Physical Activity Alters Functional Connectivity of Orbitofrontal Cortex Subdivisions in Healthy Young Adults: A Longitudinal fMRI Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedure
2.3. Magnetic Resonance Imaging
2.3.1. Data Acquisition
2.3.2. Data Analysis
2.3.3. Seed-to-Whole-Brain Analysis
2.4. Statistics
2.4.1. Physiological Data
2.4.2. Resting State fMRI Data
2.4.3. Mood Questionnaires
2.4.4. Correlations
3. Results
3.1. Participants/Demographics
3.2. Fitness
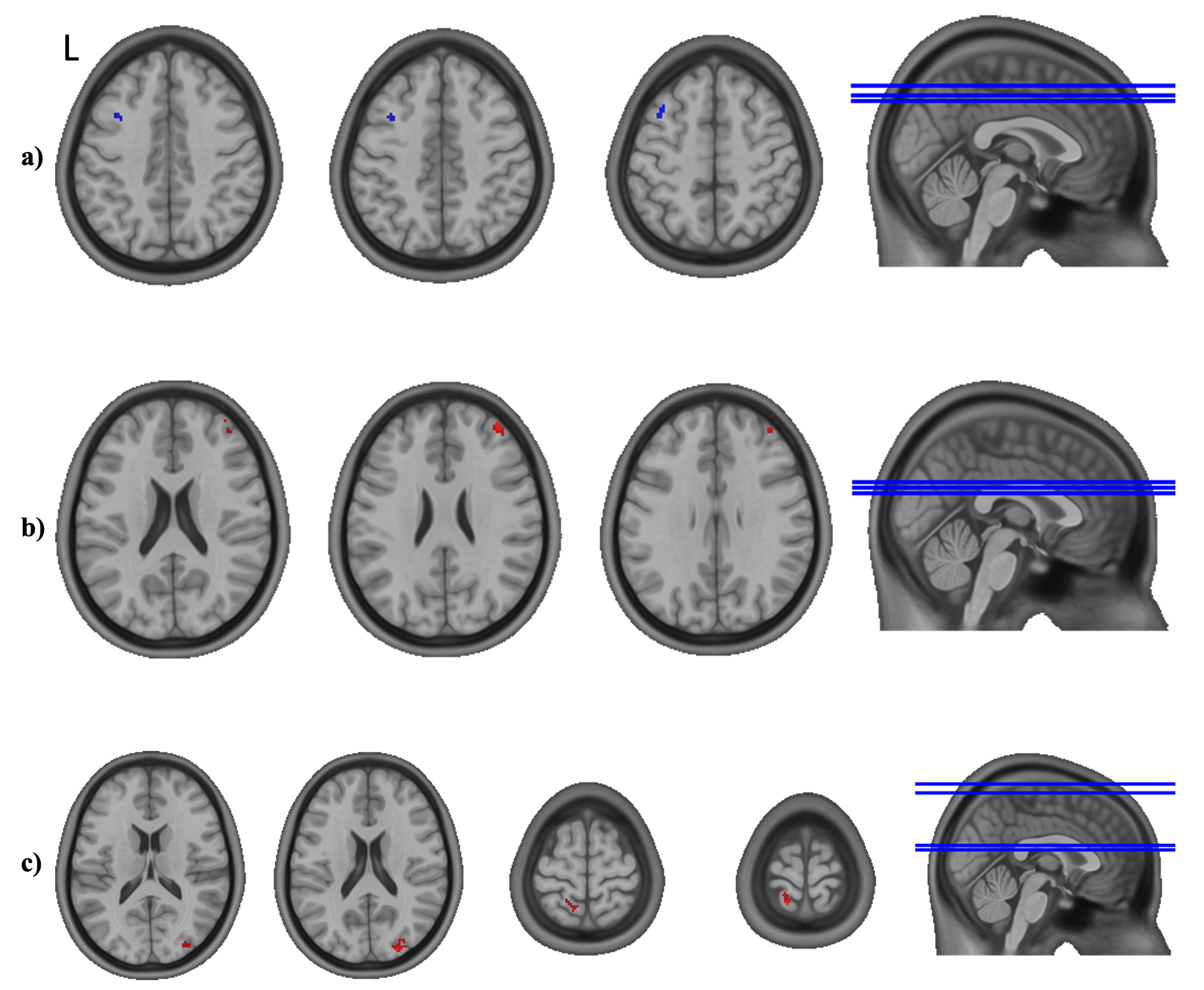
3.3. Seed-to-Whole-Brain Analysis
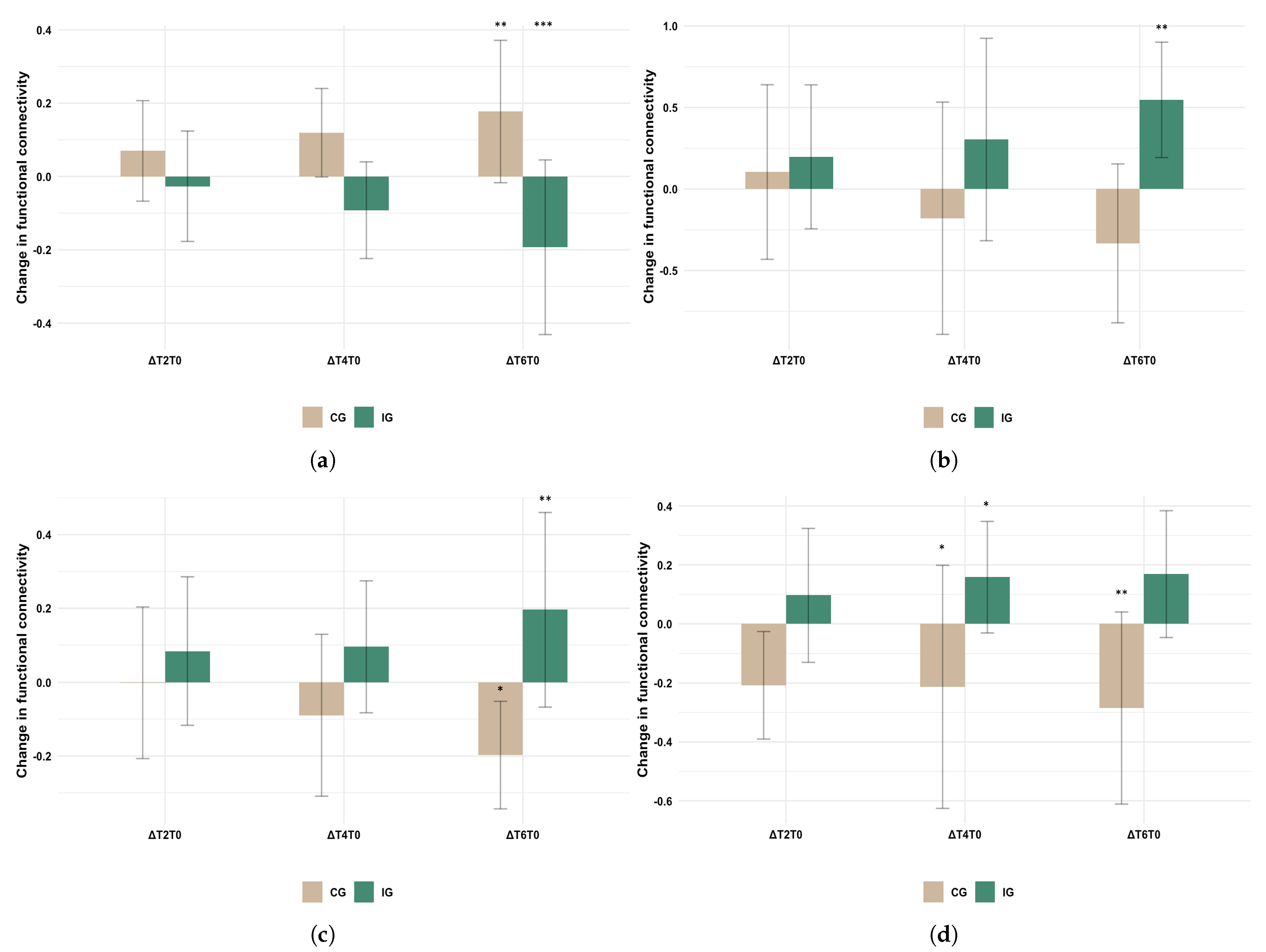
3.4. Post-Hoc Analyses
3.5. Mood Questionnaires
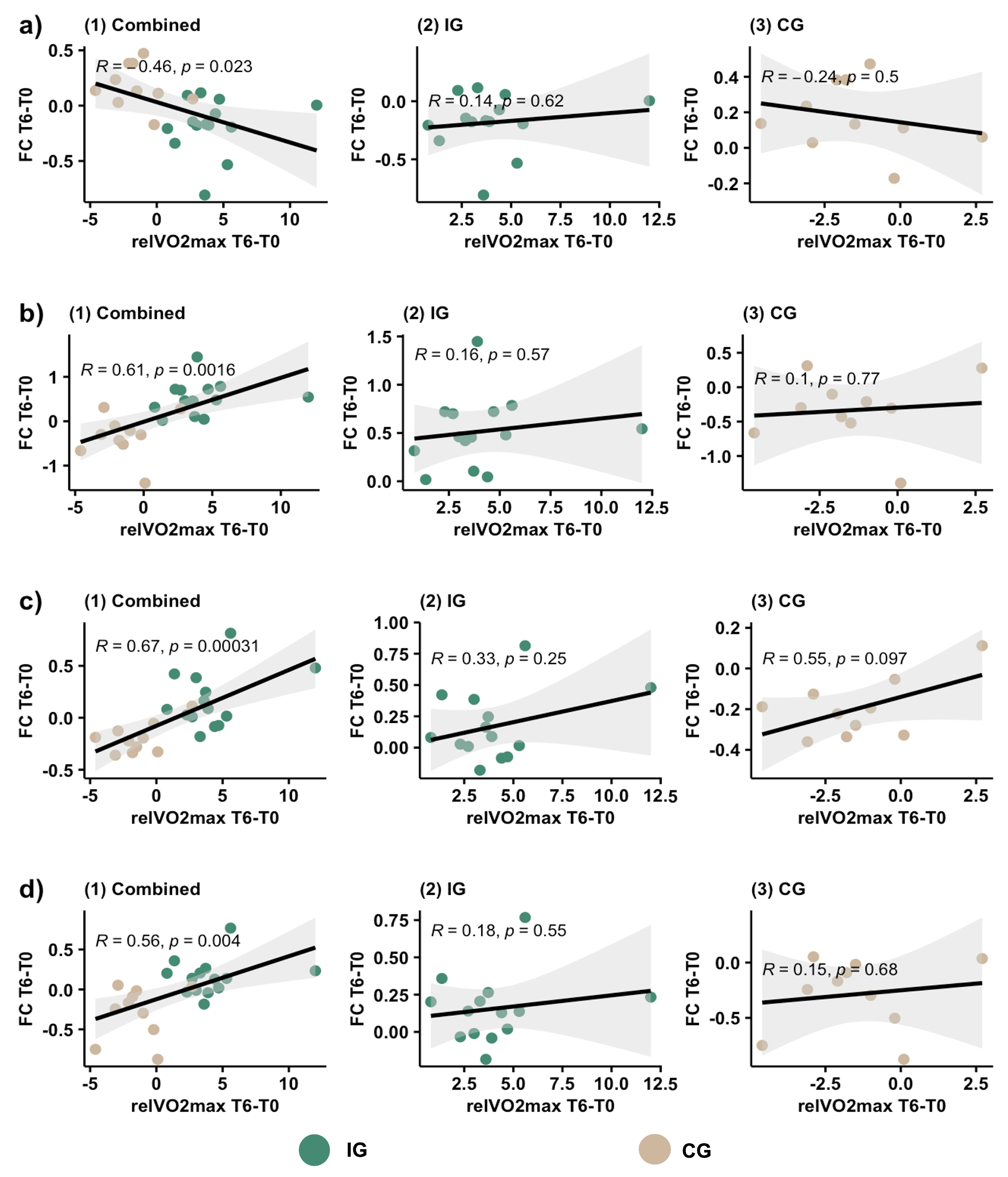
3.6. Correlation Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDI | Beck Depression Inventory |
| BOLD | Blood-oxygenation-level-dependent |
| CG | Control group |
| CSF | Cerebrospinal fluid |
| DLPFC | Dorsolateral prefrontal cortex |
| EPI | Echo-planar imaging |
| FC | Functional connectivity |
| IG | Intervention group |
| MD | Major depression |
| MFG | Middle frontal gyrus |
| OFC | Orbitofrontal cortex |
| PANAS | Positive and Negative Affect Schedule |
| PA | Physical activity |
| relVO2max | Maximal oxygen uptake (mL/min/kg) |
| fMRI | Functional magnetic resonance imaging |
| ROI | Region of interest |
| rsfMRI | Resting state fMRI |
| STAI | State-Trait-Anxiety Inventory |
| WM | White matter |
References
- Kringelbach, M.L. The Human Orbitofrontal Cortex: Linking Reward to Hedonic Experience. Nat. Rev. Neurosci. 2005, 6, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Cheng, W.; Feng, J. The Orbitofrontal Cortex: Reward, Emotion and Depression. Brain Commun. 2020, 2, fcaa196. [Google Scholar] [CrossRef] [PubMed]
- Kuusinen, V.; Cesnaite, E.; Peräkylä, J.; Ogawa, K.H.; Hartikainen, K.M. Orbitofrontal Lesion Alters Brain Dynamics of Emotion-Attention and Emotion-Cognitive Control Interaction in Humans. Front. Hum. Neurosci. 2018, 12, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mah, L.W.; Arnold, M.C.; Grafman, J. Deficits in Social Knowledge Following Damage to Ventromedial Prefrontal Cortex. JNP 2005, 17, 66–74. [Google Scholar] [CrossRef]
- Mäki-Marttunen, V.; Kuusinen, V.; Peräkylä, J.; Ogawa, K.H.; Brause, M.; Brander, A.; Hartikainen, K.M. Greater Attention to Task-Relevant Threat Due to Orbitofrontal Lesion. J. Neurotrauma 2017, 34, 400–413. [Google Scholar] [CrossRef]
