Chronic Resistance Training Effects on Serum Adipokines in Type 2 Diabetes Mellitus: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Extraction Process
2.6. Data Collection
2.7. Methodological Quality of the Included Studies
3. Results
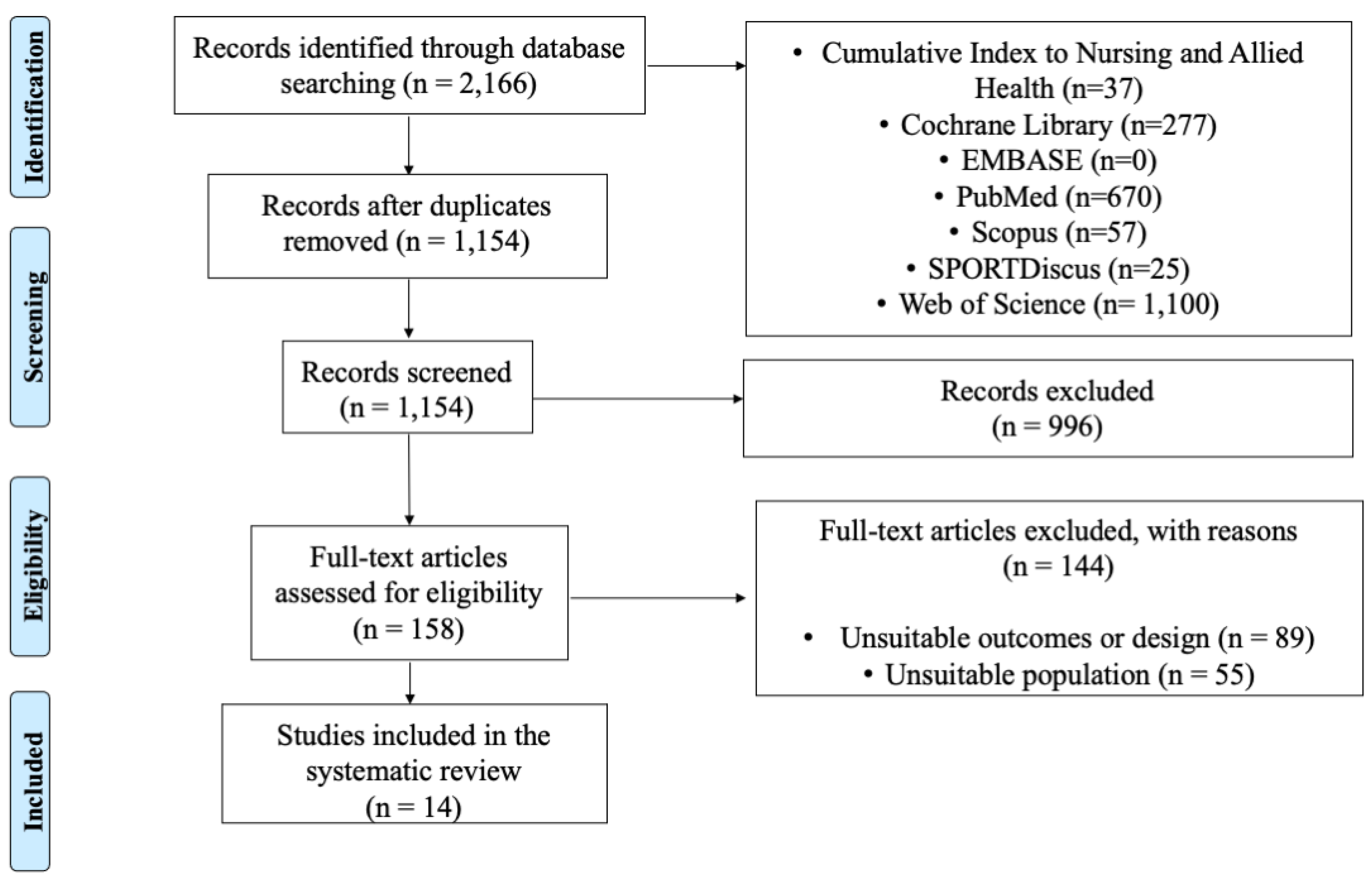
3.1. Data Selection
3.2. Studies’ Characteristics
3.3. Methodological Quality of Included Studies
3.4. Results Synthesis
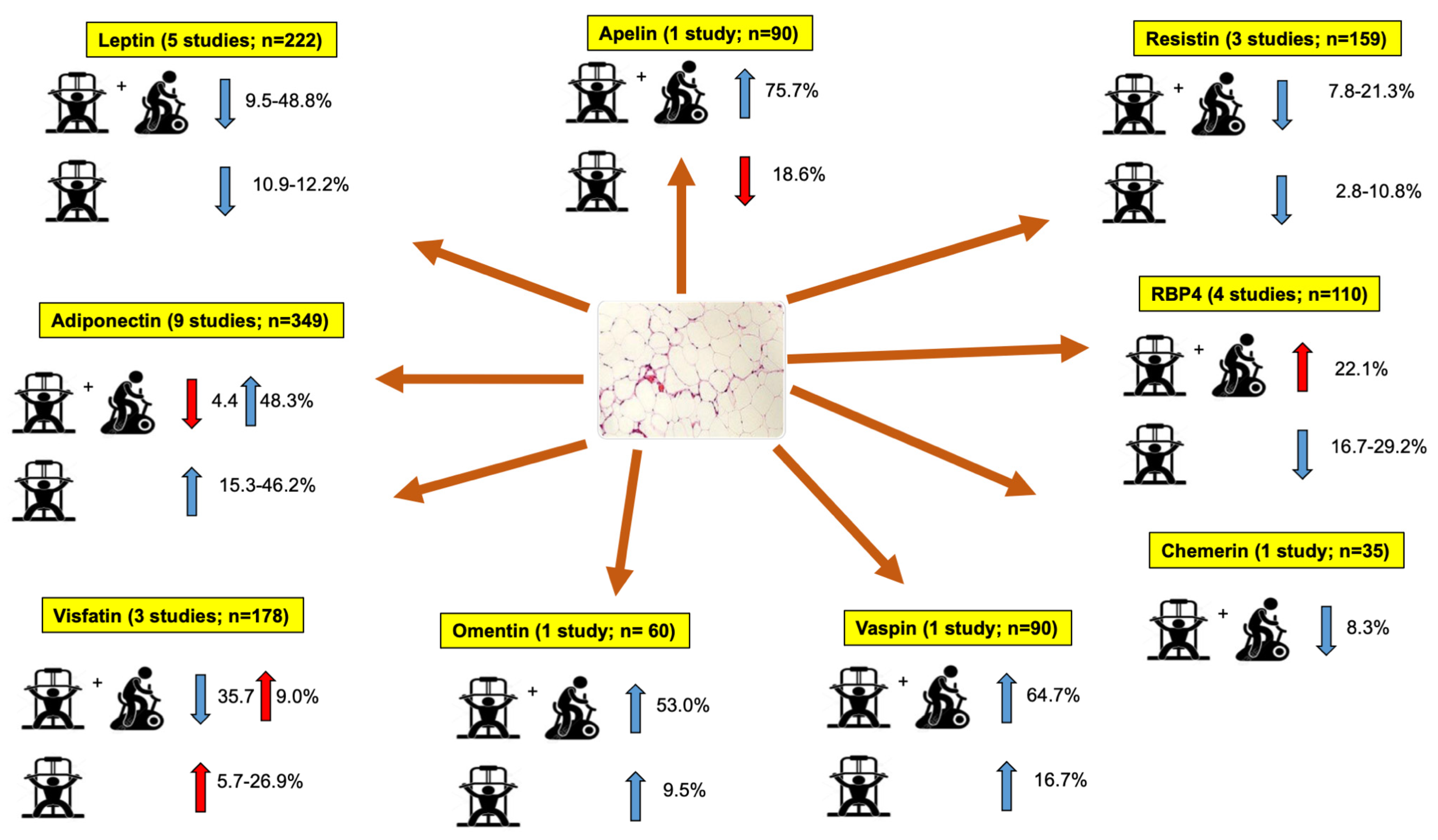
3.4.1. Leptin
3.4.2. Adiponectin
3.4.3. Visfatin
3.4.4. Apelin
3.4.5. Resistin
3.4.6. Retinol-Binding Protein 4 (RBP4)
3.4.7. Vaspin
3.4.8. Chemerin
3.4.9. Omentin
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can. J. Diabetes 2018, 42, S10–S15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2019, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.-F. Global, Regional, and National Burden and Trend of Diabetes in 195 Countries and Territories: An Analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef] [PubMed]
- Iglay, K.; Hannachi, H.; Joseph Howie, P.; Xu, J.; Li, X.; Engel, S.S.; Moore, L.M.; Rajpathak, S. Prevalence and Co-Prevalence of Comorbidities among Patients with Type 2 Diabetes Mellitus. Curr. Med. Res. Opin. 2016, 32, 1243–1252. [Google Scholar] [CrossRef]
- Anton, S.D.; Karabetian, C.; Naugle, K.; Buford, T.W. Obesity and Diabetes as Accelerators of Functional Decline: Can Lifestyle Interventions Maintain Functional Status in High Risk Older Adults? Exp. Gerontol. 2013, 48, 888–897. [Google Scholar] [CrossRef] [Green Version]
- Orlando, G.; Balducci, S.; Bazzucchi, I.; Pugliese, G.; Sacchetti, M. Neuromuscular Dysfunction in Type 2 Diabetes: Underlying Mechanisms and Effect of Resistance Training: Neuromuscular Dysfunction in Type 2 Diabetes. Diabetes Metab. Res. Rev. 2016, 32, 40–50. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- El husseny, M.W.A.; Mamdouh, M.; Shaban, S.; Ibrahim Abushouk, A.; Zaki, M.M.M.; Ahmed, O.M.; Abdel-Daim, M.M. Adipokines: Potential Therapeutic Targets for Vascular Dysfunction in Type II Diabetes Mellitus and Obesity. J. Diabetes Res. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Khan, M.; Joseph, F. Adipose Tissue and Adipokines: The Association with and Application of Adipokines in Obesity. Scientifica 2014, 2014, 328592. [Google Scholar] [CrossRef]
- Blüher, M. Clinical Relevance of Adipokines. Diabetes Metab. J. 2012, 36, 317. [Google Scholar] [CrossRef] [Green Version]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eves, N.D.; Plotnikoff, R.C. Resistance Training and Type 2 Diabetes: Considerations for Implementation at the Population Level. Diabetes Care 2006, 29, 1933–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hordern, M.D.; Dunstan, D.W.; Prins, J.B.; Baker, M.K.; Singh, M.A.F.; Coombes, J.S. Exercise Prescription for Patients with Type 2 Diabetes and Pre-Diabetes: A Position Statement from Exercise and Sport Science Australia. J. Sci. Med. Sport 2012, 15, 25–31. [Google Scholar] [CrossRef] [PubMed]
