFC compared to the left DLPFC.
On the contrary, a study examining whole-brain activity during motor sequence learning revealed the activation of the right DLPFC during the first half of learning but no activation when the task became overlearned [34]. However, it is essential to note that the significant activation of the right DLPFC observed in this study involving realistic drum content does not necessarily imply that the activation was in the learning stage. Instead, this heightened activation may be indicative of a compensatory mechanism enabling individuals to perform adequately, particularly in cases where memory networks may not be functioning optimally [35]. The DLPFC is implicated in recognizing salient events, both stimulating and inhibiting motor responses, shifting attentional focus, and directing attention to new targets [36]. Moreover, it plays a critical role in monitoring stimulus occurrence, leading to a decrease in reaction time as the inter-stimulus interval (ISI) increases (for period effect), a function also reliant on the integrity of the DLPFC [37]. Nonetheless, recent evidence suggests that the right DLPFC may operate more efficiently. It sustains attention and maintains a heightened state of alertness, induced by warning signals that briefly precede the target. These processes may be associated with the anterior cingulate cortex (ACC) or other midline frontal structures [38].
4.2. Different Prefrontal Cortex Activation with OFC
The hypothesized medial OFC (channels 7, 10) within the prefrontal lobe showed more activations in the RCE group during memory training with the virtual drum. Previous NIRS studies have demonstrated that the prefrontal cortex, including the OFC, becomes activated during tasks requiring verbal fluency [39,40]. The OFC has been previously associated with reward processing [41]. Touch, as a primary reinforcer, can evoke feelings of pleasure [42]. Notably, fMRI studies conducted by Rolls and colleagues [43] have demonstrated that reward-related brain regions, including the OFC, are activated in response to pleasant touch sensations, such as those by velvet fabric, which aligns with our present results. Furthermore, another research study on fMRI has also reported the activation of similar brain regions in response to pleasant touch experiences [44]. A controller (HTC Vive Pro, HTC, Taiwan) without an HMD was used as a display device for the execution of VR drum content. These controllers were equipped with vibration motors designed to provide tactile feedback in the form of vibrations. Research has demonstrated that incorporating vibration as a source of force feedback can yield improvements compared to relying solely on visual feedback [45]. The human sense of touch is particularly adept at detecting and interpreting these vibrations, which provide valuable information about interactions with the physical world [46]. Tactile sensations such as pressure and vibration are perceived by specialized sensory receptors called mechanoreceptors implanted into the skin. Each type of mechanoreceptor is specialized to perceive and respond to a specific type of tactile stimuli [47,48].
4.3. Strengths and Limitations
Our study has several strengths. These include a simple design with a short intervention period, a randomized trial design, and the distinction of being the first clinical study of the newly developed virtual drum beating system. In addition, this study investigated the effectiveness of the prefrontal cortex in response to the unique characteristics and requirements of older adults. However, it is crucial to acknowledge the limitations of this preliminary study. While the content and equipment of virtual drum beating represent a cognitive training method for older adults, the outcomes cannot be generalized to the elderly population due to the preliminary nature of this research conducted on healthy people (healthy adults aged 19 to 40 years). Before starting the study, we did not identify any prior studies using the same intervention (realistic cognitive exergame with virtual drum beating content) with the same primary outcome of HbO. Consequently, the sample size of 30 participants may have been insufficient for robust conclusions. Further studies should aim to include a more representative and larger sample size, specifically targeting older adults, building upon the insights gained from this initial investigation.
5. Conclusions
Recently, a novel virtual drum beating system was developed, capable of simultaneously training memory skills while engaging in drumming activities. This study used functional near-infrared spectroscopy (fNIRS) to examine the effects of realistic cognitive and behavioral tasks on brain activity. The primary outcome measure was the level of oxyhemoglobin in the dorsolateral prefrontal cortex (DLPFC). In the right DLPFC, a statistically significant mean difference in HbO of 0.417 ± 0.616 μm (p = 0.029) was observed. Notably, remarkable activations were recorded in the medial OFC on channels 7 and 10. The combination of memory training and virtual drumming provided distinct sensory inputs, encompassing visual, auditory, and vibrational cues. This stimulation resulted in heightened activity in the DLPFC and OFC regions for the RCE group. When compared to conventional cognitive training, the new memory training program, incorporating realistic cognitive exergaming with a virtual drum component, demonstrated a greater degree of brain activation, particularly in oxyhemoglobin levels within the VLPFC and OFC.