- Jackowski, A.P.; de Araújo Filho, G.M.; de Almeida, A.G.; de Araújo, C.M.; Reis, M.; Nery, F.; Batista, I.R.; Silva, I.; Lacerda, A.L. The Involvement of the Orbitofrontal Cortex in Psychiatric Disorders: An Update of Neuroimaging Findings. Rev. Bras. Psiquiatr. 2012, 34, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Rolls, E.T.; Qiu, J.; Xie, X.; Wei, D.; Huang, C.C.; Yang, A.C.; Tsai, S.J.; Li, Q.; Meng, J.; et al. Increased Functional Connectivity of the Posterior Cingulate Cortex with the Lateral Orbitofrontal Cortex in Depression. Transl. Psychiatry 2018, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Frodl, T.; Bokde, A.L.; Scheuerecker, J.; Lisiecka, D.; Schoepf, V.; Hampel, H.; Möller, H.J.; Brückmann, H.; Wiesmann, M.; Meisenzahl, E. Functional Connectivity Bias of the Orbitofrontal Cortex in Drug-Free Patients with Major Depression. Biol. Psychiatry 2010, 67, 161–167. [Google Scholar] [CrossRef]
- O’Doherty, J.; Kringelbach, M.L.; Rolls, E.T.; Hornak, J.; Andrews, C. Abstract Reward and Punishment Representations in the Human Orbitofrontal Cortex. Nat. Neurosci. 2001, 4, 95–102. [Google Scholar] [CrossRef]
- Xie, C.; Jia, T.; Rolls, E.T.; Robbins, T.W.; Sahakian, B.J.; Zhang, J.; Liu, Z.; Cheng, W.; Luo, Q.; Zac Lo, C.Y.; et al. Reward Versus Nonreward Sensitivity of the Medial Versus Lateral Orbitofrontal Cortex Relates to the Severity of Depressive Symptoms. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 259–269. [Google Scholar] [CrossRef]
- Kahnt, T.; Chang, L.J.; Park, S.Q.; Heinzle, J.; Haynes, J.D. Connectivity-Based Parcellation of the Human Orbitofrontal Cortex. J. Neurosci. 2012, 32, 6240–6250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Rolls, E.T.; Cheng, W.; Li, Y.; Gong, W.; Qiu, J.; Feng, J. Functional Connectivity of the Orbitofrontal Cortex, Anterior Cingulate Cortex, and Inferior Frontal Gyrus in Humans. Cortex 2020, 123, 185–199. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Holla, B.; Bhargav, H.; Ramakrishna, K.K.; Chikkanna, U.; Varambally, S.; Gangadhar, B.N. Integrative Medicine as “Medicine”: A Perspective. Integr. Med. Rep. 2022, 1, 86–94. [Google Scholar] [CrossRef]
- Millman, L.S.M.; Terhune, D.B.; Hunter, E.C.M.; Orgs, G. Towards a Neurocognitive Approach to Dance Movement Therapy for Mental Health: A Systematic Review. Clin. Psychol. Psychother. 2021, 28, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, S.; Tiedemann, A.; Sherrington, C.; Curtis, J.; Ward, P.B. Physical Activity Interventions for People With Mental Illness: A Systematic Review and Meta-Analysis. J. Clin. Psychiatry 2014, 75, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Cotter, J.; Elliott, R.; French, P.; Yung, A.R. A Systematic Review and Meta-Analysis of Exercise Interventions in Schizophrenia Patients. Psychol. Med. 2015, 45, 1343–1361. [Google Scholar] [CrossRef] [Green Version]
- Bothe, N.; Zschucke, E.; Dimeo, F.; Heinz, A.; Wüstenberg, T.; Ströhle, A. Acute Exercise Influences Reward Processing in Highly Trained and Untrained Men. Med. Sci. Sport. Exerc. 2013, 45, 583–591. [Google Scholar] [CrossRef]
