Best-Worst Scaling Survey of Inpatients’ Preferences in Medical Decision-Making Participation in China
Abstract
1. Introduction
1.1. Thematic Background
1.2. Methodological Background
2. Methods
2.1. Best-Worst Scaling Experiment
2.2. Generation of Best-Worst Scaling Factors
2.3. Questionnaire and Experimental Design
2.4. Survey and Data Collection
2.5. Statistical Analysis
- The BW score is the number of times an attribute is selected as the most important minus the number of times it is chosen as the least important. If the BW score is a positive number, the attribute is selected as the most important more often than the least important, or vice versa [70].
- Scaled BW score is the square root of the total best score divided by the total worst score. It designates the choice probability relative to the most essential attribute [71].
- The mean BW score equals the BW score divided by the number of respondents responding to each attribute.
3. Results
3.1. Respondents’ Demographic Characteristics
3.2. Results of the Best-Worst Scaling Survey
3.3. Heterogeneity
4. Discussion
Limitations and Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Castro, E.M.; Van Regenmortel, T.; Vanhaecht, K.; Sermeus, W.; Van Hecke, A. Patient Empowerment, Patient Participation and Patient-Centeredness in Hospital Care: A Concept Analysis Based on A Literature Review. Patient Educ. Couns. 2016, 12, 1923–1939. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Williams, N.; Elwyn, G.; Edwards, A. Knowledge is Not Power for Patients: A Systematic Review and Thematic Synthesis of Patient-Reported Barriers and Facilitators to Shared Decision Making. Patient Educ. Couns. Patient Educ. Couns. 2014, 94, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Q.; Liang, L. Study on the Theory and Practical Problem of “Patient Participation” in Clinical Decision Making. Chin. Med. Ethics. 2018, 6, 799–803. [Google Scholar]
- Müller-Engelmann, M.; Keller, H.; Donner-Banzhoff, N.; Krones., T. Shared Decision Making in Medicine: The Influence of Situational Treatment Factors. Patient Educ. Couns. 2011, 82, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P. Encouraging Patients to Take Part in Their Own Care. Nurs. Times 1995, 91, 42–43. [Google Scholar] [PubMed]
- Hoffman, T.C.; Montori, V.M.; Del Mar, C. The Connection Between Evidence-Based Medicine and Shared Decision Making. JAMA 2014, 312, 1295–1296. [Google Scholar] [CrossRef]
- Liying, C. The Impact of Patient Participation Behavior on Patient Satisfaction. Master’s Thesis, Zhejiang University, Hangzhou, China, 2013. [Google Scholar]
- Hack, T.F.; Degner, L.F.; Watson, P.; Sinha, L. Do Patients Benefit from Participating in Medical Decision Making? Longitudinal Follow-Up of Women With Breast Cancer. Psycho Oncol. 2006, 15, 9–19. [Google Scholar] [CrossRef]
- Hamann, J.; Langer, B.; Winkler, V.; Busch, R.; Cohen, R.; Leucht, S.; Kissling, W. Shared Decision Making for In Patients With Schizophrenia. Acta Psychiatr. Scand. 2006, 114, 265–273. [Google Scholar] [CrossRef]
- Hawley, S.T.; Lantz, P.M.; Janz, N.K.; Salem, B.; Morrow, M.; Schwartz, K.; Liu, L.; Katz, S.J. Factors Associated with Patient Involvement in Surgical Treatment Decision Making for Breast Cancer. Patient Educ. Couns. Patient Ed. 2007, 65, 387–395. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Taneda, A.; Sasaki, N.; Mizuno, M.; Sakata, M.; Fukui, S.; Hisanaga, F.; Bernick, P.; Ito, J.; Matsunaga, A.; et al. Efficacy of a Peer-Led, Recovery-Oriented Shared Decision-Making System: A Pilot Randomized Controlled Trial. Psychiatr. Serv. 2017, 68, 1307–1311. [Google Scholar] [CrossRef]
- Saheb, K.M.; Mcgill, E.T.; Berger, Z.D. Shared Decision-Making and Outcomes in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Patient Educ. Couns. 2017, 100, 2159–2171. [Google Scholar] [CrossRef]
- Coxeter, P.; Del, M.C.; Mcgregor, L.; Beller, E.M.; Hoffmann, T.C. Interventions to Facilitate Shared Decision Making to Address Antibiotic Use for Acute Respiratory Infections in Primary Care. Cochrane Database Syst. Rev. 2015, 11, CD010907. [Google Scholar] [CrossRef]