- AminiLari, Z.; Fararouei, M.; Amanat, S.; Sinaei, E.; Dianatinasab, S.; AminiLari, M.; Daneshi, N.; Dianatinasab, M. The Effect of 12 Weeks Aerobic, Resistance, and Combined Exercises on Omentin-1 Levels and Insulin Resistance among Type 2 Diabetic Middle-Aged Women. Diabetes Metab. J. 2017, 41, 205. [Google Scholar] [CrossRef]
- Balducci, S.; Zanuso, S.; Nicolucci, A.; Fernando, F.; Cavallo, S.; Cardelli, P.; Fallucca, S.; Alessi, E.; Letizia, C.; Jimenez, A.; et al. Anti-Inflammatory Effect of Exercise Training in Subjects with Type 2 Diabetes and the Metabolic Syndrome Is Dependent on Exercise Modalities and Independent of Weight Loss. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Fotiadis, G.; Kapelouzou, A.; Kostakis, A.; Liapis, C.D.; Vrabas, I.S. The Differential Anti-Inflammatory Effects of Exercise Modalities and Their Association with Early Carotid Atherosclerosis Progression in Patients with Type 2 Diabetes. Diabet. Med. 2013, 30, e41–e50. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.H.; Ahn, K.Y.; Lee, D.H.; Suh, Y.J.; Cho, S.G.; Choi, Y.J.; Lee, D.H.; Lee, S.Y.; Hong, S.B.; et al. Effect of Lifestyle Modification on Serum Chemerin Concentration and Its Association with Insulin Sensitivity in Overweight and Obese Adults with Type 2 Diabetes. Clin. Endocrinol. 2014, 80, 825–833. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Fenicchia, L.M.; Miller, C.S.; Ploutz-Synder, L.L.; Weinstock, R.S.; Carhart, R.; Azevedo, J.L. Resting Leptin Responses to Acute and Chronic Resistance Training in Type 2 Diabetic Men and Women. Int. J. Obes. 2001, 25, 1474–1480. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Woo, J.H.; Shin, K.O.; Kim, D.; Lee, H.J.; Kim, Y.J.; Yeo, N.H. Circuit Resistance Exercise Improves Glycemic Control and Adipokines in Females with Type 2 Diabetes Mellitus. J. Sports Sci. Med. 2009, 8, 682–688. [Google Scholar]
- Sukala, W.R.; Page, R.; Rowlands, D.S.; Krebs, J.; Lys, I.; Leikis, M.; Pearce, J.; Cheema, B.S. South Pacific Islanders Resist Type 2 Diabetes: Comparison of Aerobic and Resistance Training. Eur. J. Appl. Physiol. 2012, 112, 317–325. [Google Scholar] [CrossRef]
- Abt, G.; Boreham, C.; Davison, G.; Jackson, R.; Nevill, A.; Wallace, E.; Williams, M. Power, Precision, and Sample Size Estimation in Sport and Exercise Science Research. J. Sports Sci. 2020, 38, 1933–1935. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Drevon, D.; Fursa, S.R.; Malcolm, A.L. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav. Modif. 2017, 41, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.B. Estimating Effect Sizes From Pretest-Posttest-Control Group Designs. Organ. Res. Methods 2008, 11, 364–386. [Google Scholar] [CrossRef]
- de Morton, N.A. The PEDro Scale Is a Valid Measure of the Methodological Quality of Clinical Trials: A Demographic Study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moseley, A.M.; Rahman, P.; Wells, G.A.; Zadro, J.R.; Sherrington, C.; Toupin-April, K.; Brosseau, L. Agreement between the Cochrane Risk of Bias Tool and Physiotherapy Evidence Database (PEDro) Scale: A Meta-Epidemiological Study of Randomized Controlled Trials of Physical Therapy Interventions. PLoS ONE 2019, 14, e0222770. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Campillo, R.; Álvarez, C.; García-Hermoso, A.; Ramírez-Vélez, R.; Gentil, P.; Asadi, A.; Chaabene, H.; Moran, J.; Meylan, C.; García-de-Alcaraz, A.; et al. Methodological Characteristics and Future Directions for Plyometric Jump Training Research: A Scoping Review. Sports Med. 2018, 48, 1059–1081. [Google Scholar] [CrossRef]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef]