- Schmitt, A.; Upadhyay, N.; Martin, J.A.; Rojas, S.; Strüder, H.K.; Boecker, H. Modulation of Distinct Intrinsic Resting State Brain Networks by Acute Exercise Bouts of Differing Intensity. BPL 2019, 5, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Weng, T.B.; Pierce, G.L.; Darling, W.G.; Falk, D.; Magnotta, V.A.; Voss, M.W. The Acute Effects of Aerobic Exercise on the Functional Connectivity of Human Brain Networks. BPL 2017, 2, 171–190. [Google Scholar] [CrossRef] [Green Version]
- Peluso, M.A.M.; de Andrade, L.H.S.G. Physical Activity and Mental Health: The Association between Exercise and Mood. Clinics 2005, 60, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, A.; Upadhyay, N.; Martin, J.A.; Rojas Vega, S.; Strüder, H.K.; Boecker, H. Affective Modulation after High-Intensity Exercise Is Associated with Prolonged Amygdalar-Insular Functional Connectivity Increase. Neural Plast. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ströhle, A. Physical Activity, Exercise, Depression and Anxiety Disorders. J. Neural Transm. 2009, 116, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Bourbeau, K.; Moriarty, T.; Ayanniyi, A.; Zuhl, M. The Combined Effect of Exercise and Behavioral Therapy for Depression and Anxiety: Systematic Review and Meta-Analysis. Behav. Sci. 2020, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Stubbs, B. The Role of Exercise in Preventing and Treating Depression. Curr. Sport. Med. Rep. 2019, 18, 299–304. [Google Scholar] [CrossRef]
- Pascoe, M.C.; Parker, A.G. Physical Activity and Exercise as a Universal Depression Prevention in Young People: A Narrative Review. Early Interv. Psychiatry 2019, 13, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, N.; Schörkmaier, T.; Maurer, A.; Claus, J.; Scheef, L.; Daamen, M.; Martin, J.A.; Stirnberg, R.; Radbruch, A.; Attenberger, U.; et al. Regional Cortical Perfusion Increases Induced by a 6-Month Endurance Training in Young Sedentary Adults. Front. Aging Neurosci. 2022, 14, 951022. [Google Scholar] [CrossRef]
- Maurer, A.; Klein, J.; Claus, J.; Upadhyay, N.; Henschel, L.; Martin, J.A.; Scheef, L.; Daamen, M.; Schörkmaier, T.; Stirnberg, R.; et al. Effects of a 6-Month Aerobic Exercise Intervention on Mood and Amygdala Functional Plasticity in Young Untrained Subjects. Int. J. Environ. Res. Public Health 2022, 19, 6078. [Google Scholar] [CrossRef]
- Schmidt, K.H.; Metzler, P. WST-Wortschatztest; Gött Beltz Test: Göttingen, Germany, 1992. [Google Scholar]
- Oldfield, R. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. 20), 22–57. [Google Scholar]
