Relative Risk of Death in Bulgarian Cancer Patients during the Initial Waves of the COVID-19 Pandemic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Data Analyses
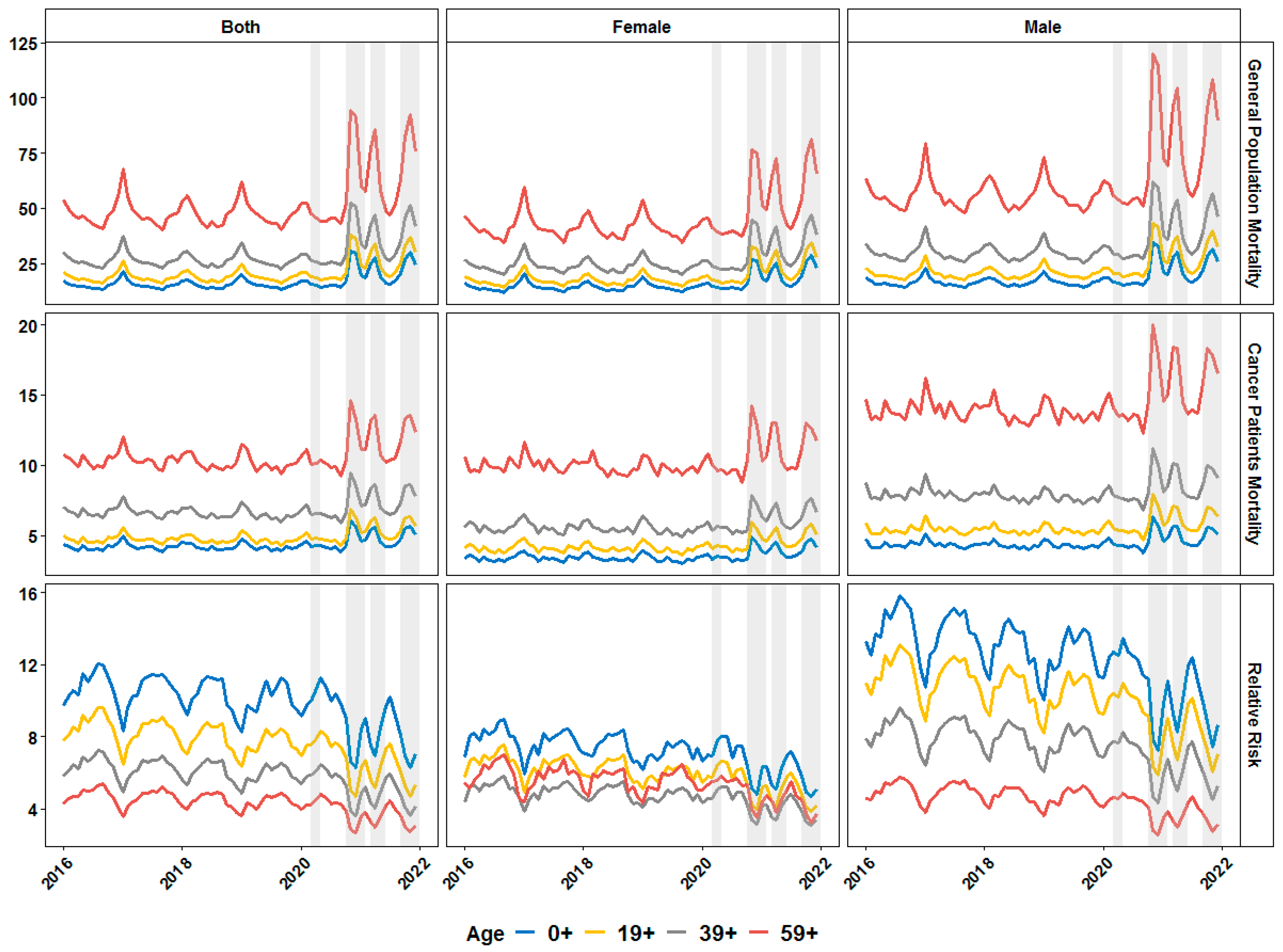
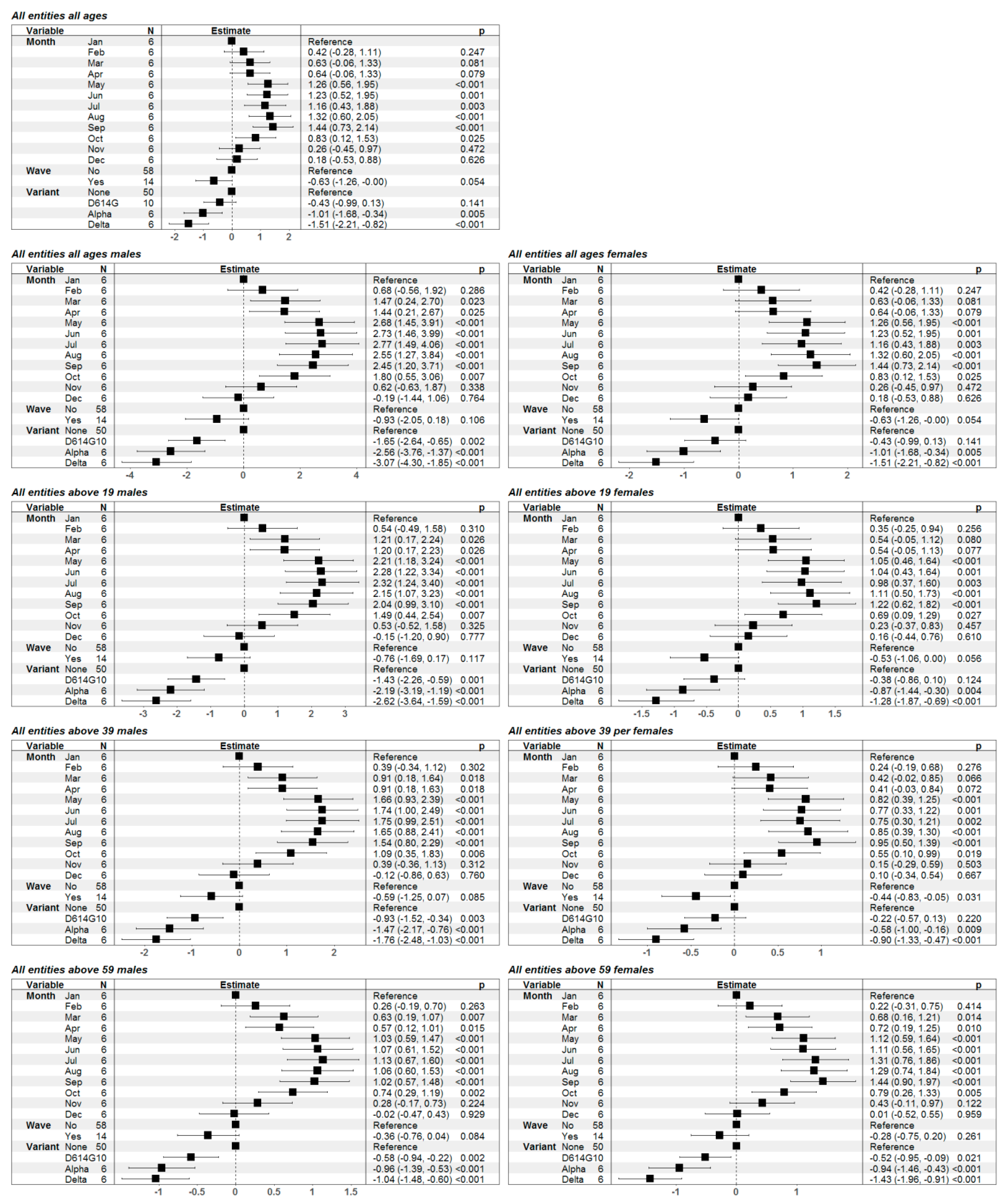
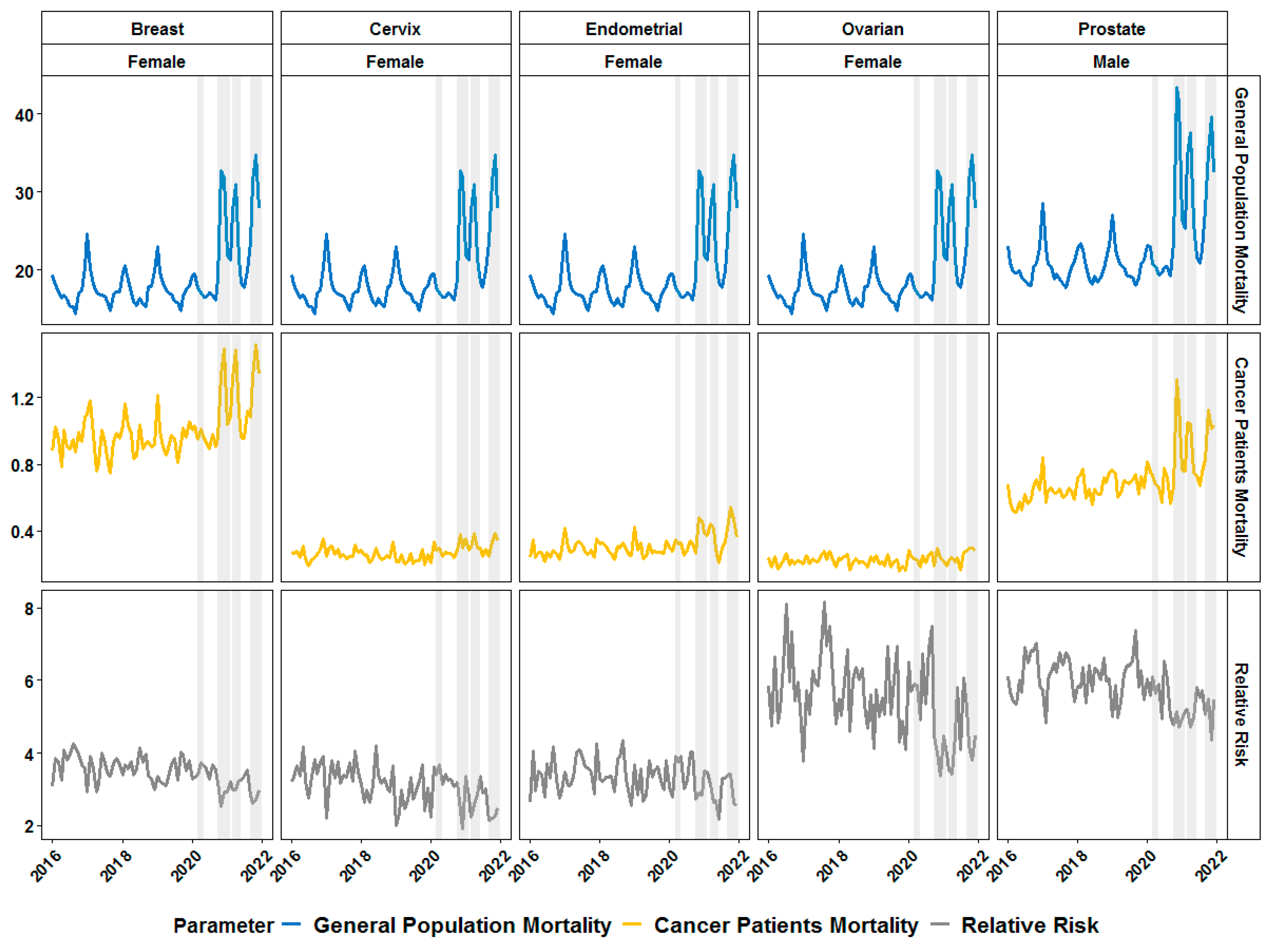
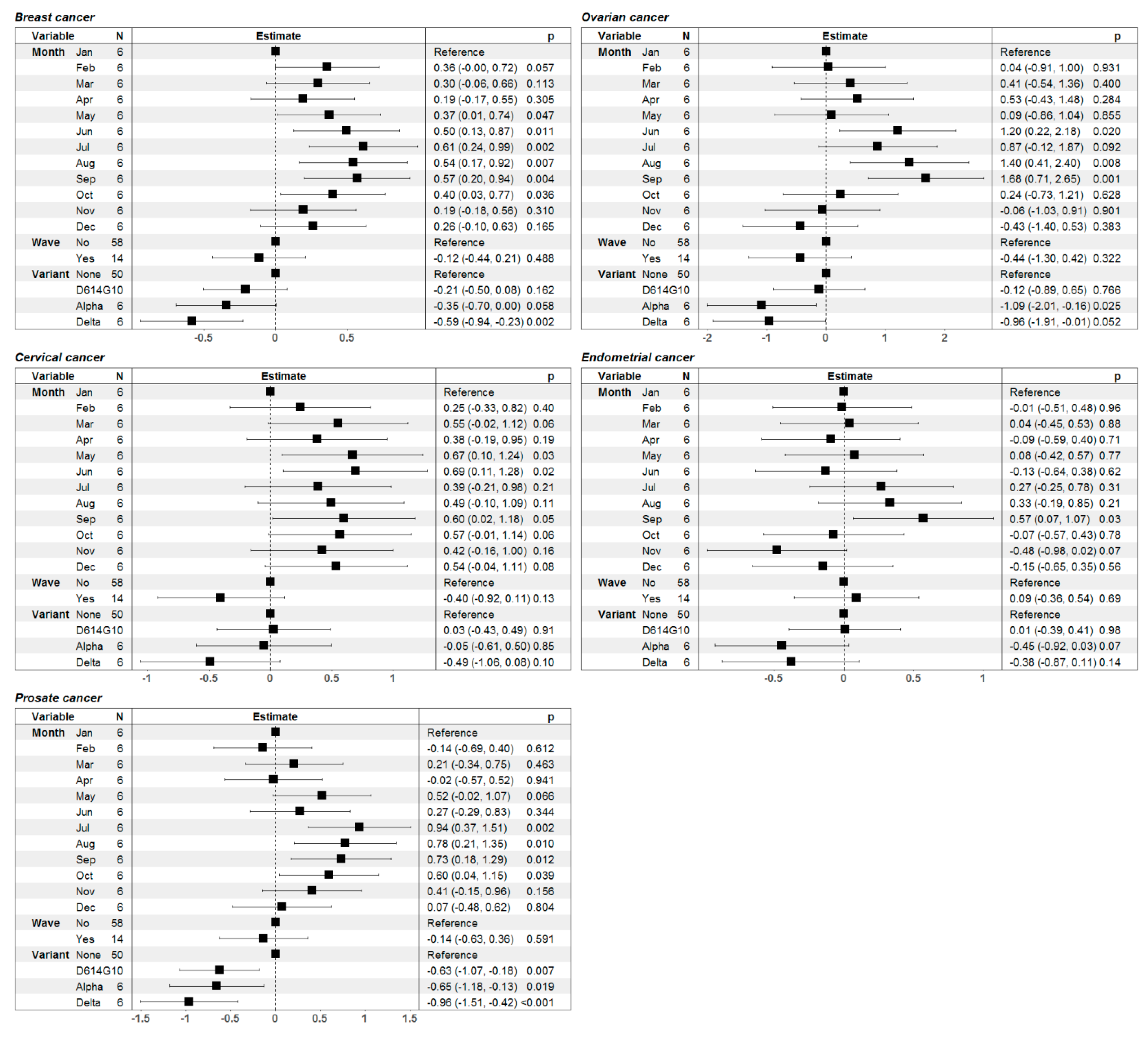
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karmakar, M.; Lantz, P.M.; Tipirneni, R. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw. Open 2021, 4, e2036462. [Google Scholar] [CrossRef]
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Chaudhry, R.; Dranitsaris, G.; Mubashir, T.; Bartoszko, J.; Riazi, S. A country level analysis measuring the impact of government actions, country preparedness and socioeconomic factors on COVID-19 mortality and related health outcomes. EClinicalMedicine 2020, 25, 100464. [Google Scholar] [CrossRef]
- Nixon, J.; Ulmann, P. The relationship between health care expenditure and health outcomes: Evidence and caveats for a causal link. Eur. J. Health Econ. 2006, 7, 7–18. [Google Scholar] [CrossRef]
- Owusu, P.A.; Sarkodie, S.A.; Pedersen, P.A. Relationship between mortality and health care expenditure: Sustainable assessment of health care system. PLoS ONE 2021, 16, e0247413. [Google Scholar] [CrossRef] [PubMed]
- Ivankova, V.; Gavurova, B.; Khouri, S. Understanding the relationships between health spending, treatable mortality and economic productivity in OECD countries. Front. Public Health 2022, 10, 1036058. [Google Scholar] [CrossRef] [PubMed]