- Annibalini, G.; Lucertini, F.; Agostini, D.; Vallorani, L.; Gioacchini, A.; Barbieri, E.; Guescini, M.; Casadei, L.; Passalia, A.; Del Sal, M.; et al. Concurrent Aerobic and Resistance Training Has Anti-Inflammatory Effects and Increases Both Plasma and Leukocyte Levels of IGF-1 in Late Middle-Aged Type 2 Diabetic Patients. Oxid. Med. Cell. Longev. 2017, 2017, 3937842 . [Google Scholar] [CrossRef]
- Brooks, N.; Layne, J.E.; Gordon, P.L.; Roubenoff, R.; Nelson, M.E.; Castaneda-Sceppa, C. Strength Training Improves Muscle Quality and Insulin Sensitivity in Hispanic Older Adults with Type 2 Diabetes. Int. J. Med. Sci. 2007, 4, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorge, M.L.M.P.; De Oliveira, V.N.; Resende, N.M.; Paraiso, L.F.; Calixto, A.; Diniz, A.L.D.; Resende, E.S.; Ropelle, E.R.; Carvalheira, J.B.; Espindola, F.S.; et al. The Effects of Aerobic, Resistance, and Combined Exercise on Metabolic Control, Inflammatory Markers, Adipocytokines, and Muscle Insulin Signaling in Patients with Type 2 Diabetes Mellitus. Metabolism 2011, 60, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.H.; Han, K.A.; Ahn, H.; Kwon, H.; Koo, B.K.; Kim, H.C.; Min, K.W. Resistance Exercise Did Not Alter Intramuscular Adipose Tissue but Reduced Retinol-Binding Protein-4 Concentration in Individuals with Type 2 Diabetes Mellitus. J. Int. Med. Res. 2010, 38, 782–791. [Google Scholar] [CrossRef] [Green Version]
- Loimaala, A.; Groundstroem, K.; Rinne, M.; Nenonen, A.; Huhtala, H.; Parkkari, J.; Vuori, I. Effect of Long-Term Endurance and Strength Training on Metabolic Control and Arterial Elasticity in Patients with Type 2 Diabetes Mellitus. Am. J. Cardiol. 2009, 103, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, A.; Hamzezadeh, S.; Tofighi, A. Investigation of Plasma Visfatin Changes in Women with Type 2 Diabetes Followed by Endurance, Resistance and Combined Exercise: The Role of Lipid Profile, Glycemic Indices and Insulin Resistance. J. Diabetes Metab. 2016, 7, 2. [Google Scholar] [CrossRef]
- Miller, E.G.; Sethi, P.; Nowson, C.A.; Dunstan, D.W.; Daly, R.M. Effects of Progressive Resistance Training and Weight Loss versus Weight Loss Alone on Inflammatory and Endothelial Biomarkers in Older Adults with Type 2 Diabetes. Eur. J. Appl. Physiol. 2017, 117, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lobo, A.M.; Donato, J. The Role of Leptin in Health and Disease. Temperature 2017, 4, 258–291. [Google Scholar] [CrossRef] [Green Version]
- Bouassida, A.; Chamari, K.; Zaouali, M.; Feki, Y.; Zbidi, A.; Tabka, Z. Review on Leptin and Adiponectin Responses and Adaptations to Acute and Chronic Exercise. Br. J. Sports Med. 2010, 44, 620–630. [Google Scholar] [CrossRef]
- Friedman, J. The Long Road to Leptin. J. Clin. Investig 2016, 126, 4727–4734. [Google Scholar] [CrossRef] [Green Version]