- Mohammadpour, S.; Yousefi, M.; Javan-Noughabi, J.; Sharifi, T.; Niknam, N.; Vakilzadeh, A.K.; Ariafar, A.; Shahidi, N.A. Shared decision making for patients with COVID-19 in a public training hospital in Mashhad, Iran. Int. J. Healthc. Manag. 2022, 1–9. [Google Scholar] [CrossRef]
- Kother, A.K.; Siebenhaar, K.U.; Alpers, G.W. Shared Decision Making during the COVID-19 Pandemic. Med. Decis. Mak. 2021, 41, 430–438. [Google Scholar] [CrossRef]
- Wang, L.P.; Xu, A.J.; Jiang, L.J.; Liu, Y.; Chen, A.Q. An Analysis on the Relationship Between Health Self-Evaluation and Healthcare Seeking Decision for the Patients With Hypertension. Chin. J. Health Pol. 2017, 10, 59–65. [Google Scholar] [CrossRef]
- Hajizadeh, N.; Uhler, L.M.; Pérez Figueroa, R.E. Understanding Patients’ and Doctors’ Attitudes About Shared Decision Making for Advance Care Planning. Health Expect. 2015, 18, 2054–2065. [Google Scholar] [CrossRef]
- Sahlsten, M.J.M.; Larsson, I.E.; PLos, K.A.E.; Lindencrona, C.S. Hindrance for Patient Participation in Nursing Care. Scand. J. Caring Sci. 2005, 19, 223–229. [Google Scholar] [CrossRef]
- Sainio, C.; Eriksson, E.; Lauri, S. Patient Participation in Decision Making About Care. Cancer Nurs. 2001, 24, 172–179. [Google Scholar] [CrossRef]
- Davis, R.E.; Dolan, G.; Thomas, S.; Atwell, C.; Mead, D.; Nehammer, S.; Moseley, L.; Edwards, A.; Elwyn, G. Exploring Doctor and Patient Views About Risk Communication and Shared Decision-Making in the Consultation. Health Expect. 2003, 6, 198–207. [Google Scholar] [CrossRef]
- Heisler, M.; Vijan, S.; Anderson, R.M.; Ubel, P.A.; Bernstein, S.J.; Hofer, T.P. When Do Patients and Their Physicians Agree on Diabetes Treatment Goals and Strategies, and What Difference Does it Make? J. Gen. Intern. Med. 2003, 18, 893–902. [Google Scholar] [CrossRef]
- Attanasio, L.B.; Kozhimannil, K.B.; Kjerulff, K.H. Factors Influencing Women’s Perceptions of Shared Decision Making During Labor and Delivery: Results from a Large-Scale Cohort Study of First Childbirth. Patient Educ. Couns. 2018, 101, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.A.; Leonhart, R.; Helmes, A.W. Communication Matters: The Impact of Communication and Participation in Decision-Making on Breast Cancer Patients’ Depression and Quality of Life. Patient Educ. Couns. 2009, 77, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhou, Y.-Y.; Gao, Y.; He, Z. Quality and Influencing Factors of Patient Involvement in Clinical Decision-Making in Diagnosis and Treatment of Stable Coronary Artery Disease. Basic Clin. Med. 2022, 42, 1572–1576. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Zhang, X.-Y.; Zhu, B.-Q.; Zhao, L. Stepping into the Cancer Patients: Qualitative Study on Elements of Patients’ Participation in Medical Decision-Making and Their Health Outcomes. Chin. Med. Ethics 2022, 35, 1230–1240. [Google Scholar] [CrossRef]
- Meng, Z.-X.; Zhao, X.; Li, C. Current Analysis of Research on Cancer Patients’ Participation in Treatment Decision Making. Modern Nurse 2022, 29, 9–13. [Google Scholar] [CrossRef]
- Xiao, L.; Peng, M.-F.; Liu, Y.-W.; Zhang, L.-L. Effect of Participation Competence on Perceived Shared Decision Making Among Cancer Patients. J. Nurs. Sci. 2022, 37, 42–45. [Google Scholar] [CrossRef]
- Hahlweg, P.; Kriston, L.; Scholl, I.; Brähler, E.; Faller, H.; Schulz, H.; Weis, J.; Koch, U.; Wegscheider, K.; Mehnert, A.; et al. Cancer Patients’ Preferred and Perceived Level of Involvement in Treatment Decision-Making: An Epidemiological Study. Acta Oncol. 2020, 59, 967–974. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Z.; Liu, P.; Li, L. Current Status of Participation in Surgery Decision-Making of Informed Primary Liver Cancer Patients and its Influence Factors. J. Nurs. 2016, 23, 6–11. [Google Scholar] [CrossRef]
- Luo, H.; Liu, G.; Lu, J.; Xue, D. Association of Shared Decision Making With Inpatient Satisfaction: A Cross-Sectional Study. BMC Med. Inform. Decis. Mak. 2021, 21, 25. [Google Scholar] [CrossRef]
- Fraenkel, L. Incorporating Patients’ Preferences into Medical Decision Making. Med. Care Res. Rev. 2013, 70, 80S–93S. [Google Scholar] [CrossRef]