- Mitkova, Z.; Doneva, M.; Gerasimov, N.; Tachkov, K.; Dimitrova, M.; Kamusheva, M.; Petrova, G. Analysis of healthcare expenditures in Bulgaria. Healthcare 2022, 10, 274. [Google Scholar] [CrossRef]
- Dimova, A.; Rohova, M.; Moutafova, E.; Atanasova, E.; Koeva, S.; Panteli, D.; van Ginneken, E.; WHO. Bulgaria: Health System Review; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Luengo-Fernandez, R.; Leal, J.; Gray, A.; Sullivan, R. Economic burden of cancer across the European Union: A population-based cost analysis. Lancet Oncol. 2013, 14, 1165–1174. [Google Scholar] [CrossRef]
- Hofmarcher, T.; Lindgren, P.; Wilking, N.; Jönsson, B. The cost of cancer in Europe 2018. Eur. J. Cancer 2020, 129, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Cazier, J.-B.; Starkey, T.; Briggs, S.E.; Arnold, R.; Bisht, V.; Booth, S.; Campton, N.A.; Cheng, V.W.; Collins, G. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020, 21, 1309–1316. [Google Scholar] [CrossRef]
- Lee, L.Y.; Cazier, J.-B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.; Curley, H.M.; Fittall, M.W. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Noordzij, M.; van Diepen, M.; Caskey, F.C.; Jager, K.J. Relative risk versus absolute risk: One cannot be interpreted without the other. Nephrol. Dial. Transplant. 2017, 32, ii13–ii18. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.G.; Dobson, A.J. Analysing Seasonal Health Data; Springer: Berlin/Heidelberg, Germany, 2010; Volume 30. [Google Scholar]
- Rangachev, A.; Marinov, G.K.; Mladenov, M. The impact and progression of the COVID-19 pandemic in Bulgaria in its first two years. Vaccines 2022, 10, 1901. [Google Scholar] [CrossRef] [PubMed]