- Arunkumar, A.; Sushil, J. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. In Comprehensive Physiology; Pollock, D.M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 1031–1063. ISBN 978-0-470-65071-4. [Google Scholar]
- Park, K.M.; Park, S.C.; Kang, S. Effects of Resistance Exercise on Adipokine Factors and Body Composition in Pre- and Postmenopausal Women. J. Exerc. Rehabil. 2019, 15, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.A.; Singh, M.A.F. Effects of Exercise on Adiponectin: A Systematic Review. Obesity 2008, 16, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Hu, W.; Wang, M.; Xiao, Y. The Role of the Adipocytokines Vaspin and Visfatin in Vascular Endothelial Function and Insulin Resistance in Obese Children. BMC Endocr. Disord. 2019, 19, 127. [Google Scholar] [CrossRef] [PubMed]
- Dakroub, A.; Nasser, S.A.; Younis, N.; Bhagani, H.; Al-Dhaheri, Y.; Pintus, G.; Eid, A.A.; El-Yazbi, A.F.; Eid, A.H. Visfatin: A Possible Role in Cardiovasculo-Metabolic Disorders. Cells 2020, 9, 2444. [Google Scholar] [CrossRef]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Aozasa, N.; Asano, Y.; Akamata, K.; Noda, S.; Masui, Y.; Yamada, D.; Tamaki, Z.; Tada, Y.; Sugaya, M.; Kadono, T.; et al. Serum Apelin Levels: Clinical Association with Vascular Involvements in Patients with Systemic Sclerosis: Significance of Serum Apelin Levels in SSc. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 37–42. [Google Scholar] [CrossRef]
- Liakos, C.I.; Sanidas, E.A.; Perrea, D.N.; Grassos, C.A.; Chantziara, V.; Viniou, N.-A.; Barbetseas, J.D.; Papadopoulos, D.P. Apelin and Visfatin Plasma Levels in Healthy Individuals with High Normal Blood Pressure: Table 1. Am. J. Hypertens. 2016, 29, 549–552. [Google Scholar] [CrossRef] [Green Version]
- Wysocka, M.B.; Pietraszek-Gremplewicz, K.; Nowak, D. The Role of Apelin in Cardiovascular Diseases, Obesity and Cancer. Front. Physiol. 2018, 9, 557. [Google Scholar] [CrossRef]
- Jang, S.-H.; Paik, I.-Y.; Ryu, J.-H.; Lee, T.-H.; Kim, D.-E. Effects of Aerobic and Resistance Exercises on Circulating Apelin-12 and Apelin-36 Concentrations in Obese Middle-Aged Women: A Randomized Controlled Trial. BMC Womens Health 2019, 19, 23. [Google Scholar] [CrossRef]
- Castan-Laurell, I.; El Boustany, R.; Pereira, O.; Potier, L.; Marre, M.; Fumeron, F.; Valet, P.; Gourdy, P.; Velho, G.; Roussel, R. Plasma Apelin and Risk of Type 2 Diabetes in a Cohort From the Community. Diabetes Care 2020, 43, e15–e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, K.; Li, Y.; Zhang, D.; Yuan, J.; Zhang, C.; Liu, Y.; Song, L.; Lin, Q.; Li, M.; Dong, J. Relation of Circulating Resistin to Insulin Resistance in Type 2 Diabetes and Obesity: A Systematic Review and Meta-Analysis. Front. Physiol. 2019, 10, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobbold, C. Type 2 Diabetes Mellitus Risk and Exercise: Is Resistin Involved? J. Sports Med. Phys. Fitness 2018, 59, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, J.S.; Lass, A.; Schupp, M. Biological Functions of RBP4 and Its Relevance for Human Diseases. Front. Physiol. 2021, 12, 659977. [Google Scholar] [CrossRef] [PubMed]