- Brennan, P.F.; Strombom, I. Improving Health Care by Understanding Patient Preferences: The Role of Computer Technology. J. Am. Med. Inform. Assoc. 1998, 5, 257–262. [Google Scholar] [CrossRef]
- Bouvy, J.C.; Cowie, L.; Lovett, R.; Morrison, D.; Livingstone, H.; Crabb, N. Use of Patient Preference Studies in HTA Decision Making: A NICE Perspective. Patient 2020, 13, 145–149. [Google Scholar] [CrossRef]
- Whitty, J.A.; Oliveira Gonçalves, A.S. A Systematic Review Comparing the Acceptability, Validity and Concordance of Discrete Choice Experiments and Best–Worst Scaling for Eliciting Preferences in Healthcare. Patient 2018, 11, 301–317. [Google Scholar] [CrossRef]
- Starmer, C. Developments in Non-Expected Utility Theory: The Hunt for a Descriptive Theory of Choice Under Risk. J. Econ. Lit. 2000, 38, 332–382. [Google Scholar] [CrossRef]
- Van Houtven, G.; Johnson, F.R.; Kilambi, V.; Hauber, A.B. Eliciting Benefit–Risk Preferences and Probability-Weighted Utility Using Choice-Format Conjoint Analysis. Med. Decis. Making 2011, 31, 469–480. [Google Scholar] [CrossRef]
- Cheung, K.L.; Wijnen, B.F.; Hollin, I.L.; Janssen, E.M.; Bridges, J.F.; Evers, S.M.; Hiligsmann, M. Using Best–Worst Scaling to Investigate Preferences in Health Care. Pharmacoeconomics 2016, 34, 1195–1209. [Google Scholar] [CrossRef]
- Flynn, T.N.; Louviere, J.J.; Peters, T.J.; Coast, J. Best–Worst Scaling: What it Can Do for Health Care Research and How to Do It. J. Health Econ. 2007, 26, 171–189. [Google Scholar] [CrossRef]
- Louviere, J.J.; Flynn, T.N.; Marley, A.A.J. Best-Worst Scaling: Theory, Methods and Applications; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Crossnohere, N.L.; Janse, S.; Janssen, E.; Bridges, J.F.P. Comparing the Preferences of Patients and the General Public for Treatment Outcomes in Type 2 Diabetes Mellitus. Patient 2021, 14, 89–100. [Google Scholar] [CrossRef]
- Zhang, L.-F.; Huang, J.-J.; Wang, H.; Jiang, S. An Overview on Theoretic and Applied Research of Best-Worst Scaling. J. Stat. Info. 2019, 34, 24–30. [Google Scholar]
- Hensher, D.A.; Rose, J.M.; Greene, W.H. Applied Choice Analysis: A Primer; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Kaya Pezük, Ş.; Senel, G. An Application of Soft Multisets to a Decision-Making Problem Concerning Side Effects of COVID-19 Vaccines. J. New Theory 2021, 103–113. [Google Scholar] [CrossRef]
- Turbitt, E.; D’Amanda, C.; Hyman, S.; Weber, J.D.; Bridges, J.F.; Peay, H.L.; Biesecker, B.B. Parent Clinical Trial Priorities for Fragile X Syndrome: A Best-Worst Scaling. Eur. J. Hum. Gen. 2021, 29, 1245–1251. [Google Scholar] [CrossRef]
- Paquin, R.S.; Fischer, R.; Mansfield, C.; Mange, B.; Beaverson, K.; Ganot, A.; Martin, A.S.; Morris, C.; Rensch, C.; Ricotti, V.; et al. Priorities When Deciding on Participation in Early-Phase Gene Therapy Trials for Duchenne Muscular Dystrophy: A Best-Worst Scaling Experiment in Caregivers and Adult Patients. Orphanet J. Rare Dis. 2019, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.H.; Zhou, L.; Wong, E.L.-Y.; Wang, D.; Xiang, G.C.; Xu, C. A Best-Worst Scaling Survey of Medical Students’ Perspective on Implementing Shared Decision-Making in China. BMC Med. Educ. 2020, 20, 486. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Tsuge, T. Best-Worst Scaling Survey of Preferences Regarding the Adverse Effects of Tobacco Use in China. SSM Popul. Health 2017, 3, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.H.; Zhou, L.M.; Wong, E.L.; Wang, D. Investigating Medical Student’s Preferences for Internet-Based Healthcare Services: A Best-Worst Scaling Survey. Front. Public Health 2021, 9, 757310. [Google Scholar] [CrossRef] [PubMed]
- Molassiotis, A.; Emsley, R.; Ashcroft, D.; Caress, A.; Ellis, J.; Wagland, R.; Bailey, C.D.; Haines, J.; Williams, M.L.; Lorigan, P.; et al. Applying Best–Worst Scaling Methodology to Establish Delivery Preferences of a Symptom Supportive Care Intervention in Patients With Lung Cancer. Lung Cancer 2012, 77, 199–204. [Google Scholar] [CrossRef]
