A Prospective Cohort Study of COVID-19: Evaluation of the Early Role of IL-1 and IL-6 Antagonists in Improving the Outcome of the Illness and Reduction in the Risk of Death
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Study Design
2.5. Statistical Analysis
2.6. Ethical Approval
3. Results
3.1. The Usual Regimen Did Not Improve the Biochemical Parameters Significantly
3.2. Anakinra or Tocilizumab Significantly Decreased the Tested Parameters
3.3. Anakinra Improved the Ferritin and D-Dimer Levels in Comparison to Tocilizumab and the Usual Treatment Regimen
3.4. Anakinra Improved the Illness Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Rao, Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.P.; Sun, Y.L.; Wang, Y.F.; Yazici, D.; Azkur, D.; Ogulur, I.; Azkur, A.K.; Yang, Z.W.; Chen, X.X.; Zhang, A.Z.; et al. Recent developments in the immunopathology of COVID-19. Allergy 2023, 78, 369–388. [Google Scholar] [CrossRef]
- Cao, Z.; Gao, W.; Bao, H.; Feng, H.; Mei, S.; Chen, P.; Gao, Y.; Cui, Z.; Zhang, Q.; Meng, X.; et al. VV116 versus Nirmatrelvir-Ritonavir for Oral Treatment of COVID-19. N. Engl. J. Med. 2023, 388, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Krishnan, U.M. The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie 2020, 179, 85–100. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Kirtipal, N.; Bharadwaj, S.; Kang, S.G. From SARS to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses. Infect. Genet. Evol. 2020, 85, 104502. [Google Scholar] [CrossRef]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef]
- Botta, M.; Caritg, O.; van Meenen, D.M.P.; Pacheco, A.; Tsonas, A.M.; Mooij, W.E.; Burgener, A.; Manrique Hehl, T.; Shrestha, G.S.; Horn, J.; et al. Oxygen Consumption with High-Flow Nasal Oxygen versus Mechanical Ventilation- An International Multicenter Observational Study in COVID-19 Patients (PROXY-COVID). Am. J. Trop. Med. Hyg. 2023, 1–7. [Google Scholar]
- Forchette, L.; Sebastian, W.; Liu, T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr. Med. Sci. 2021, 41, 1037–1051. [Google Scholar] [CrossRef]
- Svoboda, J.; Tkadlec, J.; Pavlogiannis, A.; Chatterjee, K.; Nowak, M.A. Infection dynamics of COVID-19 virus under lockdown and reopening. Sci. Rep. 2022, 12, 1526. [Google Scholar] [CrossRef]
- Rivera, L.C.; Mohamed, S.; Salar, T.; Mostafa, M.R.; Najim, M.; Malik, M.A.; Renjithal, S.L.M.; Magdi, M. Organizing Pneumonia: An Unusual Sequela of COVID-19 Infection. Eur. J. Case Rep. Intern. Med. 2023, 10, 003787. [Google Scholar] [CrossRef] [PubMed]
- Maliki, I.; Elmsellem, H.; Hafez, B.; EL Moussaoui, A.; Reda Kachmar, M.; Ouahbi, A. The psychological properties of the Arabic BDI-II and the psychological state of the general Moroccan population during the mandatory quarantine due to the COVID-19 pandemic. Casp. J. Environ. Sci. 2021, 19, 139–150. [Google Scholar]
- Ahmad, M.F.; Mahakkanukrauh, P.; Das, S. The Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Virus in the Vaginal Fluid of Females With Severe Coronavirus Disease 2019 (COVID-19) Infection: Scientific Facts. Clin. Infect. Dis. 2022, 74, 2262–2263. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.M.; Chan, Y.F.; Jamaluddin, M.F.H.; Hasan, M.S.; Pang, Y.K.; Ponnampalavanar, S.; Syed Omar, S.F.; Sam, I.C. Rhinovirus/enterovirus was the most common respiratory virus detected in adults with severe acute respiratory infections pre-COVID-19 in Kuala Lumpur, Malaysia. PLoS ONE 2022, 17, e0273697. [Google Scholar] [CrossRef] [PubMed]