- Hautzinger, M.; Bailer, M.; Worall, H.; Keller, F. Beck-Depressions-Inventar (BDI); Huber: Bern, Switzerland, 1994. [Google Scholar]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerstrom, K.O. The Fagerstrom Test for Nicotine Dependence: A Revision of the Fagerstrom Tolerance Questionnaire. Addiction 1991, 86, 1119–1127. [Google Scholar] [CrossRef]
- Bentley, D.J.; Newell, J.; Bishop, D. Incremental Exercise Test Design and Analysis: Implications for Performance Diagnostics in Endurance Athletes. Sport. Med. 2007, 37, 575–586. [Google Scholar] [CrossRef]
- Krohne, H.W.; Egloff, B.; Kohlmann, C.W.; Tausch, A. Untersuchungen Mit Einer Deutschen Version Der “Positive and Negative Affect Schedule” (PANAS). Diagnostica 1996, 42, 139–156. [Google Scholar]
- Spielberger, C.; Goruch, R.; Lushene, R.; Vagg, P.; Jacobs, G. Manual for the State-Trait Inventory STAI (Form Y); Mind Garden: Palo Alto, CA, USA, 1983. [Google Scholar]
- Setsompop, K.; Gagoski, B.A.; Polimeni, J.R.; Witzel, T.; Wedeen, V.J.; Wald, L.L. Blipped-Controlled Aliasing in Parallel Imaging for Simultaneous Multislice Echo Planar Imaging with Reduced g -Factor Penalty: Blipped-CAIPI for Simultaneous Multislice EPI. Magn. Reson. Med. 2012, 67, 1210–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stirnberg, R.; Stöcker, T. Segmented K-space Blipped-controlled Aliasing in Parallel Imaging for High Spatiotemporal Resolution EPI. Magn. Reson. Med. 2021, 85, 1540–1551. [Google Scholar] [CrossRef] [PubMed]
- Stirnberg, R.; Huijbers, W.; Brenner, D.; Poser, B.A.; Breteler, M.; Stöcker, T. Rapid Whole-Brain Resting-State fMRI at 3 T: Efficiency-optimized Three-Dimensional EPI versus Repetition Time-Matched Simultaneous-Multi-Slice EPI. NeuroImage 2017, 163, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.; Stirnberg, R.; Pracht, E.D.; Stöcker, T. Two-Dimensional Accelerated MP-RAGE Imaging with Flexible Linear Reordering. Magn. Reson Mater. Phy. 2014, 27, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Esteban, O.; Birman, D.; Schaer, M.; Koyejo, O.O.; Poldrack, R.A.; Gorgolewski, K.J. MRIQC: Advancing the Automatic Prediction of Image Quality in MRI from Unseen Sites. PLoS ONE 2017, 12, e0184661. [Google Scholar] [CrossRef] [Green Version]
- Esteban, O.; Markiewicz, C.J.; Blair, R.W.; Moodie, C.A.; Isik, A.I.; Erramuzpe, A.; Kent, J.D.; Goncalves, M.; DuPre, E.; Snyder, M.; et al. fMRIPrep: A Robust Preprocessing Pipeline for Functional MRI. Nat. Methods 2019, 16, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Gorgolewski, K.; Burns, C.D.; Madison, C.; Clark, D.; Halchenko, Y.O.; Waskom, M.L.; Ghosh, S.S. Nipype: A Flexible, Lightweight and Extensible Neuroimaging Data Processing Framework in Python. Front. Neuroinform. 2011, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Glasser, M.F.; Sotiropoulos, S.N.; Wilson, J.A.; Coalson, T.S.; Fischl, B.; Andersson, J.L.; Xu, J.; Jbabdi, S.; Webster, M.; Polimeni, J.R.; et al. The Minimal Preprocessing Pipelines for the Human Connectome Project. NeuroImage 2013, 80, 105–124. [Google Scholar] [CrossRef] [Green Version]