- Mühlbacher, A.C.; Sadler, A.; Lamprecht, B.; Juhnke, C. Patient Preferences in the Treatment of Hemophilia: A Best–Worst Scaling Case 3 Analysis. Value Health 2020, 23, 862–869. [Google Scholar] [CrossRef]
- Mühlbacher, A.; Johnson, F.R. Choice Experiments to Quantify Preferences for Health and Healthcare: State of the Practice. Appl. Health Econ. Health Policy 2016, 14, 253–266. [Google Scholar] [CrossRef]
- Hensher, D.A.; Rose, J.M.; Greene, W.H. Applied Choice Analysis, 2nd ed.; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Mcfadden, D. Conditional Logit Analysis of Qualitative Choice Behavior. In Frontiers in Econometrics; Zarembka, P., Ed.; Academic Press: Salt Lake City, UT, USA, 1974. [Google Scholar]
- Hein, K.A.; Jaeger, S.R.; Carr, B.T.; Delahunty, C.M. Comparison of Five Common Acceptance and Preference Methods. Food Qual. Prefer. 2008, 19, 651–661. [Google Scholar] [CrossRef]
- Cohen, I.L. Criterion-Related Validity of the PDD Behavior Inventory. J. Autism Dev. Disord. 2003, 33, 47–53. [Google Scholar] [CrossRef]
- Jaeger, T.F. Categorical Data Analysis: Away from Anovas (Transformation or Not) and Towards Logit Mixed Models. J. Mem. Lang. 2008, 5, 434–446. [Google Scholar] [CrossRef]
- Adamsen, J.M.; Rundle-Thiele, S.; Whitty, J.A. Best-Worst Scaling Reflections on Presentation, Analysis, and Lessons Learnt from Case 3 BWS Experiments. Market Soc. Res. 2013, 2, 9–27. [Google Scholar]
- Qian, L.-R.; Wang, W.-J. On Patients’ Rights and Legal Protection. Chin. Med. Ethics 2011, 24, 3. [Google Scholar] [CrossRef]
- Bi, Y.-T.; Lin, W.-J.; Cheng, X.-B. Strengthening Hospital System Culture to Build a Harmonious Doctor-Patient Environment. Lab Med. Clin. 2011, 8, 366–368. [Google Scholar]
- Medveckis, A.; Pigozne, T. Satisfaction With Environment of Regional Health Care Institution: Parents’ Opinions and Societal Stereotypes. Econ. Sci. Rural. Dev. Conf. Proc. 2018, 52, 84–92. [Google Scholar] [CrossRef]
- Brown, J.B.; Brett, P.; Stewart, M.; Marshall, J.N. Roles and Influence of People Who Accompany Parents on Visits to the Doctor. Canadian Fam. Phys. 1998, 44, 1644. [Google Scholar]
- Prusiński, T. The Role of Doctors’ Authority in Patients’ Treatment Decisions. Am. J. Health Behav. 2022, 46, 503–514. [Google Scholar] [CrossRef]
- Wang, M.; Hung, L.; Lo, Y.T. Glycemic Control in Type 2 Diabetes: Role of Health Literacy and Shared Decision-Making. Patient Prefer. Adherence 2019, 13, 871–879. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, H.; Shang, L.; Li, D.; Wang, R.; Zhang, R.; Xu, Y. Preferences and Perceived Involvement in Treatment Decision-Making Among Chinese Patients With Chronic Hepatitis. Med. Decis. Making 2011, 31, 245–253. [Google Scholar] [CrossRef]
- Kraetschmer, N.; Sharpe, N.; Urowitz, S.; Deber, R.B. How Does Trust Affect Patient Preferences for Participation in Decision-Making? Health Expect. 2004, 7, 317–326. [Google Scholar] [CrossRef]
- Viklund, P.; Lagergren, J. A Care Pathway for Patients With Oesophagael Cancer. Eur. J. Cancer Care 2010, 16, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Soutar, G.N.; Louviere, J. Measuring Values Using Best-Worst Scaling: The LOV Example. Psychol. Mark. 2007, 24, 1043–1058. [Google Scholar] [CrossRef]
- Lawson, J. Design and Analysis of Experiments With R; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Lancsar, E.; Louviere, J. Conducting Discrete Choice Experiments to Inform Healthcare Decision Making: A User’s Guide. Pharmacoeconomics 2008, 26, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Beusterien, K.; Kennelly, M.J.; Bridges, J.F.P.; Amos, K.; Williams, M.J.; Vasavada, S. Use of Best-Worst Scaling to Assess Patient Perceptions of Treatments for Refractory Overactive Bladder. Neurourol. Urodyn. 2016, 35, 1028–1033. [Google Scholar] [CrossRef]
- Uy, E.J.B.; Bautista, D.C.; Xin, X.; Cheung, Y.B.; Thio, S.T.; Thumboo, J. Using Best-Worst Scaling Choice Experiments to Elicit the Most Important Domains of Health for Health-Related Quality of Life in Singapore. PLoS ONE 2018, 13, e0189687. [Google Scholar] [CrossRef]
- Croissant, Y. Estimation of Random Utility Models in R: The Mlogit Package. J. Stat. Softw. 2020, 95, 1–41. [Google Scholar] [CrossRef]