- Mrcela, D.; Markic, J.; Zhao, C.; Viskovic, D.V.; Milic, P.; Copac, R.; Li, Y. Changes following the Onset of the COVID-19 Pandemic in the Burden of Hospitalization for Respiratory Syncytial Virus Acute Lower Respiratory Infection in Children under Two Years: A Retrospective Study from Croatia. Viruses 2022, 14, 2746. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.O.; Abdalrahman, N.A.; Shanab, E.A.I.; Mohammed, M.M.A.; Ibrahim, M.M.; Abdalrahman, I.B. The outcome of COVID-19 patients in the intensive care unit in Sudan: A cross-sectional study. Health Sci. Rep. 2023, 6, e1161. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.; Qiu, J.; Li, L.; Jia, X.; Kai, G. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020, 53, 66–70. [Google Scholar] [CrossRef]
- Soy, M.; Keser, G.; Atagunduz, P. Pathogenesis and treatment of cytokine storm in COVID-19. Turk. J. Biol. 2021, 45, 372–389. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the; Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Yongzhi, X. COVID-19-associated cytokine storm syndrome and diagnostic principles: An old and new Issue. Emerg. Microbes Infect. 2021, 10, 266–276. [Google Scholar] [CrossRef]
- Aldawsari, M.F.; Alalaiwe, A.; Khafagy, E.S.; Al Saqr, A.; Alshahrani, S.M.; Alsulays, B.B.; Alshehri, S.; Abu Lila, A.S.; Danish Rizvi, S.M.; Hegazy, W.A.H. Efficacy of SPG-ODN 1826 Nanovehicles in Inducing M1 Phenotype through TLR-9 Activation in Murine Alveolar J774A.1 Cells: Plausible Nano-Immunotherapy for Lung Carcinoma. Int. J. Mol. Sci. 2021, 22, 6833. [Google Scholar] [CrossRef]
- Khayyat, A.N.; Abbas, H.A.; Mohamed, M.F.A.; Asfour, H.Z.; Khayat, M.T.; Ibrahim, T.S.; Youns, M.; Khafagy, E.-S.; Abu Lila, A.S.; Safo, M.K.; et al. Not Only Antimicrobial: Metronidazole Mitigates the Virulence of Proteus mirabilis Isolated from Macerated Diabetic Foot Ulcer. Appl. Sci. 2021, 11, 6847. [Google Scholar] [CrossRef]
- Askoura, M.; Almalki, A.J.; Lila, A.S.A.; Almansour, K.; Alshammari, F.; Khafagy, E.-S.; Ibrahim, T.S.; Hegazy, W.A.H. Alteration of Salmonella enterica Virulence and Host Pathogenesis through Targeting sdiA by Using the CRISPR-Cas9 System. Microorganisms 2021, 9, 2564. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Subbian, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e00299-20. [Google Scholar] [CrossRef]
- Soy, M.; Keser, G.; Atagunduz, P.; Tabak, F.; Atagunduz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Fara, A.; Mitrev, Z.; Rosalia, R.A.; Assas, B.M. Cytokine storm and COVID-19: A chronicle of pro-inflammatory cytokines. Open Biol. 2020, 10, 200160. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, S.; Song, X. Cytokine storm with rapidly elevated interleukin-6 indicates sudden death in patients with critical COVID-19. Cytokine Growth Factor Rev. 2021, 58, 30–31. [Google Scholar] [CrossRef]
- Conti, P.; Caraffa, A.; Tete, G.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Di Emidio, P.; Ronconi, G. Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. J. Biol. Regul. Homeost. Agents 2020, 34, 1629–1632. [Google Scholar] [CrossRef]
- Jafrin, S.; Aziz, M.A.; Islam, M.S. Elevated Levels of Pleiotropic Interleukin-6 (IL-6) and Interleukin-10 (IL-10) are Critically Involved With the Severity and Mortality of COVID-19: An Updated Longitudinal Meta-Analysis and Systematic Review on 147 Studies. Biomark. Insights 2022, 17, 11772719221106600. [Google Scholar] [CrossRef]
- Mojtabavi, H.; Saghazadeh, A.; Rezaei, N. Interleukin-6 and severe COVID-19: A systematic review and meta-analysis. Eur. Cytokine Netw. 2020, 31, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kitsos, D.; Tzartos, J.; Korres, G.; Giannopapas, V.; Riga, M.; Stergiou, C.; Tsoga, A.; Grigoropoulos, C.; Paraskevas, G.; Zompola, C.; et al. IL-6 Serum Levels in COVID-19 Patients With Vertigo. Cureus 2023, 15, e35042. [Google Scholar] [CrossRef] [PubMed]