- Marti-Soler, H.; Gonseth, S.; Gubelmann, C.; Stringhini, S.; Bovet, P.; Chen, P.-C.; Wojtyniak, B.; Paccaud, F.; Tsai, D.-H.; Zdrojewski, T. Seasonal variation of overall and cardiovascular mortality: A study in 19 countries from different geographic locations. PLoS ONE 2014, 9, e113500. [Google Scholar] [CrossRef] [PubMed]
- Shivarov, V.; Shivarov, H.; Yordanov, A. Seasonal Pattern of Cerebrovascular Fatalities in Cancer Patients. Healthcare 2023, 11, 456. [Google Scholar] [CrossRef]
- Simonsen, L. The global impact of influenza on morbidity and mortality. Vaccine 1999, 17, S3–S10. [Google Scholar] [CrossRef]
- Shivarov, V.; Shivarov, H.; Yordanov, A. Seasonality of Deaths Due to Heart Diseases among Cancer Patients. Medicina 2022, 58, 1651. [Google Scholar] [CrossRef]
- Audi, A.; AlIbrahim, M.; Kaddoura, M.; Hijazi, G.; Yassine, H.M.; Zaraket, H. Seasonality of respiratory viral infections: Will COVID-19 follow suit? Front. Public Health 2020, 8, 576184. [Google Scholar] [CrossRef]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Lavena Marzio, M.A.; Agnoletti, C.; Bengolea, A.; Ceirano, A.; Espinosa, F.; Saavedra, E.; Sanguine, V. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS ONE 2020, 15, e0241955. [Google Scholar] [CrossRef]
- Turtle, L.; Thorpe, M.; Drake, T.M.; Swets, M.; Palmieri, C.; Russell, C.D.; Ho, A.; Aston, S.; Wootton, D.G.; Richter, A. Outcome of COVID-19 in hospitalised immunocompromised patients: An analysis of the WHO ISARIC CCP-UK prospective cohort study. PLoS Med. 2023, 20, e1004086. [Google Scholar] [CrossRef]