- Thurstone, L.L. A Law Of Comparative Judgment. Psychol. Rev. 1927, 34, 273–286. [Google Scholar] [CrossRef]
- Mühlbacher, A.C.; Zweifel, P.; Kaczynski, A.; Johnson, F.R. Experimental Measurement of Preferences in Health Care Using Best-Worst Scaling (BWS): Theoretical and Statistical Issues. Health Econ. Rev. 2016, 6, 5. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment For Statistical Computing; R Foundation For Statistical Computing: Vienna, Austria, 2013; Available online: https://www.r-project.org/ (accessed on 1 July 2021).
- Wang, J.-Q. Overview on Fuzzy Multi-Criteria Decision-Making Approach. Control. Decis. 2008, 601–606. [Google Scholar] [CrossRef]
- Mohr, W.; Rädke, A.; Michalowsky, B.; Hoffmann, W. Elicitation of Quantitative Choice-Based Preferences for Person-Centered Care Among People Living With Dementia in Comparison to Physicians’ Judgements in Germany: Study Protocol for the Mixed-Methods Predemcare-Study. BMC Geriatr. 2022, 22, 567. [Google Scholar] [CrossRef]
- Wang, S. A Research of the Relationship Among Patient Participation, Doctor-Patient Psychological Contract Violation and Patients’ Trust. Master’s Thesis, Zhejiang Normal University, Jinhua, China, 2010. [Google Scholar]
- Peng, Y.; Yin, P.; Deng, Z.; Wang, R. Patient-Physician Interaction and Trust in Online Health Community: The Role of Perceived Usefulness of Health Information and Services. Int. J. Environ. Res. Public Health 2019, 17, 139. [Google Scholar] [CrossRef]
- Peek, M.E.; Gorawara-Bhat, R.; Quinn, M.T.; Odoms-Young, A.; Wilson, S.C.; Chin, M.H. Patient Trust in Physicians and Shared Decision-Making Among African-Americans With Diabetes. Health Commun. 2013, 28, 616–623. [Google Scholar] [CrossRef]
- Wu, Q. Study on Process, Influencing Factors and Information Processing Feature of Atrial Fibrillation Patient Engagement in Treatment Decision Making. Ph.D. Thesis, Naval Medical University, Shanghai, China, 2019. [Google Scholar]
- Trachtenberg, F.; Dugan, E.; Hall, M.A. How Patients’ Trust Relates to Their Involvement in Medical Care. J. Fam. Pract. 2005, 54, 344–352. [Google Scholar]
- Liu, J.; Du, X.; Ma, C.; Zhang, P.; Wu, X. Effect of the Trust Degree to Doctor on Clinical Decision Preference for Patients With Cardiovascular Disease. China Herald. 2014, 11, 135–138. [Google Scholar]
- Wu, F.; Wen, H.; Tao, W.; Zhou, H. Family Participation in Medical Decision-Making. Chin. J Manag. Sci. 2020, 10, 68–72. [Google Scholar] [CrossRef]
- Zadro, J.R.; Traeger, A.C.; Décary, S.; O’Keeffe, M. Problem With Patient Decision Aids. BMJ Evid. Based Med. 2020, 26, 180–183. [Google Scholar] [CrossRef]
- Hardy, C.; Penn, S.; Morris, T. Attitudes of Prostate Cancer Patients Towards the Diagnosis and Treatment of Their Disease: Findings from a Multinational Survey. Curr. Med. Res. Opin. 2007, 23, 2107–2116. [Google Scholar] [CrossRef]
- Nease, R.F.; Brooks, W.B. Patient Desire for Information and Decision Making in Health Care Decisions: The Autonomy Preference Index and the Health Opinion Survey. J. Gen. Intern. Med. 1995, 10, 593–600. [Google Scholar] [CrossRef]
- Zhao, D.H.; Rao, K.Q.; Zhang, Z.R. Patient Trust in Physicians: Empirical Evidence from Shanghai, China. Chin. Med. J. 2016, 129, 814–818. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Zhang, Q.-W.; Wang, H.-Q.; Dong, X.-X.; Chen, X.-Y.; Fan, Z.-Y.; Wei, X.; Yin, Q.-H.; Shang, L.-L.; Zhang, M.-M. A Survey Analysis of the Feasibility of a Bidirectional Way for Encouraging Shared Decision-Making Between Physicians and Patients. Med. Philos. B. 2013, 34, 94–97. [Google Scholar]
- Nacoti, M.; Ciocca, A.; Giupponi, A.; Brambillasca, P.; Lussana, F.; Pisano, M.; Goisis, G.; Bonacina, D.; Fazzi, H.; Naspro, R.; et al. At the epicenter of the Covid-19 pandemic and humanitarian crises in Italy: Changing perspectives on preparation and mitigation. N. Engl. J. Med. 2020, 1, 1–5. [Google Scholar]
- Abrams, E.M.; Shaker, M.; Oppenheimer, J.; Davis, R.S.; Bukstein, D.A.; Greenhawt, M. The challenges and opportunities for shared decision making highlighted by COVID-19. J. Allergy Clin. Immunol. 2020, 8, 2474–2480.e1. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis; National Academies Press: Washington, DC, USA, 2013. [Google Scholar]