- Sasson, J.; Moreau, G.B.; Petri, W.A., Jr. The Role of IL-13 and the Type 2 Immune Pathway in COVID-19: A Review. Ann. Allergy Asthma Immunol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Pinzon, R.T.; Wijaya, V.O.; Buana, R.B. Interleukin-6 (IL-6) inhibitors as therapeutic agents for coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Infect. Public Health 2021, 14, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Udomsinprasert, W.; Jittikoon, J.; Sangroongruangsri, S.; Chaikledkaew, U. Circulating Levels of Interleukin-6 and Interleukin-10, But Not Tumor Necrosis Factor-Alpha, as Potential Biomarkers of Severity and Mortality for COVID-19: Systematic Review with Meta-analysis. J. Clin. Immunol. 2021, 41, 11–22. [Google Scholar] [CrossRef]
- Yu, S.Y.; Koh, D.H.; Choi, M.; Ryoo, S.; Huh, K.; Yeom, J.S.; Yoon, Y.K. Clinical efficacy and safety of interleukin-6 receptor antagonists (tocilizumab and sarilumab) in patients with COVID-19: A systematic review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 1154–1165. [Google Scholar] [CrossRef]
- Conti, P.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Ronconi, G. Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: A promising inhibitory strategy. J. Biol. Regul. Homeost. Agents 2020, 34, 1971–1975. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef]
- Kaps, L.; Labenz, C.; Grimm, D.; Schwarting, A.; Galle, P.R.; Schreiner, O. Treatment of cytokine storm syndrome with IL-1 receptor antagonist anakinra in a patient with ARDS caused by COVID-19 infection: A case report. Clin. Case Rep. 2020, 8, 2990–2994. [Google Scholar] [CrossRef]
- Askoura, M.; Abbas, H.A.; Al Sadoun, H.; Abdulaal, W.H.; Abu Lila, A.S.; Almansour, K.; Alshammari, F.; Khafagy, E.-S.; Ibrahim, T.S.; Hegazy, W.A.H. Elevated Levels of IL-33, IL-17 and IL-25 Indicate the Progression from Chronicity to Hepatocellular Carcinoma in Hepatitis C Virus Patients. Pathogens 2022, 11, 57. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, W.A.H.; Henaway, M. Hepatitis C virus pathogenesis: Serum IL-33 level indicates liver damage. Afr. J. Microbiol. Res. 2015, 9, 1386–1393. [Google Scholar] [CrossRef] [Green Version]
- Rondovic, G.; Djordjevic, D.; Udovicic, I.; Stanojevic, I.; Zeba, S.; Abazovic, T.; Vojvodic, D.; Abazovic, D.; Khan, W.; Surbatovic, M. From Cytokine Storm to Cytokine Breeze: Did Lessons Learned from Immunopathogenesis Improve Immunomodulatory Treatment of Moderate-to-Severe COVID-19? Biomedicines 2022, 10, 2620. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Guo, M.; Zheng, Y.; Zhang, Y.; De, Y.; Xu, C.; Zhang, L.; Sun, R.; Lv, Y.; Liang, Y.; et al. Current Evidence of Interleukin-6 Signaling Inhibitors in Patients With COVID-19: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 615972. [Google Scholar] [CrossRef]
- Bovet, M.; Wadsack, D.; Kosely, F.; Zink, W.; Zahn, R. Fatal course of COVID-19 despite IL-6 receptor blockade in cytokine storm: Perimyocarditis and coagulopathy after administration of tocilizumab. Anaesthesist 2021, 70, 121–126. [Google Scholar] [CrossRef]
- Emsley, H.; Smith, C.; Georgiou, R.; Vail, A.; Hopkins, S.; Rothwell, N.; Tyrrell, P. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1366–1372. [Google Scholar] [CrossRef] [Green Version]
- Möller, B.; Villiger, P.M. Inhibition of IL-1, IL-6, and TNF-α in immune-mediated inflammatory diseases. In Springer Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 391–408. [Google Scholar]
- Scherger, S.; Henao-Martínez, A.; Franco-Paredes, C.; Shapiro, L. Rethinking interleukin-6 blockade for treatment of COVID-19. Med. Hypotheses 2020, 144, 110053. [Google Scholar] [CrossRef]