- Džakula, A.; Banadinović, M.; Lovrenčić, I.L.; Vajagić, M.; Dimova, A.; Rohova, M.; Minev, M.; Scintee, S.G.; Vladescu, C.; Farcasanu, D. A comparison of health system responses to COVID-19 in Bulgaria, Croatia and Romania in 2020. Health Policy 2022, 126, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Steinert, J.I.; Sternberg, H.; Prince, H.; Fasolo, B.; Galizzi, M.M.; Büthe, T.; Veltri, G.A. COVID-19 vaccine hesitancy in eight European countries: Prevalence, determinants, and heterogeneity. Sci. Adv. 2022, 8, eabm9825. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, G.; Valkov, T.; Batselova, H.; Kounchev, O.; Momekov, G.; Argirova, R. Nationwide analysis of the impact of COVID-19 in patients with a cardiovascular, oncological or chronic pulmonary disease in the context of an Eastern European country with a low vaccination rate, Bulgaria: March 2020–April 2022. BMJ Open 2023, 13, e068431. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yin, F.; Nie, L.; Wang, Y.; Qin, J.; Chen, J. Estrogen and estrogen receptor modulators: Potential therapeutic strategies for COVID-19 and breast cancer. Front. Endocrinol. 2022, 13, 829879. [Google Scholar] [CrossRef] [PubMed]
- Lasagna, A.; Zuccaro, V.; Ferraris, E.; Corbella, M.; Bruno, R.; Pedrazzoli, P. COVID-19 and breast cancer: May the microbiome be the issue? Future Med. 2020, 17, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Parmar, H.S.; Nayak, A.; Gavel, P.K.; Jha, H.C.; Bhagwat, S.; Sharma, R. Cross talk between COVID-19 and breast cancer. Curr. Cancer Drug Targets 2021, 21, 575–600. [Google Scholar] [PubMed]