Author Contributions
B.-S.K. made substantial contributions to the experimental design, data analysis, and drafting of the manuscript. Y.-G.N. made substantial contributions to the data collection, data analysis, and drafting of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Culture Sports and Tourism R&D Program through the Korea Creative Content Agency grant funded by the Ministry of Culture Sports and Tourism in 2023 (Project Number SR 202106002).
Institutional Review Board Statement
This research protocol was approved by the Institutional Review Board (IRB) of Dongguk University Ilsan Hospital. This study was performed following protocols approved by the IRB (No. DUIH 2022-02-005-004) and included only subjects who provided written informed consent. Trial registration: KCT0007463 Clinical Research Information Service (CRIS), Republic of Korea.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Gates, N.J.; Vernooij, R.W.; Di Nisio, M.; Karim, S.; March, E.; Martinez, G.; Rutjes, A.W. Computerised cognitive training for preventing dementia in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2019, 3, CD012279. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, B.P.; Dattatri, N.; Le, J.; Lappinga, C.; Collins, N.L. Cognitive Training with Neurofeedback Using fNIRS Improves Cognitive Function in Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 5531. [Google Scholar] [CrossRef] [PubMed]
- Bahar-Fuchs, A.; Martyr, A.; Goh, A.M.; Sabates, J.; Clare, L. Cognitive training for people with mild to moderate dementia. Cochrane Database Syst. Rev. 2019, 3, CD013069. [Google Scholar] [CrossRef] [PubMed]
- Belleville, S. Cognitive training for persons with mild cognitive impairment. Int. Psychogeriatr. 2008, 20, 57–66. [Google Scholar] [CrossRef]
- Manera, V.; Chapoulie, E.; Bourgeois, J.; Guerchouche, R.; David, R.; Ondrej, J.; Drettakis, G.; Robert, P. A feasibility study with image-based rendered virtual reality in patients with mild cognitive impairment and dementia. PLoS ONE 2016, 11, e0151487. [Google Scholar] [CrossRef]
- Dunn, J.; Yeo, E.; Moghaddampour, P.; Chau, B.; Humbert, S. Virtual and augmented reality in the treatment of phantom limb pain: A literature review. NeuroRehabilitation 2017, 40, 595–601. [Google Scholar] [CrossRef]
- Teo, W.P.; Muthalib, M.; Yamin, S.; Hendy, A.M.; Bramstedt, K.; Kotsopoulos, E.; Perrey, S.; Ayaz, H. Does a combination of virtual reality, neuromodulation and neuroimaging provide a comprehensive platform for neurorehabilitation?–A narrative review of the literature. Front. Hum. Neurosci. 2016, 10, 284. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Di Santo, S.G.; Franchini, F.; Arlati, S.; Zangiacomi, A.; Greci, L.; Moretti, S.; Jesuthasan, N.; Marzorati, M.; Rizzo, G.; et al. Effects of combined physical and cognitive virtual reality-based training on cognitive impairment and oxidative stress in MCI patients: A pilot study. Front. Aging Neurosci. 2018, 10, 282. [Google Scholar] [CrossRef]
- Slater, M. Immersion and the illusion of presence in virtual reality. Br. J. Psychol. 2018, 109, 431–433. [Google Scholar] [CrossRef]
- Howett, D.; Castegnaro, A.; Krzywicka, K.; Hagman, J.; Marchment, D.; Henson, R.; Rio, M.; King, J.A.; Burgess, N.; Chan, D. Differentiation of mild cognitive impairment using an entorhinal cortex-based test of virtual reality navigation. Brain 2019, 142, 1751–1766. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Park, J.; Choi, Y.; Choe, M. Virtual reality sickness questionnaire (VRSQ): Motion sickness measurement index in a virtual reality environment. Appl. Ergon. 2018, 69, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.S.; Drexler, J.M.; Compton, D.E.; Stanney, K.M.; Lanham, D.S.; Harm, D.L. Configural scoring of simulator sickness, cybersickness and space adaptation syndrome: Similarities and differences. In Virtual and Adaptive Environments: Applications, Implications, and Human Performance Issues, 1st ed.; Hettinger, L.J., Haas, M.W., Eds.; Taylor & Francis Group: Oxfordshire, UK, 2003; pp. 247–275. [Google Scholar]