- Yao, J. Patient-Physician Shared Decision-Making in Ophthalmology Care. Med. Philos. 2018, 39, 22–23. [Google Scholar] [CrossRef]
- Li, J.-W.; Zhang, R.-H.; Yang, L. Study on Participation Behaviors of Patients: Dimensions, Motivation and Countermeasures. Med. Philos. 2014, 35, 46–49. [Google Scholar] [CrossRef]
- Mohammed, E.S.; Seedhom, A.E.; Ghazawy, E.R. Awareness and Practice of Patient Rights from a Patient Perspective: An Insight from Upper Egypt. Int. J. Qual. Health Care. 2018, 30, 145–151. [Google Scholar] [CrossRef]
- Lee, Y.K.; Ng, C.J.; Lee, P.Y.; Tong, W.T.; Sa’at, H. Shared decision-making in Malaysia: Legislation, patient involvement, implementation and the impact of COVID-19. Z. Fur Evidenz Fortbild. Und Qual. Im Gesundh. 2022, 171, 89–92. [Google Scholar] [CrossRef]
- Cong, Y. Rethinking on the Doctor-Patient-Relationship. Chin. Med. Ethics. 2017, 30, 666–669. [Google Scholar] [CrossRef]

| Factor | Abbreviation | Description | |
|---|---|---|---|
| 1 | Sound medical laws and regulations | Law | The government has comprehensive laws and administrative regulations and a complete legal system to protect patients’ rights [58]. |
| 2 | Sound hospital rules and regulations | Rule | The hospital the patient visits has clear and complete rules and regulations that govern health workers’ behaviors and clarify codes of conduct and reward and punishment mechanisms [59]. |
| 3 | Medical environment | Environment | The medical environment should positively influence patients, that is, it should be convenient, comfortable, and patient-centered, and help patients’ recovery, satisfy their needs, and ease their pain [60]. |
| 4 | Influence of the surrounding people | People | Comments by people around patients (e.g., families, friends, and colleagues) on the hospital or the physician, as well as successful cases of other people participating in decision-making [61]. |
| 5 | Physicians’ attitudes | Attitude | The physician provides humane care, has good peer relationships, adheres to work ethics, and is passionate, sincere, calm, and careful [18]. |
| 6 | Physicians’ professional expertise | Expertise | The physician shows a high level of clinical expertise and skills and is capable of achieving patients’ goals in terms of treatment [62]. |
| 7 | Physicians’ communication ability | Communication | The physician can deliver necessary information with correct, accurate, and plain language, show humaneness and compassion, and listen to the patient [20]. |
| 8 | Consultation time duration | Time | The physician has enough time for examination, diagnosis, treatment, and patient communication regarding illness and treatment [17]. |
| 9 | Patients’ health literacy | Literacy | Patients can obtain and understand health information and use it to maintain or improve their well-being [63]. |
| 10 | Patients’ awareness of their illness | Awareness | Patients can identify and understand their illnesses correctly and accurately [64]. |
| 11 | Patients’ trust in physicians | Trust | Patients and physicians trust and respect each other; patients believe that physicians will do their best with regard to treatment [65]. |
| 12 | Patients’ ability to participate | Ability | Patients can obtain medical information related to disease, treatment, and recovery before consultation, communicate or collaborate with physicians during the interaction, and have adequate ability to make decisions and protect their rights [27]. |
| 13 | Patients’ ability to bear the disease burden | Burden | Patients can bear health or economic burdens related to pain, disability, and premature death resulting from their diseases [66]. |
| Factor | Choice Task (CT) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT1 | CT2 | CT3 | CT4 | CT5 | CT6 | CT7 | CT8 | CT9 | CT10 | CT11 | CT12 | CT13 | |