- Avants, B.; Epstein, C.; Grossman, M.; Gee, J. Symmetric Diffeomorphic Image Registration with Cross-Correlation: Evaluating Automated Labeling of Elderly and Neurodegenerative Brain. Med. Image Anal. 2008, 12, 26–41. [Google Scholar] [CrossRef] [Green Version]
- Greve, D.N.; Fischl, B. Accurate and Robust Brain Image Alignment Using Boundary-Based Registration. NeuroImage 2009, 48, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Fonov, V.; Evans, A.; McKinstry, R.; Almli, C.; Collins, D. Unbiased Nonlinear Average Age-Appropriate Brain Templates from Birth to Adulthood. NeuroImage 2009, 47, S102. [Google Scholar] [CrossRef]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to Detect, Characterize, and Remove Motion Artifact in Resting State fMRI. NeuroImage 2014, 84, 320–341. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A Component Based Noise Correction Method (CompCor) for BOLD and Perfusion Based fMRI. NeuroImage 2007, 37, 90–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satterthwaite, T.D.; Elliott, M.A.; Gerraty, R.T.; Ruparel, K.; Loughead, J.; Calkins, M.E.; Eickhoff, S.B.; Hakonarson, H.; Gur, R.C.; Gur, R.E.; et al. An Improved Framework for Confound Regression and Filtering for Control of Motion Artifact in the Preprocessing of Resting-State Functional Connectivity Data. NeuroImage 2013, 64, 240–256. [Google Scholar] [CrossRef] [Green Version]
- Lanczos, C. Evaluation of Noisy Data. J. Soc. Ind. Appl. Math. Ser. B Numer. Anal. 1964, 1, 76–85. [Google Scholar] [CrossRef]
- Cox, R.W. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef]
- Cox, R.W.; Hyde, J.S. Software Tools for Analysis and Visualization of fMRI Data. NMR Biomed. 1997, 10, 171–178. [Google Scholar] [CrossRef]
- Caballero-Gaudes, C.; Reynolds, R.C. Methods for Cleaning the BOLD fMRI Signal. NeuroImage 2017, 154, 128–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciric, R.; Wolf, D.H.; Power, J.D.; Roalf, D.R.; Baum, G.L.; Ruparel, K.; Shinohara, R.T.; Elliott, M.A.; Eickhoff, S.B.; Davatzikos, C.; et al. Benchmarking of Participant-Level Confound Regression Strategies for the Control of Motion Artifact in Studies of Functional Connectivity. NeuroImage 2017, 154, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Muschelli, J.; Nebel, M.B.; Caffo, B.S.; Barber, A.D.; Pekar, J.J.; Mostofsky, S.H. Reduction of Motion-Related Artifacts in Resting State fMRI Using aCompCor. NeuroImage 2014, 96, 22–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Yao, J.; Lv, Y.; Zhao, X.; Zhang, X.; Lei, J.; Li, Y.; Sui, Y. Aberrant Functional Connectivity of the Orbitofrontal Cortex Is Associated With Excited Symptoms in First-Episode Drug-Naïve Patients With Schizophrenia. Front. Psychiatry 2022, 13, 922272. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Cheng, W.; Du, J.; Wei, D.; Qiu, J.; Dai, D.; Zhou, Q.; Xie, P.; Feng, J. Functional Connectivity of the Right Inferior Frontal Gyrus and Orbitofrontal Cortex in Depression. Soc. Cogn. Affect. Neurosci. 2020, 15, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickerson, L.D.; Smith, S.M.; Öngür, D.; Beckmann, C.F. Using Dual Regression to Investigate Network Shape and Amplitude in Functional Connectivity Analyses. Front. Neurosci. 2017, 11, 115. [Google Scholar] [CrossRef] [Green Version]
- Devlin, J.T.; Russell, R.P.; Davis, M.H.; Price, C.J.; Wilson, J.; Moss, H.E.; Matthews, P.M.; Tyler, L.K. Susceptibility-Induced Loss of Signal: Comparing PET and fMRI on a Semantic Task. NeuroImage 2000, 11, 589–600. [Google Scholar] [CrossRef] [Green Version]
- Weiskopf, N.; Hutton, C.; Josephs, O.; Deichmann, R. Optimal EPI Parameters for Reduction of Susceptibility-Induced BOLD Sensitivity Losses: A Whole-Brain Analysis at 3 T and 1.5 T. NeuroImage 2006, 33, 493–504. [Google Scholar] [CrossRef]
- Rorden, C.; Brett, M. Stereotaxic Display of Brain Lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2021. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.6.1. 2021. Available online: https://cran.r-project.org/package=emmeans (accessed on 22 December 2022).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Chen, G.; Saad, Z.S.; Britton, J.C.; Pine, D.S.; Cox, R.W. Linear Mixed-Effects Modeling Approach to FMRI Group Analysis. NeuroImage 2013, 73, 176–190. [Google Scholar] [CrossRef] [Green Version]
- Cox, R.W.; Chen, G.; Glen, D.R.; Reynolds, R.C.; Taylor, P.A. fMRI Clustering and False-Positive Rates. Proc. Natl. Acad. Sci. USA 2017, 114, E3370–E3371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, R.W.; Chen, G.; Glen, D.R.; Reynolds, R.C.; Taylor, P.A. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect. 2017, 7, 152–171. [Google Scholar] [CrossRef]
- Chen, G.; Taylor, P.A.; Stoddard, J.; Cox, R.W.; Bandettini, P.A.; Pessoa, L. Sources of Information Waste in Neuroimaging: Mishandling Structures, Thinking Dichotomously, and Over-Reducing Data. Aperture Neuro 2022, 2021, 46. [Google Scholar] [CrossRef]