- Muscat, M.; Marinova, L.; Mankertz, A.; Gatcheva, N.; Mihneva, Z.; Santibanez, S.; Kunchev, A.; Filipova, R.; Kojouharova, M. The measles outbreak in Bulgaria, 2009–2011: An epidemiological assessment and lessons learnt. Eurosurveillance 2016, 21, 30152. [Google Scholar] [CrossRef]
- Samson, K.K.; Haynatzki, G.; Soliman, A.S.; Valerianova, Z. Temporal changes in the cervical cancer burden in Bulgaria: Implications for eastern european countries going through transition. Cancer Epidemiol. 2016, 44, 154–160. [Google Scholar] [CrossRef]
- Karcheva, M.; Yordanov, A.; Kostadinov, S. An overview of cervical cancer epidemiology and prevention in Bulgaria. Germs 2020, 10, 322. [Google Scholar] [CrossRef]
- Cronin, A.; Ibrahim, N. A scoping review of literature exploring factors affecting vaccine uptake within Roma communities across Europe. Expert Rev. Vaccines 2022, 21, 1429–1442. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.M.; Majd, H.; Richter, M.N.; Ghazizadeh, Z.; Zekavat, S.M.; Navickas, A.; Ramirez, J.T.; Asgharian, H.; Simoneau, C.R.; Bonser, L.R. Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell 2020, 27, 876–889.e812. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.L.; Tucker, M.D.; Bakouny, Z.; Labaki, C.; Hsu, C.-Y.; Shyr, Y.; Armstrong, A.J.; Beer, T.M.; Bijjula, R.R.; Bilen, M.A. Association between androgen deprivation therapy and mortality among patients with prostate cancer and COVID-19. JAMA Netw. Open 2021, 4, e2134330. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Zhong, X.; Liaw, B.; Tremblay, D.; Tsao, C.