| Environment | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Communication | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Law | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Trust | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Expertise | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| People | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Awareness | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Time | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Ability | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 |
| Rule | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 |
| Attitude | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Literacy | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| Burden | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Characteristics | Category | Frequency (n) | Composition Ratio (%) | Characteristic | Category | Frequency (n) | Composition Ratio (%) |
|---|---|---|---|---|---|---|---|
| Monthly family income (CNY) | <10,000 | 310 | 38.1 | Number of hospitalizations in the last year | 0 | 525 | 64.5 |
| 10,00020,000 | 304 | 37.3 | 1~2 | 226 | 27.8 | ||
| >20,000 | 200 | 24.6 | ≥3 | 63 | 7.7 | ||
| Age (y) | ≤25 | 77 | 9.5 | Department of hospitalization | Internal medicine | 176 | 21.6 |
| 26~35 | 204 | 25.1 | Surgery | 378 | 46.4 | ||
| 36~45 | 186 | 22.9 | Gynecology | 86 | 10.6 | ||
| 46~55 | 165 | 20.3 | Otorhinolaryngology | 82 | 10.1 | ||
| ≥56 | 182 | 22.4 | Other | 92 | 11.3 | ||
| Gender | Male | 404 | 49.6 | Academic degree | Middle school and below | 467 | 57.4 |
| Female | 410 | 50.4 | College | 145 | 17.8 | ||
| Undergraduate | 164 | 20.1 | |||||
| Masters or above | 38 | 4.7 |
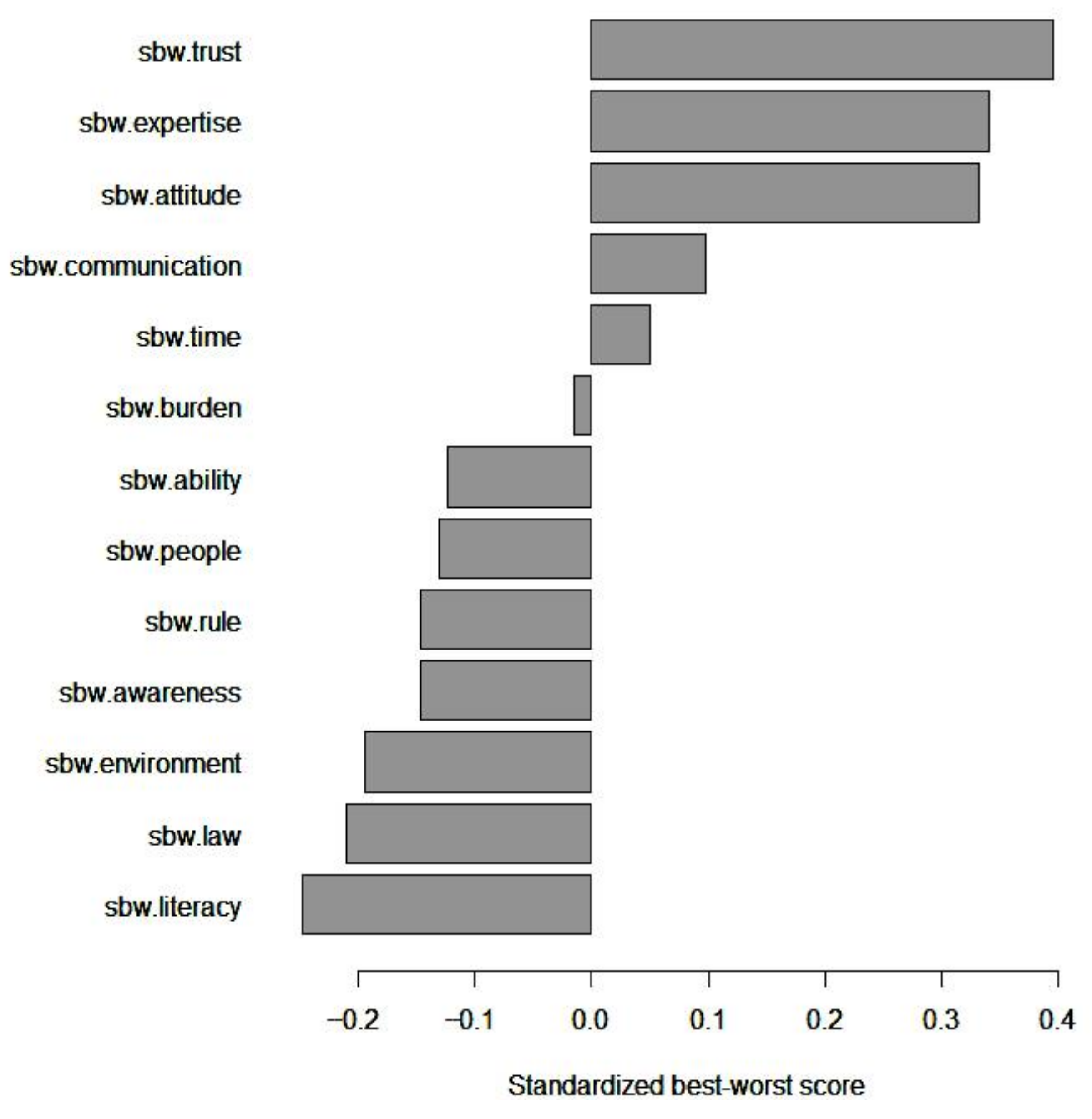
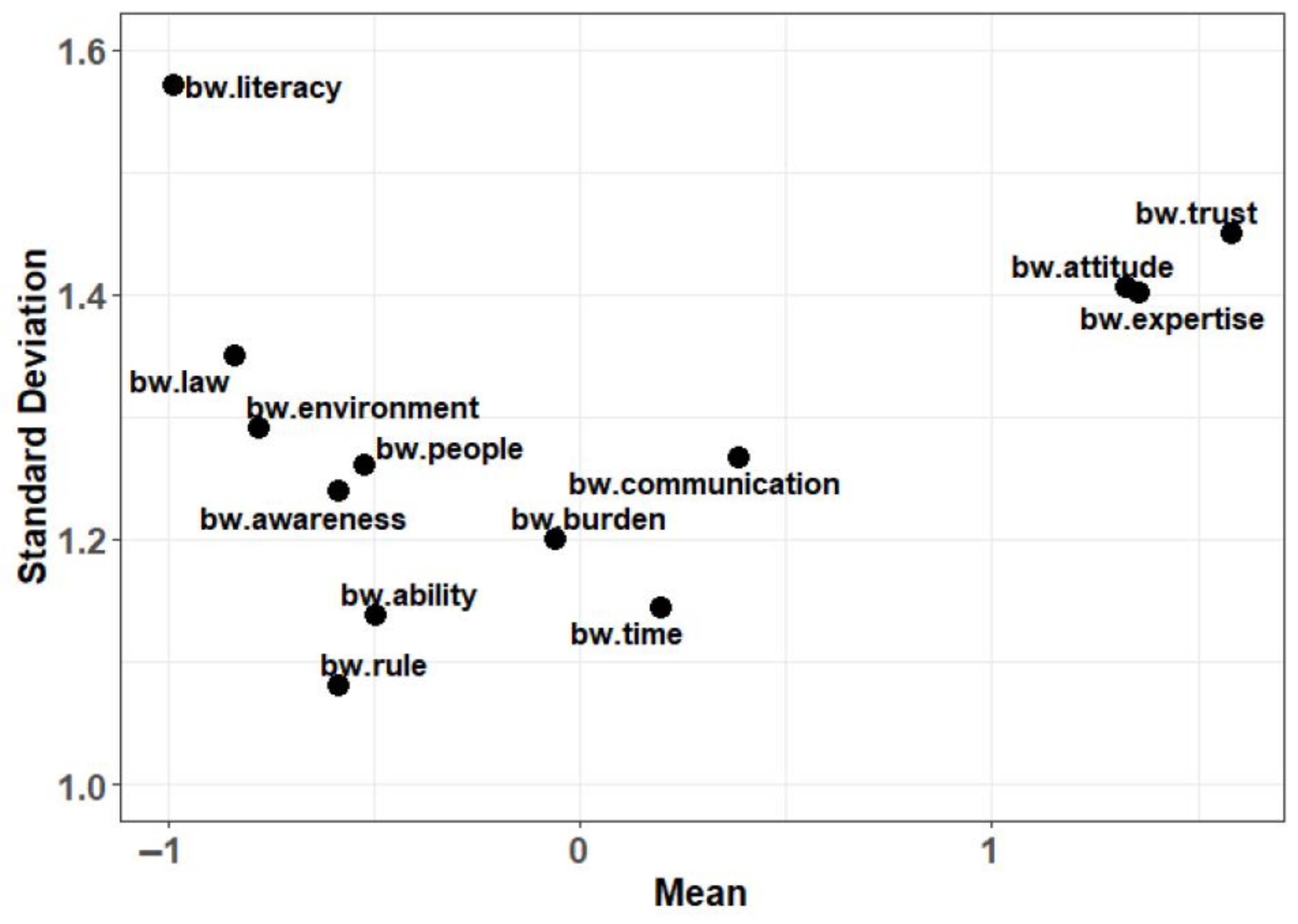
| B | W | BW Score | Mean BW Score | Mean Std. BW Score | Scaled BW Score | Std. Scaled BW Score | Rank | |
|---|---|---|---|---|---|---|---|---|
| Environment | 594 | 1227 | −633 | −0.778 | −0.194 | 0.696 | 0.279 | 11 |
| Communication | 966 | 650 | 316 | 0.388 | 0.097 | 1.219 | 0.489 | 4 |
| Law | 404 | 1,087 | −683 | −0.839 | −0.210 | 0.610 | 0.245 | 12 |
| Trust | 1534 | 247 | 1,287 | 1.581 | 0.395 | 2.492 | 1 | 1 |
| Expertise | 1595 | 489 | 1,106 | 1.359 | 0.340 | 1.806 | 0.725 | 2 |
| People | 517 | 943 | −426 | −0.523 | −0.131 | 0.740 | 0.297 | 8 |
| Awareness | 534 | 1,012 | −478 | −0.587 | −0.147 | 0.726 | 0.291 | 10 |
| Time | 898 | 737 | 161 | 0.198 | 0.049 | 1.104 | 0.443 | 5 |
| Ability | 597 | 1000 | −403 | −0.495 | −0.124 | 0.773 | 0.310 | 7 |
| Rule | 346 | 821 | −475 | −0.584 | −0.146 | 0.649 | 0.260 | 9 |
| Attitude | 1393 | 313 | 1,080 | 1.327 | 0.332 | 2.110 | 0.847 | 3 |
| Literacy | 481 | 1285 | −804 | −0.988 | −0.247 | 0.612 | 0.246 | 13 |
| Burden | 723 | 771 | −48 | −0.059 | −0.015 | 0.968 | 0.389 | 6 |
| Conditional Logit Model | Mixed Logit Model | |||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | z-Value | SP | B | SE | z-Value | SP | |
| Trust | 1.339 | 0.038 | 35.57 *** | 0.1579 | 1.404 | 0.041 | 34.15 *** | 0.1644 |
| Expertise | 1.205 | 0.037 | 32.64 *** | 0.1382 | 1.250 | 0.035 | 35.42 *** | 0.1409 |
| Attitude | 1.196 | 0.037 | 32.20 *** | 0.1369 | 1.237 | 0.039 | 31.48 *** | 0.1392 |
| Communication | 0.712 | 0.036 | 19.82 *** | 0.0843 | 0.723 | 0.034 | 21.4 *** | 0.0832 |
| Time | 0.604 | 0.036 | 16.93 *** | 0.0757 | 0.612 | 0.035 | 17.35 *** | 0.0744 |
| Burden | 0.465 | 0.035 | 13.12 *** | 0.0659 | 0.469 | 0.036 | 12.94 *** | 0.0646 |
| Ability | 0.256 | 0.035 | 7.25 *** | 0.0535 | 0.258 | 0.035 | 7.29 *** | 0.0523 |
| People | 0.242 | 0.036 | 6.80 *** | 0.0527 | 0.245 | 0.035 | 6.91 *** | 0.0516 |
| Rule | 0.218 | 0.036 | 6.10 *** | 0.0515 | 0.221 | 0.039 | 5.74 *** | 0.0504 |
| Awareness | 0.210 | 0.035 | 5.93 *** | 0.0511 | 0.211 | 0.036 | 5.81 *** | 0.0499 |
| Environment | 0.103 | 0.035 | 2.93 ** | 0.0459 | 0.104 | 0.034 | 3.05 ** | 0.0448 |
| Law | 0.086 | 0.036 | 2.41 * | 0.0451 | 0.086 | 0.036 | 2.42 * | 0.0440 |
| Literacy | Reference | 0.0414 | Reference | 0.0404 | ||||
| Environment | Communication | Law | Trust | Expertise | People | Awareness | Time | Ability | Rule | Attitude | Literacy | Burden | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