- Mattern, A.; Zellmann, T.; Beck-Sickinger, A.G. Processing, Signaling, and Physiological Function of Chemerin: Processing, Signaling, and Physiological Function of Chemerin. IUBMB Life 2014, 66, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Mahdirejei, H.A. Concentrations, Metabolic Parameters Levels and Physical. CELL J. 2014, 16, 8. [Google Scholar]
- Leandro, A.; Queiroz, M.; Azul, L.; Seiça, R.; Sena, C.M. Omentin: A Novel Therapeutic Approach for the Treatment of Endothelial Dysfunction in Type 2 Diabetes. Free Radic. Biol. Med. 2021, 162, 233–242. [Google Scholar] [CrossRef]
- Helfer, G.; Wu, Q.-F. Chemerin: A Multifaceted Adipokine Involved in Metabolic Disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef] [Green Version]
- Zehsaz, F.; Farhangi, N.; Ghahramani, M. The Response of Circulating Omentin-1 Concentration to 16-Week Exercise Training in Male Children with Obesity. Phys. Sportsmed. 2016, 44, 355–361. [Google Scholar] [CrossRef]
| Study ¶ | Sample n (Sex), Age, Time in DM2, Comorbidities | Exercise Type | Duration (W) | Frequency (W) | Exercise Protocol |
|---|---|---|---|---|---|
| Aminilari et al., 2017 [14] | 45 (F) 45–60 years >2 years Without cardiovascular or chronic conditions | RT AET RT + AET C | 12 | 3 | RT: 6 exercises, 3 × 8/50–55% RM AET: 20–25 min/50–55% HRmax RT + AET: 6 exercises, 1–2 × 8/50–55% RM + 10–12 min/50–55% HRmax C |
| Annibalini et al., 2017 [30] | 16 (M) 55–70 years 7.8–10.1 Without cardiovascular diseases or medication | RT + AET C | 16 | 3 | RT + AET: 4 exercises, 2–4 × 12–20/40–60% RM + 30–60 min/40–65% HRR C |
| Balducci et al., 2010 [15] | 82 (34 F, 48 M) 50–70 years 7.8–10.1 Without cardiovascular diseases or medication | AET 1 AET 2 RT + AET C | 52 | 2 | AET 1: unspecified AET 2: 70–80% VO2max, 60 min RT + AET: 70–80% VO2max, 40 min + 4 exercises/80% 1RM 20 min C |
| Brooks et al., 2007 [31] | 62 (22 F, 40 M) >55 years 8–11 years Without cardiovascular or chronic conditions | RT C | 16 | 3 | RT: 5 exercises, 3 × 8/60–80% RM, 35 min C |
| Jorge et al., 2011 [32] | 48 (30 F, 18 M) 53.9 ± 9.9 years 5–7 years Without cardiovascular complications | RT AET RT + AET C | 12 | 3 | RT: Circuit of 7 exercises, 60 min. Intensity unspecified AET: Lactic threshold HR, 60 min RT + AET: Circuit of 7 exercises, 30 min Intensity unspecified + Lactic threshold HR, 30 min C: Stretching, 60 min |
| Kadoglou et al., 2013 [16] | 90 (65 F, 25 M) 56–70 years 5–7 years Without cardiovascular, orthopedic, immune or cytokine-derived disruptions | RT AET RT + AET C | 24 | 4 | RT: 8 exercises, 2–3 × 8–10/60–80% RM, 60 min AET: 60–75% HRmax, 60 min RT + AET: 1 session of RT, 1 session of AET, 2 sessions RT + AET C: Leisure time activities, 150 min/week |
| Kanaley et al., 2001 [18] | 30 (15 F, 15 M) 45–55 years Non-detailed Without cardiovascular or other metabolic diseases | RT RT | 6 | 3 | Diabetics: 1 exercise per muscle group, 3 × 8–12/80% 3RM Non–diabetics: 1 exercise per muscle group, 3 × 8–12/80% 3RM |
| Kang et al., 2009 [19] | 15 (F) 51.5 ± 2.2 years Non-detailed Without complex metabolic alterations | RT AET | 12 | 3 | RT: 3 × 12/50–55% HRR AET: 20–25 min/50–55% HRmax |