- Zhang, Z.; Olszewska-Guizzo, A.; Husain, S.F.; Bose, J.; Choi, J.; Tan, W.; Wang, J.; Xuan Tran, B.; Wang, B.; Jin, Y.; et al. Brief relaxation practice induces significantly more prefrontal cortex activation during arithmetic tasks comparing to viewing greenery images as revealed by functional near-infrared spectroscopy (fNIRS). Int. J. Environ. Res. Public Health 2020, 17, 8366. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q.; Supekar, K.; Menon, V. Typical and atypical development of functional human brain networks: Insights from resting-state FMRI. Front. Syst. Neurosci. 2010, 4, 1447. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.N.; Hoffman, M.D.; Trost, S.L.; Culotta, M.L.; Eilbott, J.; Tsuzuki, D.; Pelphrey, K.A. Cortical activation during action observation, action execution, and interpersonal synchrony in adults: A functional near-infrared spectroscopy (fNIRS) study. Front. Hum. Neurosci. 2017, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Egetemeir, J.; Stenneken, P.; Koehler, S.; Fallgatter, A.J.; Herrmann, M.J. Exploring the neural basis of real-life joint action: Measuring brain activation during joint table setting with functional near-infrared spectroscopy. Front. Hum. Neurosci. 2021, 5, 95. [Google Scholar] [CrossRef]
- Khoe, H.C.; Low, J.W.; Wijerathne, S.; Ann, L.S.; Salgaonkar, H.; Lomanto, D.; Choi, J.; Baek, J.; Tam, W.W.; Pei, H.; et al. Use of prefrontal cortex activity as a measure of learning curve in surgical novices: Results of a single blind randomised controlled trial. Surg. Endosc. 2020, 34, 5604–5615. [Google Scholar] [CrossRef]
- Jueptner, M.; Stephan, K.M.; Frith, C.D.; Brooks, D.J.; Frackowiak, R.S.; Passingham, R.E. Anatomy of motor learning. I. Frontal cortex and attention to action. J. Neurophysiol. 1997, 77, 1313–1324. [Google Scholar] [CrossRef]
- Mori, T.; Takeuchi, N.; Izumi, S.I. Prefrontal cortex activation during a dual task in patients with stroke. Gait Posture 2018, 59, 193–198. [Google Scholar] [CrossRef]
- Moberly, A.C.; Pisoni, D.B.; Harris, M.S. Visual working memory span in adults with cochlear implants: Some preliminary findings. World J. Otorhinolaryngol.-Head Neck Surg. 2017, 3, 224–230. [Google Scholar] [CrossRef]
- Delpy, D.T.; Cope, M.; van der Zee, P.; Arridge, S.; Wray, S.; Wyatt, J.S. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 1988, 33, 1433. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.H.; Nah, Y.; Park, S.H.; Han, S. Prefrontal cortical activation in Internet Gaming Disorder Scale high scorers during actual real-time internet gaming: A preliminary study using fNIRS. J. Behav. Addict. 2022, 11, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Strangman, G.; Culver, J.P.; Thompson, J.H.; Boas, D.A. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage 2002, 17, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Bray, S.; Bryant, D.M.; Glover, G.H.; Reiss, A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage 2011, 54, 2808–2821. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, A.; Kessels, R.P.; Heskamp, L.; Simons, E.M.; Dautzenberg, P.L.; Claassen, J.A. Prefrontal activation may predict working-memory training gain in normal aging and mild cognitive impairment. Brain Imaging Behav. 2017, 11, 141–154. [Google Scholar] [CrossRef]
- Belleville, S.; Clement, F.; Mellah, S.; Gilbert, B.; Fontaine, F.; Gauthier, S. Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain 2011, 134, 1623–1634. [Google Scholar] [CrossRef]
- Milham, M.P.; Banich, M.T.; Barad, V. Competition for priority in processing increases prefrontal cortex’s involvement in top-down control: An event-related fMRI study of the stroop task. Cogn. Brain Res. 2003, 17, 212–222. [Google Scholar] [CrossRef]
- Liston, C.; Matalon, S.; Hare, T.A.; Davidson, M.C.; Casey, B.J. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron 2006, 50, 643–653. [Google Scholar] [CrossRef]