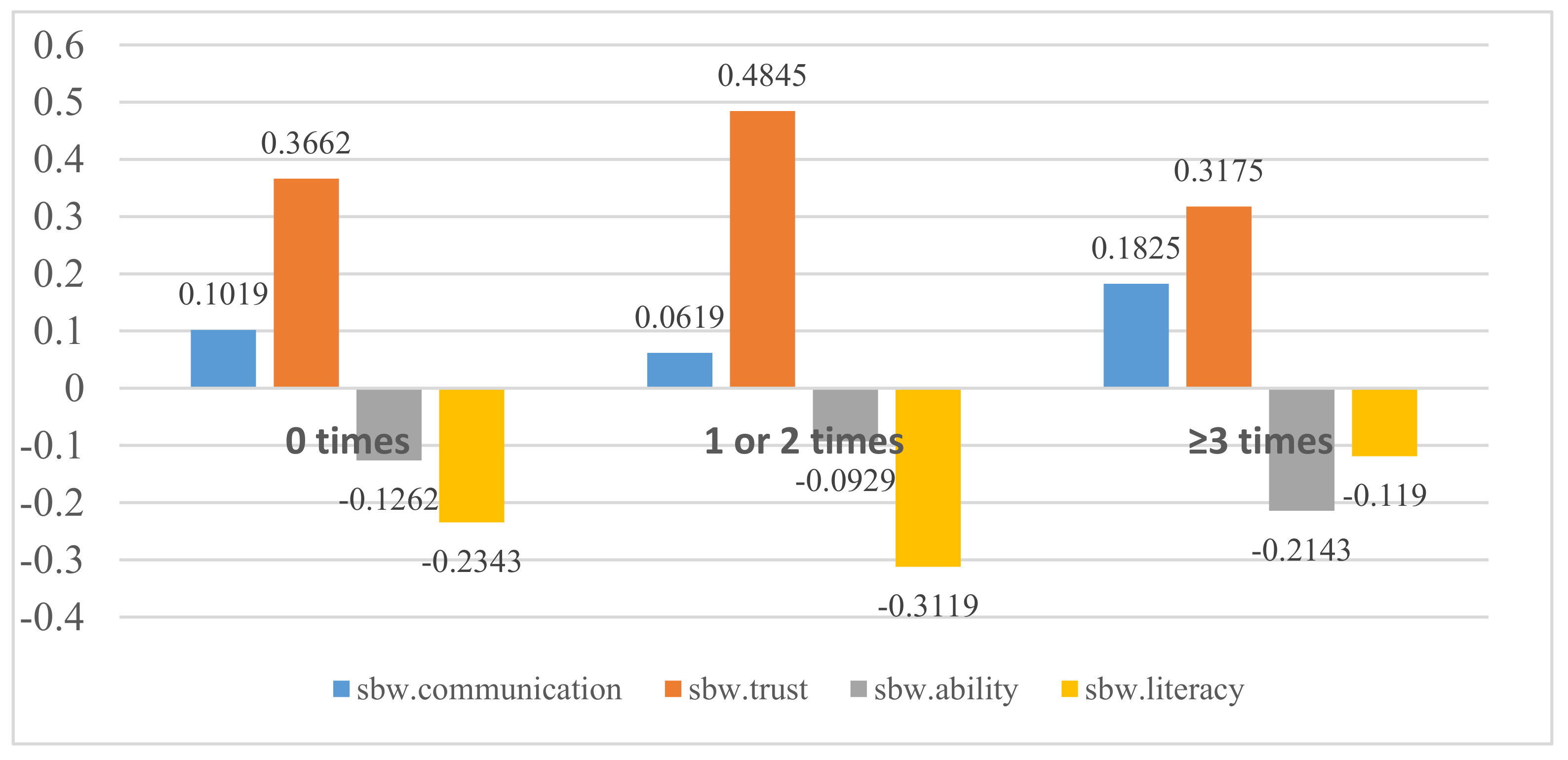
| Number of hospitalizations in the last year (times) | 0 | −0.181 | 0.102 | −0.217 | 0.366 *** | 0.334 | −0.123 | −0.151 | 0.038 | −0.126 | −0.131 | 0.331 | −0.234 * | −0.007 |
| 1 or 2 | −0.215 | 0.062 | −0.198 | 0.485 | 0.371 | −0.135 | −0.132 | 0.060 | −0.093 | −0.179 | 0.321 | −0.312 | −0.034 | |
| ≥3 | −0.234 | 0.183 * | −0.191 | 0.318 ** | 0.278 | −0.179 | −0.167 | 0.111 | −0.214 * | −0.151 | 0.373 | −0.119 ** | −0.008 | |
| F-value | 1.377 | 3.769 * | 0.365 | 10.223 *** | 1.940 | 0.890 | 0.447 | 2.063 | 4.573 * | 2.540 | 0.544 | 6.813 ** | 0.665 | |
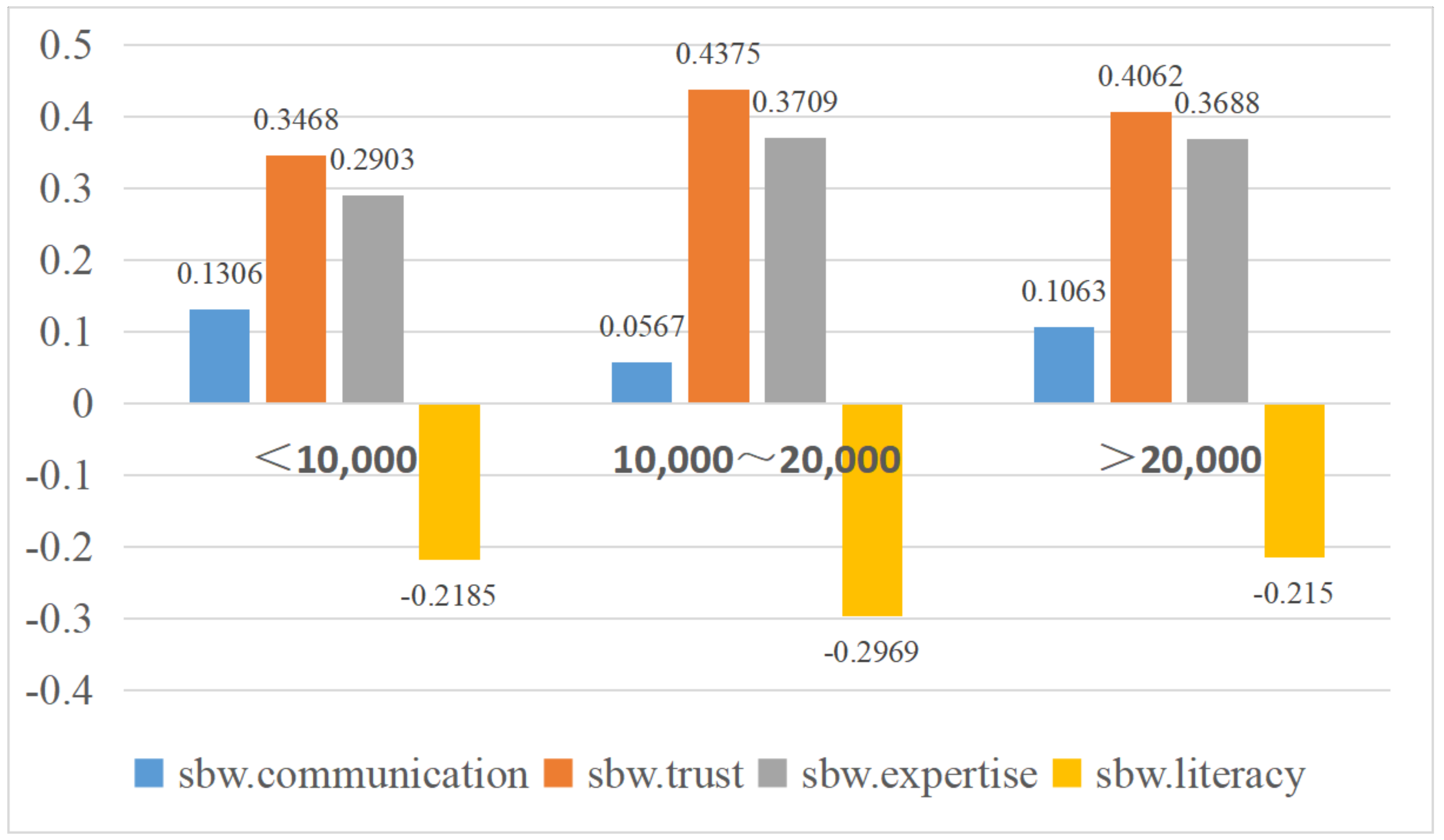
| Monthly family income (CNY) | <10,000 | −0.186 | 0.131 | −0.186 | 0.347 | 0.290 | −0.112 | −0.139 | 0.052 | −0.151 | −0.148 | 0.338 | −0.219 | −0.018 |
| 10,000~20,000 | −0.194 | 0.057 * | −0.220 | 0.438 ** | 0.371 * | −0.126 | −0.163 | 0.067 | −0.108 | −0.122 | 0.326 | −0.297 * | −0.029 | |
| >20,000 | −0.209 | 0.106 | −0.230 | 0.406 | 0.369 * | −0.168 | −0.135 | 0.018 | −0.106 | −0.179 | 0.331 | −0.215 | 0.011 | |
| F-value | 0.316 | 4.324 * | 1.261 | 4.980 ** | 5.018 ** | 1.943 | 0.658 | 1.868 | 2.268 | 2.721 | 0.093 | 3.962 * | 1.101 | |
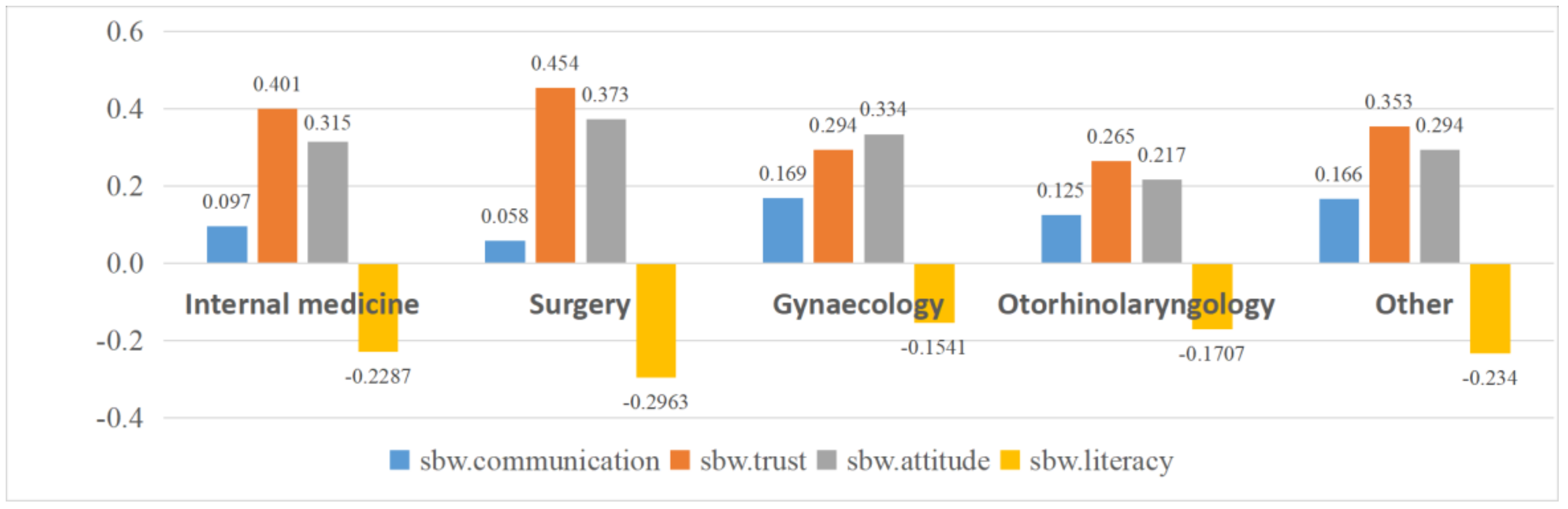
| Department | Internal medicine | −0.183 | 0.097 | −0.185 | 0.401 | 0.288 | −0.139 | −0.158 | 0.068 | −0.141 | −0.149 | 0.315 | −0.229 | 0.014 |
| Surgery | −0.189 | 0.058 | −0.214 | 0.454 | 0.342 | −0.120 | −0.157 | 0.038 | −0.107 | −0.152 | 0.373 | −0.296 | −0.030 | |
| Gynecology | −0.224 | 0.169 | −0.250 | 0.294 ** | 0.352 | −0.128 | −0.076 | −0.006 | −0.134 | −0.122 | 0.334 | −0.154 * | −0.055 | |
| Otolaryngology | −0.195 | 0.125 | −0.171 | 0.265 ** | 0.369 | −0.131 | −0.156 | 0.101 | −0.113 | −0.131 | 0.217 ** | −0.171 | −0.009 | |
| Others | −0.209 | 0.166 * | −0.236 | 0.353 | 0.391 | −0.163 | −0.141 | 0.068 | −0.163 | −0.150 | 0.294 | −0.234 | 0.025 | |
| F-value | 0.304 | 3.812 ** | 0.983 | 7.388 *** | 1.620 | 0.389 | 1.328 | 1.910 | 0.998 | 0.288 | 3.934 ** | 3.639 ** | 1.443 | |
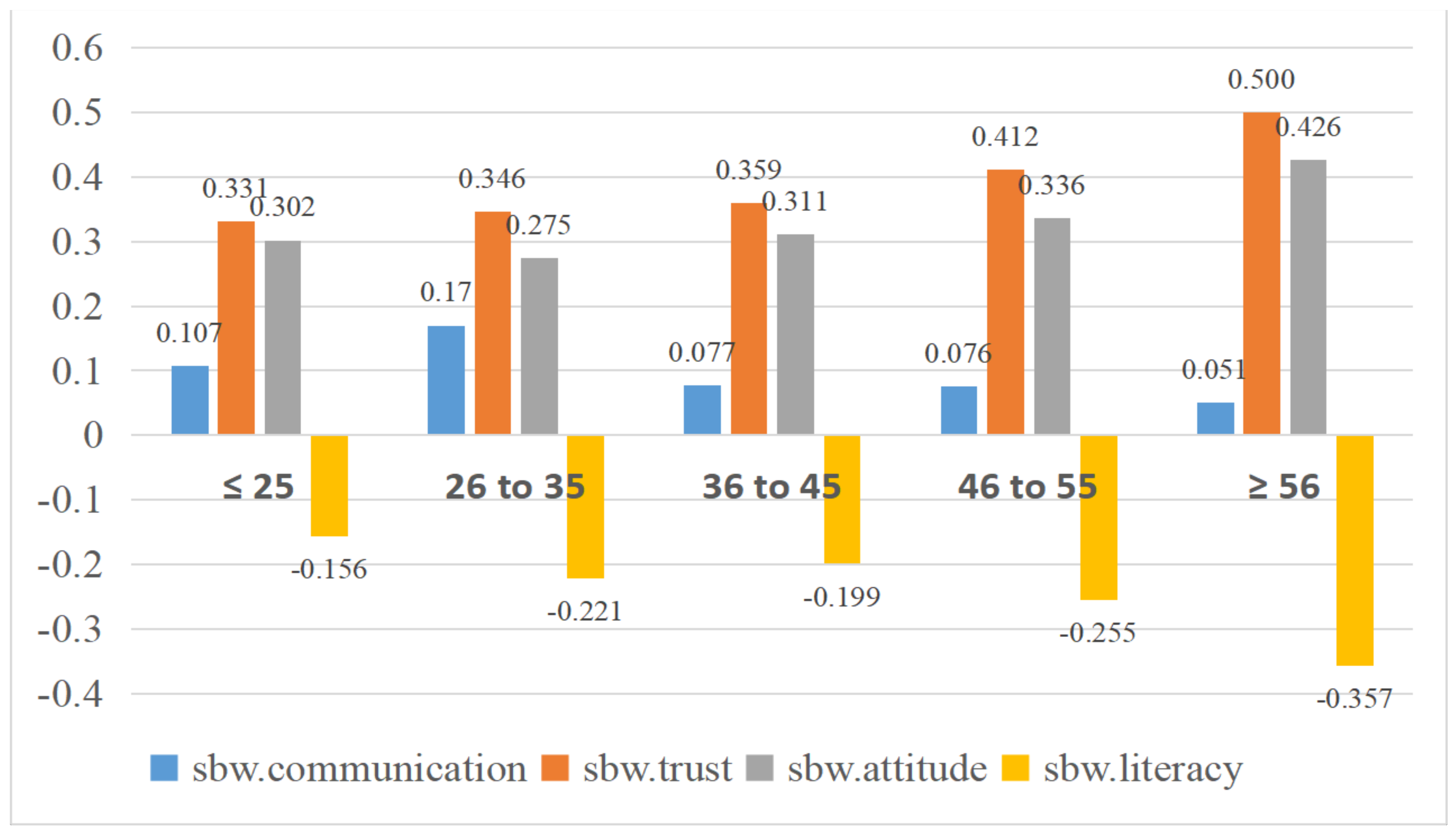
| Age (years) | ≤25 | −0.175 | 0.107 | −0.169 | 0.331 ** | 0.292 | −0.127 | −0.166 | −0.010 | −0.130 | −0.097 | 0.302 | −0.156 *** | −0.003 |
| 26 to 35 | −0.168 | 0.170 ** | −0.200 | 0.346 *** | 0.374 | −0.162 | −0.129 | 0.048 | −0.154 | −0.147 | 0.275 *** | −0.221 ** | −0.032 | |
| 36 to 45 | −0.200 | 0.077 | −0.203 | 0.359 ** | 0.332 | −0.100 | −0.133 | 0.052 | −0.106 | −0.152 | 0.311 * | −0.199 ** | −0.038 | |
| 46 to 55 | −0.214 | 0.076 | −0.230 | 0.412 | 0.342 | −0.152 | −0.155 | 0.080 | −0.103 | −0.147 | 0.336 | −0.255 | 0.008 | |
| ≥56 | −0.209 | 0.051 | −0.227 | 0.500 | 0.327 | −0.111 | −0.166 | 0.045 | −0.124 | −0.158 | 0.426 | −0.357 | 0.003 | |
| F-value | 0.663 | 4.162 ** | 0.612 | 6.067 *** | 0.921 | 1.309 | 0.540 | 1.321 | 0.997 | 0.734 | 5.025 ** | 5.694 *** | 0.849 | |
| Mean in the difference of the total | −0.194 | 0.097 | −0.210 | 0.395 | 0.340 | −0.131 | −0.147 | 0.049 | −0.124 | −0.146 | 0.332 | −0.247 | −0.015 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Chen, H.; Gao, Y.; Xiang, Y.; Wang, F.; Ni, Z.; Wang, X.; Huang, X. Best-Worst Scaling Survey of Inpatients’ Preferences in Medical Decision-Making Participation in China. Healthcare 2023, 11, 323. https://doi.org/10.3390/healthcare11030323
Sun T, Chen H, Gao Y, Xiang Y, Wang F, Ni Z, Wang X, Huang X. Best-Worst Scaling Survey of Inpatients’ Preferences in Medical Decision-Making Participation in China. Healthcare. 2023; 11(3):323. https://doi.org/10.3390/healthcare11030323
Chicago/Turabian StyleSun, Tao, Hanlin Chen, Yuan Gao, Yingru Xiang, Feng Wang, Ziling Ni, Xiaohe Wang, and Xianhong Huang. 2023. "Best-Worst Scaling Survey of Inpatients’ Preferences in Medical Decision-Making Participation in China" Healthcare 11, no. 3: 323. https://doi.org/10.3390/healthcare11030323
APA StyleSun, T., Chen, H., Gao, Y., Xiang, Y., Wang, F., Ni, Z., Wang, X., & Huang, X. (2023). Best-Worst Scaling Survey of Inpatients’ Preferences in Medical Decision-Making Participation in China. Healthcare, 11(3), 323. https://doi.org/10.3390/healthcare11030323