| Kim et al., 2014 [17] | 35 (19 F, 17 M) 48.34 ± 8.4 years 7.9–10 years Without cardiovascular or chronic conditions | RT + AET C | 12 | 3 | RT + AET: Circuit with unspecified exercises, 3 × 20/50% 1RM, 40 min + 30 min/50–70% V02max C |
| Ku et al., 2010 [33] | 44 (F) 56.4 ± 7.1 years 5.8–6.6 years Without cardiovascular or renal conditions | RT AET C | 12 | 5 | RT:10 exercises, 3 × 15–20 with elastic bands EA: 3.6–5.2 METs/30 min. C |
| Loimaala et al., 2009 [34] | 50 (M) 58.3 ± 1.81 years Non-detailed Non-detailed | RT + AET C | 24 | 4 (2 days RT, 2 days AET) | RT + AET: 8 exercises, 3–4 × 10–12/80% RM + 60–80% VO2max (time unspecified) C |
| Mehdizadeh et al., 2016 [35] | 40 (F) 45–60 years 6.9–9.1 years Without acute or chronic conditions | RT AET RT + AET C | 12 | 3 | RT: 3 × 10/50–65% RM AET: 20–50 min/60–80% HRmax RT + AET: 3 × 10/50–65% RM + 20–50 min/60–80% HRmax C |
| Miller et al., 2017 [36] | 29 (16 F, 13 M) 67.2 ± 5.2 years 7.6–8.8 years Non-detailed | RT C | 48 | 3 | RT: 3 × 8/60–85% RM, 45 min C |
| Sukala et al., 2012 [20] | 18 (13 F, 5 M) 49.0 ± 5.0 years 2.6–3.3 years Without acute or chronic conditions | RT AET | 16 | 3 | RT: 8 exercises, 2–3 × 6–8, 40–60 min AET: 60–65% HRR, 40–60 min |
| Criteria * | Aminilari et al., 2017 [14] | Annibalini et al., 2017 [30] | Balducci et al., 2010 [15] | Brooks et al., 2007 [31] | Jorge et al., 2011 [32] | Kadoglou et al., 2013 [16] | Kanaley et al., 2001 [18] | Kang et al., 2009 [19] | Kim et al., 2014 [17] | Ku et al., 2010 [33] | Loimaala et al., 2009 [34] | Mehdizadeh et al., 2016 [35] | Miller et al., 2017 [36] | Sukala et al., 2012 [20] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| 4 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| 9 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 10 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 11 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total | 6 | 7 | 8 | 6 | 7 | 7 | 6 | 7 | 7 | 7 | 5 | 6 | 5 | 6 |
| Study ¶ | Adipokine | Main Outcomes: Pre (ng/mL, μg/mL) | Main Outcomes: Post (ng/mL, μg/mL) | p-Value | Effect Size |
|---|---|---|---|---|---|
| Aminilari et al., 2017 [14] | Omentin | RT: 29.00 (±4.90) AET: 27.67 (±7.60) RT + AET: 31.90 (±4.12) C: 24.17 (±5.75) | RT: 31.76 (±5.26) AET: 29.09 (±5.78) RT + AET: 48.82 (±65.48) C: 21.41 (±6.71) | RT: 0.59 AET: 0.66 RT + AET: 0.001 * C: 0.11 | RT/C: 0.99 AET/C: 0.61 RT + AET/C: 3.77 |
| Annibalini et al., 2017 [30] | Leptin Adiponectin RBP4 | RT + AET: 5.4 (±1.8) C: 5.7 (±2.7) RT + AET: 2.3 (±0.9) C: 2.9 (±1.0) RT + AET: 30.6 (±9.7) C: 27.7 (±4.0) | RT + AET: 3.6 (±1.5) C: 5.2 (±2.5) RT + AET: 2.2 (±1.0) C: 2.8 (±1.6) RT + AET: 22.0 (±4.4) C: 26.7 (±5.1) | RT + AET/C: 0.006 * RT + AET/C: 0.897 RT + AET/C: 0.003 * | RT + AET/C: −0.54 RT + AET/C: 0.01 RT + AET/C: −0.96 |