- Atal, S.; Fatima, Z. IL-6 inhibitors in the treatment of serious COVID-19: A promising therapy? Pharm. Med. 2020, 34, 223–231. [Google Scholar] [CrossRef]
- Khayyat, A.N.; Abbas, H.A.; Khayat, M.T.; Shaldam, M.A.; Askoura, M.; Asfour, H.Z.; Khafagy, E.-S.; Abu Lila, A.S.; Allam, A.N.; Hegazy, W.A.H. Secnidazole Is a Promising Imidazole Mitigator of Serratia marcescens Virulence. Microorganisms 2021, 9, 2333. [Google Scholar] [CrossRef]
- Bertoni, A.; Penco, F.; Mollica, H.; Bocca, P.; Prigione, I.; Corcione, A.; Cangelosi, D.; Schena, F.; Del Zotto, G.; Amaro, A.; et al. Spontaneous NLRP3 inflammasome-driven IL-1-beta secretion is induced in severe COVID-19 patients and responds to anakinra treatment. J. Allergy Clin. Immunol. 2022, 150, 796–805. [Google Scholar] [CrossRef]
- Cavalli, G.; Dagna, L. The right place for IL-1 inhibition in COVID-19. Lancet Respir. Med. 2021, 9, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Della-Torre, E.; Criscuolo, E.; Lanzillotta, M.; Locatelli, M.; Clementi, N.; Mancini, N.; Dagna, L.; COVID-BioB study group. IL-1 and IL-6 inhibition affects the neutralising activity of anti-SARS-CoV-2 antibodies in patients with COVID-19. Lancet Rheumatol. 2021, 3, e829–e831. [Google Scholar] [CrossRef] [PubMed]
- Della-Torre, E.; Lanzillotta, M.; Campochiaro, C.; Cavalli, G.; De Luca, G.; Tomelleri, A.; Boffini, N.; De Lorenzo, R.; Ruggeri, A.; Rovere-Querini, P.; et al. Respiratory Impairment Predicts Response to IL-1 and IL-6 Blockade in COVID-19 Patients With Severe Pneumonia and Hyper-Inflammation. Front. Immunol. 2021, 12, 675678. [Google Scholar] [CrossRef] [PubMed]
- Franzetti, M.; Forastieri, A.; Borsa, N.; Pandolfo, A.; Molteni, C.; Borghesi, L.; Pontiggia, S.; Evasi, G.; Guiotto, L.; Erba, M.; et al. IL-1 Receptor Antagonist Anakinra in the Treatment of COVID-19 Acute Respiratory Distress Syndrome: A Retrospective, Observational Study. J. Immunol. 2021, 206, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Makaremi, S.; Asgarzadeh, A.; Kianfar, H.; Mohammadnia, A.; Asghariazar, V.; Safarzadeh, E. The role of IL-1 family of cytokines and receptors in pathogenesis of COVID-19. Inflamm. Res. 2022, 71, 923–947. [Google Scholar] [CrossRef]
- Renieris, G.; Karakike, E.; Gkavogianni, T.; Droggiti, D.E.; Stylianakis, E.; Andriopoulou, T.; Spanou, V.M.; Kafousopoulos, D.; Netea, M.G.; Eugen-Olsen, J.; et al. IL-1 Mediates Tissue-Specific Inflammation and Severe Respiratory Failure in COVID-19. J. Innate Immun. 2022, 14, 644–657. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Netea, M.G. Blocking IL-1 to prevent respiratory failure in COVID-19. Crit. Care 2020, 24, 445. [Google Scholar] [CrossRef]
- Cassat, J.E.; Skaar, E.P. Iron in infection and immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Haschka, D.; Hoffmann, A.; Weiss, G. Iron in immune cell function and host defense. Semin. Cell Dev. Biol. 2021, 115, 27–36. [Google Scholar] [CrossRef]
- Alroomi, M.; Rajan, R.; Omar, A.A.; Alsaber, A.; Pan, J.; Fatemi, M.; Zhanna, K.D.; Aboelhassan, W.; Almutairi, F.; Alotaibi, N.; et al. Ferritin level: A predictor of severity and mortality in hospitalized COVID-19 patients. Immun. Inflamm. Dis. 2021, 9, 1648–1655. [Google Scholar] [CrossRef]
- Dahan, S.; Segal, G.; Katz, I.; Hellou, T.; Tietel, M.; Bryk, G.; Amital, H.; Shoenfeld, Y.; Dagan, A. Ferritin as a marker of severity in COVID-19 patients: A fatal correlation. Isr. Med. Assoc. J. IMAJ 2020, 22, 494–500. [Google Scholar]
- Lin, Z.; Long, F.; Yang, Y.; Chen, X.; Xu, L.; Yang, M. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef] [PubMed]