- Saad, Z.S.; Reynolds, R.C. SUMA. NeuroImage 2012, 62, 768–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, Z.; Reynolds, R.; Argall, B.; Japee, S.; Cox, R. SUMA: An Interface for Surface-Based Intra- and Inter-Subject Analysis with AFNI. In Proceedings of the 2004 2nd IEEE International Symposium on Biomedical Imaging: Macro to Nano (IEEE Cat No. 04EX821), Arlington, VA, USA, 18 April 2004; Volume 2, pp. 1510–1513. [Google Scholar]
- McNab, F.; Leroux, G.; Strand, F.; Thorell, L.; Bergman, S.; Klingberg, T. Common and Unique Components of Inhibition and Working Memory: An fMRI, within-Subjects Investigation. Neuropsychologia 2008, 46, 2668–2682. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qin, W.; Zhuo, C.; Xu, L.; Zhu, J.; Liu, X.; Yu, C. Selective Functional Disconnection of the Orbitofrontal Subregions in Schizophrenia. Psychol. Med. 2017, 47, 1637–1646. [Google Scholar] [CrossRef]
- Eshel, N.; Maron-Katz, A.; Wu, W.; Abu-Amara, D.; Marmar, C.R.; Etkin, A. Neural Correlates of Anger Expression in Patients with PTSD. Neuropsychopharmacology 2021, 46, 1635–1642. [Google Scholar] [CrossRef]
- Kwon, E.; Hummer, T.; Andrews, K.D.; Finn, P.; Aalsma, M.; Bailey, A.; Hanquier, J.; Wang, T.; Hulvershorn, L. Functional Connectivity in Frontostriatal Networks Differentiate Offspring of Parents with Substance Use Disorders from Other High-Risk Youth. Drug Alcohol Depend. 2021, 219, 108498. [Google Scholar] [CrossRef]
- Zald, D.H.; McHugo, M.; Ray, K.L.; Glahn, D.C.; Eickhoff, S.B.; Laird, A.R. Meta-Analytic Connectivity Modeling Reveals Differential Functional Connectivity of the Medial and Lateral Orbitofrontal Cortex. Cereb. Cortex 2014, 24, 232–248. [Google Scholar] [CrossRef]
- Zou, K.; Gao, Q.; Long, Z.; Xu, F.; Sun, X.; Chen, H.; Sun, X. Abnormal Functional Connectivity Density in First-Episode, Drug-Naive Adult Patients with Major Depressive Disorder. J. Affect. Disord. 2016, 194, 153–158. [Google Scholar] [CrossRef]
- Kandola, A.A.; del Pozo Cruz, B.; Osborn, D.P.J.; Stubbs, B.; Choi, K.W.; Hayes, J.F. Impact of Replacing Sedentary Behaviour with Other Movement Behaviours on Depression and Anxiety Symptoms: A Prospective Cohort Study in the UK Biobank. BMC Med. 2021, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, L.; Carballedo, A.; Lavelle, G.; Doolin, K.; Doyle, M.; Amico, F.; McCarthy, H.; Gormley, J.; Lord, A.; O’Keane, V.; et al. Longitudinal Functional Connectivity Changes Correlate with Mood Improvement after Regular Exercise in a Dose-Dependent Fashion. Eur. J. Neurosci. 2016, 43, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.J.; Fredrickson, B.L. Measurement Issues in Emotion Research. In Well-Being: The Foundations of Hedonic Psychology; Russell Sage Foundation: New York, NY, USA, 1999; pp. 40–60. [Google Scholar]
- Elliott, R.; Zahn, R.; Deakin, J.F.W.; Anderson, I.M. Affective Cognition and Its Disruption in Mood Disorders. Neuropsychopharmacology 2011, 36, 153–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