| Balducci et al., 2010 [15] | Leptin Adiponectin Resistin | AET1: 15.81 (±4.92) AET 2: 15.74 (±4.26) RT + AET: 15.28 (±4.92) C: 17.65 (±4.58) AET 1: 19.47 (±4.92) AET 2: 15.61 (±3.10) RT + AET: 14.57 (±1.91) C: 17.13 (±2.87) AET 1: 4.89 (±0.68) AET 2: 4.66 (±0.25) RT + AET: 4.61 (±0.29) C: 4.73 (±0.53) | AET 1: 14.27 (±4.87) AET 2: 12.55 (±2.90 RT + AET: 7.81 (±1.24) C: 15.60 (±4.64) AET 1: 19.10 (±3.67) AET 2: 20.24 (±2.19 RT + AET: 21.61 (±3.58) C: 17.14 (±2.53) AET 1: 4.67 (±0.83) AET 2: 3.98 (±0.34) RT + AET: 3.63 (±0.29) C: 4.16 (±0.37) | AET 1/AET2/RT + AET/C: 0.22 AET 1/AET2/RT + AET/C: 0.10 AET 1/AET2/RT + AET/C: 0.46 | AET 1/C: 0.09 AET 2/C: −0.25 RT + AET/C: −1.13 AET 1/C: −0.09 AET 2/C: 1.54 RT + AET/C: 2.87 AET 1/C: 0.57 AET 2/C: −0.26 RT + AET/C: −0.93 |
| Brooks et al., 2007 [31] | Adiponectin | RT: 5.1 (±1.32) C: 8.3 (±1.12) | RT: 6.6 (±1.35) C: 6.7 (±1.15) | RT/C: <0.001 * | RT/C: 2.61 |
| Jorge et al., 2011 [32] | Resistin Visfatin Adiponectin | RT: 8.54 (±1.46) AET: 7.34 (±1.36) RT + AET: 8.21 (±3.13) C: 8.24 (±1.66) RT: 112.11 (±42.85) AET: 112.24 (±45.83) RT + AET: 116.19 (±75.41) C: 103.57 (±55.06) RT: 4.45 (±4.12) AET: 5.58 (±5.73) RT + AET: 5.98 (±3.43) C: 5.07 (±5.50) | RT: 7.62 (±1.68) AET: 7.19 (±1.08) RT + AET: 7.57 (±2.89) C: 8.02 (±1.43) RT: 142.25 (±51.04) AET: 131.54 (±58.38) RT + AET: 127.46 (±45.22) C: 134.12 (±72.06) RT: 5.13 (±4.30) AET: 3.38 (±2.22) RT + AET: 6.58 (±5.44) C: 3.75 (±2.93) | RT: >0.05 AET: >0.05 RT + AET: >0.05 C: >0.05 RT: <0.05 * AET: <0.05 * RT + AET: <0.05 * C: <0.05 * RT: >0.05 AET: >0.05 RT + AET: >0.05 C: >0.05 | RT/C: −0.43 AET/C: 0.045 RT + AET/C: −0.89 RT/C: −0.003 AET/C: −021 RT + AET/C: −0.28 RT/C: 0.39 AET/C: −0.15 RT + AET/C: −0.18 |
| Kadoglou et al., 2013 [16] | Vaspin Apelin Visfatin | RT: 0.96 (±0.31) AET: 1.17 (±0.32) RT + AET: 0.99 (±0.28) C: 1.08 (±0.31) RT: 0.59 (±0.19) AET: 0.76 (±0.21) RT + AET: 0.74 (±0.21) C: 0.68 (±0.19) RT: 30.98 (±8.42) AET: 34.92 (±7.89) RT + AET: 35.64 (±8.24) C: 30.08 (±9.14) | RT: 1.12 (±0.39) AET: 1.69 (±1.08) RT + AET: 1.63 (±0.43) C: 1.16 (±0.38) RT: 0.48 (±0.29) AET: 1.27 (±0.40) RT + AET: 1.30 (±0.32) C: 0.71 (±0.31) RT: 32.76 (±8.97) AET: 23.64 (±9.11) RT + AET: 22.92 (±5.44) C: 29.73 (±9.49) | AET/RT + AET: <0.001 * AET/RT + AET: 0.260 AET/RT + AET: <0.001* | AET/RT + AET: 0.68 AET/RT + AET: −0.23 AET/RT + AET: 0.18 |
| Kanaley et al., 2001 [18] | Leptin | Diabetics: 41.4 (±8.9) Non-diabetics: 11.4 (±3.0) | Diabetics: 36.9 (±8.80) Non-diabetics: 11.9 (±8.8) | Diabetics: <0.05 * | Diabetics/Non-diabetics: −0.77 |
| Kang et al., 2009 [19] | RBP4 Adiponectin | RT: 49.26 (±8.30) AET: 35.36 (±4.01) RT: 6.92 (±2.35) AET: 6.17 (±1.06) | RT: 34.87 (±2.93) AET: 31.46 (±5.36) RT: 10.11 (±2.82) AET: 8.35 (±1.44) | RT: <0.001 * AET: <0.001 * RT: <0.05 * AET: <0.05 * | RT: 34.87/AET: −1.55 RT/AET: 0.53 |
| Kim et al., 2014 [17] | RBP4 Chemerin Adiponectin | RT + AET: 62.4 (±13.2) C: 62 (±20) RT + AET: 97.6 (±28.9) C: 103.2 (±12.7) RT + AET: 3.1 (±1.0) C: 3.8 (±1.6) | RT + AET: 76.2 (±14.6) C: 64.2 (±15.7) RT + AET: 89.5 (±24.1) C: 111.4 (±18.2) RT + AET: 3.6 (±1.3) C: 3.4 (±1.2) | RT + AET/C: >0.05 RT + AET/C: 0.021 * RT + AET/C: >0.05 | RT + AET/C: 0.67 RT + AET/C: −0.70 RT + AET/C: 0.66 |