-K.; Galsky, M.; Oh, W. Does androgen deprivation therapy protect against severe complications from COVID-19? Ann. Oncol. 2020, 31, 1419–1420. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Heberer, K.; Gao, A.; Becker, D.J.; Loeb, S.; Makarov, D.V.; Gulanski, B.; DuVall, S.L.; Aslan, M.; Lee, J. A population-level analysis of the protective effects of androgen deprivation therapy against COVID-19 disease incidence and severity. Front. Med. 2022, 9, 774773. [Google Scholar] [CrossRef] [PubMed]
- Gedeborg, R.; Styrke, J.; Loeb, S.; Garmo, H.; Stattin, P. Androgen deprivation therapy and excess mortality in men with prostate cancer during the initial phase of the COVID-19 pandemic. PLoS ONE 2021, 16, e0255966. [Google Scholar] [CrossRef]
- Jiménez-Alcaide, E.; García-Fuentes, C.; Hernández, V.; De la Peña, E.; Pérez-Fernández, E.; Castro, A.; Caballero-Perea, B.; Guijarro, A.; Llorente, C. Influence of androgen deprivation therapy on the severity of COVID-19 in prostate cancer patients. Prostate 2021, 81, 1349–1354. [Google Scholar] [CrossRef]
- Schoenbeck, K.L.; Flynn, K.E. Health-related quality of life of patients with chronic myeloid leukemia as measured by patient-reported outcomes: Current state and future directions. Curr. Hematol. Malig. Rep. 2021, 16, 491–499. [Google Scholar] [CrossRef]
- Teh, J.S.; Coussement, J.; Neoh, Z.C.; Spelman, T.; Lazarakis, S.; Slavin, M.A.; Teh, B.W. Immunogenicity of COVID-19 vaccines in patients with hematologic malignancies: A systematic review and meta-analysis. Blood Adv. 2022, 6, 2014–2034. [Google Scholar] [CrossRef]