- Kerns, J.G.; Cohen, J.D.; MacDonald, A.W., III; Cho, R.Y.; Stenger, V.A.; Carter, C.S. Anterior cingulate conflict monitoring and adjustments in control. Science 2004, 303, 1023–1026. [Google Scholar] [CrossRef]
- Goto, K.; Hoshi, Y.; Sata, M.; Kawahara, M.; Takahashi, M.; Murohashi, H. Role of the prefrontal cortex in the cognitive control of reaching movements: Near-infrared spectroscopy study. J. Biomed. Opt. 2011, 16, 127003. [Google Scholar] [CrossRef][Green Version]
- Halsband, U.; Lange, R.K. Motor learning in man: A review of functional and clinical studies. J. Physiol. Paris 2006, 99, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Hikosaka, O.; Miyauchi, S.; Takino, R.; Sasaki, Y.; Pütz, B. Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J. Neurosci. 1998, 18, 1827–1840. [Google Scholar] [CrossRef]
- Toni, I.; Krams, M.; Turner, R.; Passingham, R.E. The time course of changes during motor sequence learning: A whole-brain fMRI study. Neuroimage 1998, 8, 50–61. [Google Scholar] [CrossRef]
- Gigi, A.; Babai, R.; Penker, A.; Hendler, T.; Korczyn, A.D. Prefrontal compensatory mechanism may enable normal semantic memory performance in mild cognitive impairment (MCI). J. Neuroimaging 2010, 20, 163–168. [Google Scholar] [CrossRef]
- Birbaumer, N.; Elbert, T.; Canavan, A.G.; Rockstroh, B. Slow potentials of the cerebral cortex and behavior. Physiol. Rev. 1990, 70, 1–41. [Google Scholar] [CrossRef]
- Cui, R.Q.; Egkher, A.; Huter, D.; Lang, W.; Lindinger, G.; Deecke, L. High resolution spatiotemporal analysis of the contingent negative variation in simple or complex motor tasks and a non-motor task. Clin. Neurophysiol. 2000, 111, 1847–1859. [Google Scholar] [CrossRef]
- Critchley, H.D.; Mathias, C.J.; Josephs, O.; O’Doherty, J.; Zanini, S.; Dewar, B.K.; Cipolotti, L.; Shallice, T.; Dolan, R.J. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain 2003, 126, 2139–2152. [Google Scholar] [CrossRef]
- Dresler, T.; Schecklmann, M.; Ernst, L.H.; Pohla, C.; Warrings, B.; Fischer, M.; Polak, T.; Fallgatter, A.J. Recovery of cortical functioning in abstinent alcohol-dependent patients: Prefrontal brain oxygenation during verbal fluency at different phases during withdrawal. World J. Biol. Psychiatry 2012, 13, 135–145. [Google Scholar] [CrossRef]
- Kahlaoui, K.; Di Sante, G.; Barbeau, J.; Maheux, M.; Lesage, F.; Ska, B.; Joanette, Y. Contribution of NIRS to the study of prefrontal cortex for verbal fluency in aging. Brain Lang. 2012, 121, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.D. Orbitofrontal cortex and its contribution to decision-making. Annu. Rev. Neurosci. 2007, 30, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The functions of the orbitofrontal cortex. Brain Cogn 2004, 55, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; O’Doherty, J.; Kringelbach, M.L.; Francis, S.; Bowtell, R.; McGlone, F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb. Cortex. 2003, 13, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.P.; Zeng, X.Z.; Liu, J.Y.; Wang, J.Y.; Guo, J.Y.; Luo, F. Dynamic changes in brain activations and functional connectivity during affectively different tactile stimuli. Cell. Mol. Neurobiol. 2008, 28, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Yamanoi, Y.; Wakita, K.; Kato, R. Investigation of a cognitive strain on hand grasping induced by sensory feedback for myoelectric hand. In Proceedings of the 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 16–21 May 2016; pp. 3549–3554. [Google Scholar]
- Klatzky, R.L.; Lederman, S.J.; Hamilton, C.; Grindley, M.; Swendsen, R.H. Feeling textures through a probe: Effects of probe and surface geometry and exploratory factors. Percept. Psychophys. 2003, 65, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Culbertson, H.; Schorr, S.B.; Okamura, A.M. Haptics: The present and future of artificial touch sensation. Annu. Rev. Control Robot. Auton. Syst. 2018, 1, 385–409. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z. Mechanoreception for soft robots via intuitive body cues. Soft Robot. 2020, 7, 198–217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).