| Ku et al., 2010 [33] | Leptin Adiponectin RBP4 | RT: 8.8 (±4) AET: 9.86 (±3.06) C: 11.6 (±5.8) RT: 4.98 (±2.52) AET: 3.86 (±2) C: 4.83 (±1.99) RT: 98.5 (±28.8) AET: 87.0 (±25.4) C: 95.0 (±20.5) | RT: 7.73 (±4.05) AET: 6.13 (±4.00) C: 11.50 (±4.92) RT: 7.28 (±3.72) AET: 6.76 (±1.24) C: 6.82 (±2.39) RT: 82.1 (±27.1) AET: 84.7 (±15.3) C: 96.2 (±28.7) | RT/AET/C: >0.05 RT/AET/C: >0.05 RT/AET/C: >0.05 | RT/C: −0.19 AET/C: −0.77 RT/C: 0.39 AET/C: 0.44 RT/C: −0.68 AET/C: −0.147 |
| Loimaala et al., 2009 [34] | Leptin | RT + AET: 7.4 (±4.1) C: 7.4 (±3.8) | RT + AET: 6.7 (±3) C: 7.9 (±3) | RT + AET: 0.43 C: 0.98 | RT + AET/C: −0.29 |
| Mehdizadeh et al., 2016 [35] | Visfatin | RT: 18.67 (±1.25) AET: 25.76 (±5.18) RT + AET: 21.61 (±2.66) C: 20. 24 (±2.37) | RT: 24.94 (±4.71) AET: 15.35 (±1.35) RT + AET: 15.80 (±1.88) C: 21.90 (±2.53) | RT/AET/RT + AET/C: >0.05 | RT/C: 2.33 AET/C: −2.87 RT + AET/C: −2.84 |
| Miller et al., 2017 [36] | Resistin Adiponectin | RT: 10.54 (±5.64) C: 10.99 (±3.86) RT: 1.68 (±0.66) C: 2.67(±0.95) | RT: 10.24 (±5.34) C: 8.97 (±3.91) RT: 1.94 (±0.93) C: 2.70 (±0.97) | RT: >0.05 C: >0.05 RT: <0.05 * C: >0.05 | RT/C: 0.34 RT/C: 0.28 |
| Sukala et al., 2012 [20] | Adiponectin | RT: 5.6 (±1.9) AET: 6.7 (±3.3) | RT: 5.6 (±2.2) AET: 6.7 (±3.2) | RT/AET: >0.05 | RT/AET: <0.001 |
| Aerobic Training | Resistance Training | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sessions per Week/Total Weeks | Intensity | Duration | Combined | Intensity | Duration | Sets | Repetitions | Exercises | |
| Leptin, adiponectin and resistin | 2/52 | 70–80% VO2max | 40 min | yes | 80% 1RM | 20 min | -- | -- | Upper limb pull, horizontal push, knee extension, trunk flexion |
| Apelin, vaspin and visfatin | 4/24 | 60–75% HRmax | 60 min | yes | 60–80% 1RM | 60 min | 2–3 | 8–10 | Seated leg press, knee extension, knee flexion, chest press, lat pulldown, overhead press, bicep curl, tricep extension |
| RBP4 | 3/12 | -- | -- | -- | Circuit 55% HRR | -- | 3 | 8 | Stair climbing, stationary cycling, resistance exercises (lat pull-down, abdominal, leg curl, leg extension, bicep curl) |
| Chemerin | 3/12 | 50–70% VO2max | 30 min | yes | 50% 1RM | -- | 3 | 20 | -- |
| Omentin | 3/12 | 55% HRmax | 10–12 min | yes | 50–55% 1RM | -- | 1–2 | 8–10 | Leg extension, prone leg curl, abdominal crunch, biceps, triceps, seated calf raise |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Martínez, P.; Ramirez-Campillo, R.; Alix-Fages, C.; Gene-Morales, J.; García-Ramos, A.; Colado, J.C. Chronic Resistance Training Effects on Serum Adipokines in Type 2 Diabetes Mellitus: A Systematic Review. Healthcare 2023, 11, 594. https://doi.org/10.3390/healthcare11040594
Jiménez-Martínez P, Ramirez-Campillo R, Alix-Fages C, Gene-Morales J, García-Ramos A, Colado JC. Chronic Resistance Training Effects on Serum Adipokines in Type 2 Diabetes Mellitus: A Systematic Review. Healthcare. 2023; 11(4):594. https://doi.org/10.3390/healthcare11040594
Chicago/Turabian StyleJiménez-Martínez, Pablo, Rodrigo Ramirez-Campillo, Carlos Alix-Fages, Javier Gene-Morales, Amador García-Ramos, and Juan C. Colado. 2023. "Chronic Resistance Training Effects on Serum Adipokines in Type 2 Diabetes Mellitus: A Systematic Review" Healthcare 11, no. 4: 594. https://doi.org/10.3390/healthcare11040594