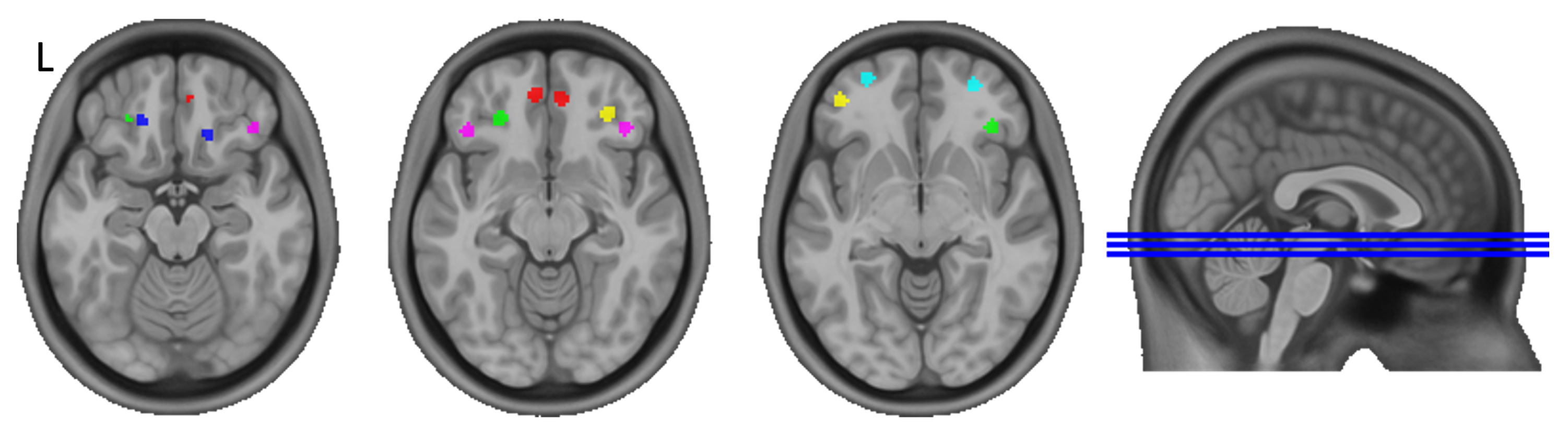

| Left Hemisphere (L) | Right Hemisphere (R) | |||||
|---|---|---|---|---|---|---|
| Seed No. | x | y | z | x | y | z |
| 1 | −7 | 49 | −12 | 6 | 47 | −13 |
| 2 | −19 | 35 | −19 | 16 | 28 | −19 |
| 3 | −26 | 36 | −13 | 37 | 32 | −8 |
| 4 | −43 | 30 | −11 | 40 | 31 | −14 |
| 5 | −42 | 46 | −8 | 31 | 39 | −12 |
| 6 | −28 | 57 | −8 | 28 | 54 | −8 |
| Variables | IG (N = 18) | CG (N = 10) | p-Value |
|---|---|---|---|
| Sex (male/female) | 7/11 | 6/4 | 0.433 b |
| Age (years) | 23.9 ± 3.9 | 23.7 ± 4.2 | 0.879 |
| Height (cm) | 174 ± 12.1 | 177 ± 7.9 | 0.441 |
| Weight (kg) | 69.9 ± 15.1 | 71.2 ± 14.1 | 0.649 a |
| BMI (kg/m2) | 23.1 ± 3.7 | 22.7 ± 3.6 | 0.762 |
| HRmax (1/min) | 198 ± 7.6 | 201 ± 8.5 | 0.468 |
| relVO2max (mL/min/kg) | 38.5 ± 3.4 | 41.7 ± 7.5 | 0.232 |
| Education (years) | 16.3 ± 3.1 | 15.8 ± 3.1 | 0.781 a |
| BDI | 2.6 ± 3.4 | 1.4 ± 1.5 | 0.704 a |
| STAI trait | 33.9 ± 9.3 | 31.4 ± 6.1 | 0.624 a |
| FTND | 0.2 ± 0.9 | 0.0 ± 0.0 | n/a c |
| WST IQ | 107 ± 9.9 | 107 ± 8.8 | 0.937 |
| EHI_LQ | 74.2 ± 16.2 | 79.5 ± 13.3 | 0.390 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claus, J.; Upadhyay, N.; Maurer, A.; Klein, J.; Scheef, L.; Daamen, M.; Martin, J.A.; Stirnberg, R.; Radbruch, A.; Attenberger, U.; et al. Physical Activity Alters Functional Connectivity of Orbitofrontal Cortex Subdivisions in Healthy Young Adults: A Longitudinal fMRI Study. Healthcare 2023, 11, 689. https://doi.org/10.3390/healthcare11050689
Claus J, Upadhyay N, Maurer A, Klein J, Scheef L, Daamen M, Martin JA, Stirnberg R, Radbruch A, Attenberger U, et al. Physical Activity Alters Functional Connectivity of Orbitofrontal Cortex Subdivisions in Healthy Young Adults: A Longitudinal fMRI Study. Healthcare. 2023; 11(5):689. https://doi.org/10.3390/healthcare11050689
Chicago/Turabian StyleClaus, Jannik, Neeraj Upadhyay, Angelika Maurer, Julian Klein, Lukas Scheef, Marcel Daamen, Jason Anthony Martin, Rüdiger Stirnberg, Alexander Radbruch, Ulrike Attenberger, and et al. 2023. "Physical Activity Alters Functional Connectivity of Orbitofrontal Cortex Subdivisions in Healthy Young Adults: A Longitudinal fMRI Study" Healthcare 11, no. 5: 689. https://doi.org/10.3390/healthcare11050689
APA StyleClaus, J., Upadhyay, N., Maurer, A., Klein, J., Scheef, L., Daamen, M., Martin, J. A., Stirnberg, R., Radbruch, A., Attenberger, U., Stöcker, T., & Boecker, H. (2023). Physical Activity Alters Functional Connectivity of Orbitofrontal Cortex Subdivisions in Healthy Young Adults: A Longitudinal fMRI Study. Healthcare, 11(5), 689. https://doi.org/10.3390/healthcare11050689