- Keppler-Hafkemeyer, A.; Greil, C.; Wratil, P.R.; Shoumariyeh, K.; Stern, M.; Hafkemeyer, A.; Ashok, D.; Hollaus, A.; Lupoli, G.; Priller, A. Potent high-avidity neutralizing antibodies and T cell responses after COVID-19 vaccination in individuals with B cell lymphoma and multiple myeloma. Nat. Cancer 2023, 4, 81–95. [Google Scholar] [CrossRef]
- De Angelis, R.; Minicozzi, P.; Sant, M.; Dal Maso, L.; Brewster, D.H.; Osca-Gelis, G.; Visser, O.; Maynadié, M.; Marcos-Gragera, R.; Troussard, X. Survival variations by country and age for lymphoid and myeloid malignancies in Europe 2000–2007: Results of EUROCARE-5 population-based study. Eur. J. Cancer 2015, 51, 2254–2268. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shivarov, V.; Grigorova, D.; Yordanov, A. Relative Risk of Death in Bulgarian Cancer Patients during the Initial Waves of the COVID-19 Pandemic. Healthcare 2023, 11, 2594. https://doi.org/10.3390/healthcare11182594
Shivarov V, Grigorova D, Yordanov A. Relative Risk of Death in Bulgarian Cancer Patients during the Initial Waves of the COVID-19 Pandemic. Healthcare. 2023; 11(18):2594. https://doi.org/10.3390/healthcare11182594
Chicago/Turabian StyleShivarov, Velizar, Denitsa Grigorova, and Angel Yordanov. 2023. "Relative Risk of Death in Bulgarian Cancer Patients during the Initial Waves of the COVID-19 Pandemic" Healthcare 11, no. 18: 2594. https://doi.org/10.3390/healthcare11182594
APA StyleShivarov, V., Grigorova, D., & Yordanov, A. (2023). Relative Risk of Death in Bulgarian Cancer Patients during the Initial Waves of the COVID-19 Pandemic. Healthcare, 11(18), 2594. https://doi.org/10.3390/healthcare11182594