- Mahroum, N.; Alghory, A.; Kiyak, Z.; Alwani, A.; Seida, R.; Alrais, M.; Shoenfeld, Y. Ferritin–from iron, through inflammation and autoimmunity, to COVID-19. J. Autoimmun. 2022, 126, 102778. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, C.; de Montmollin, E.; Buetti, N.; Goldgran-Toledano, D.; Reignier, J.; Schwebel, C.; Domitile, J.; Neuville, M.; Ursino, M.; Siami, S. Impact of early corticosteroids on 60-day mortality in critically ill patients with COVID-19: A multicenter cohort study of the OUTCOMEREA network. PLoS ONE 2021, 16, e0255644. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Vargas, C.; Azocar, F.; Villarroel, F.; Cofré, M.; Oppliger, H.; Ríos, F.; Raijmakers, M.; Silva-Ayarza, I.; Beltrán, C. Steroids and mortality in non-critically ill COVID-19 patients: A propensity score-weighted study in a Chilean cohort. Int. J. Infect. Dis. 2021, 112, 124–129. [Google Scholar] [CrossRef]
- Corominas, H.; Castellví, I.; Pomar, V.; Antonijoan, R.; Mur, I.; Matas, L.; Gich, I.; de Benito, N.; Laiz, A.; Castillo, D. Effectiveness and safety of intravenous tocilizumab to treat COVID-19-associated hyperinflammatory syndrome: Covizumab-6 observational cohort. Clin. Immunol. 2021, 223, 108631. [Google Scholar] [CrossRef]
- Hashimoto, S.; Yoshizaki, K.; Uno, K.; Kitajima, H.; Arai, T.; Tamura, Y.; Morishita, H.; Matsuoka, H.; Han, Y.; Minamoto, S. Prompt reduction in CRP, IL-6, IFN-γ, IP-10, and MCP-1 and a relatively low basal ratio of ferritin/CRP is possibly associated with the efficacy of tocilizumab Monotherapy in severely to critically ill patients with COVID-19. Front. Med. 2021, 8, 734838. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Hassan, A. Dexamethasone for the treatment of coronavirus disease (COVID-19): A review. SN Compr. Clin. Med. 2020, 2, 2637–2646. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.; Mafham, M.; Bell, J.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E. Effect of dexamethasone in hospitalized patients with COVID-19–preliminary report. MedRxiv 2020. [Google Scholar] [CrossRef]
- Albani, F.; Fusina, F.; Granato, E.; Capotosto, C.; Ceracchi, C.; Gargaruti, R.; Santangelo, G.; Schiavone, L.; Taranto, M.S.; Tosati, C. Corticosteroid treatment has no effect on hospital mortality in COVID-19 patients. Sci. Rep. 2021, 11, 1–6. [Google Scholar] [CrossRef]
- Guz, D.; Gafter-Gvili, A.; Lev, N.; Levin, G.S.; Lev, S. Tocilizumab Treatment Effect on Iron Homeostasis in Severe COVID-19 Patients. Acta Haematol. 2022, 145, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Millán, I.; Sattui, S.E.; Lakhanpal, A.; Zisa, D.; Siegel, C.H.; Crow, M.K. Use of anakinra to prevent mechanical ventilation in severe COVID-19: A case series. Arthritis Rheumatol. 2020, 72, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Huet, T.; Cavalli, G.; Gori, A.; Kyprianou, M.; Pickkers, P.; Eugen-Olsen, J.; Clerici, M.; Veas, F.; Chatellier, G. Effect of anakinra on mortality in patients with COVID-19: A systematic review and patient-level meta-analysis. Lancet Rheumatol. 2021, 3, e690–e697. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Mansouritorghabeh, H. D-dimer level in COVID-19 infection: A systematic review. Expert Rev. Hematol. 2020, 13, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Alzoughool, F.; Alanagreh, L.a.; Abumweis, S.; Atoum, M. Cerebrovascular comorbidity, high blood levels of C-reactive protein and D-dimer are associated with disease outcomes in COVID-19 patients. Clin. Hemorheol. Microcirc. 2021, 77, 311–322. [Google Scholar] [CrossRef]
- Takahashi, H.; Iwasaki, Y.; Watanabe, T.; Ichinose, N.; Oda, T. Pulmonary embolism after dexamethasone treatment for COVID-19: A case report. BMC Infect. Dis. 2022, 22, 277. [Google Scholar] [CrossRef] [PubMed]
- Wenban, C.; Heer, R.S.; Baktash, V.; Kandiah, P.; Katsanouli, T.; Pandey, A.; Goindoo, R.; Ajaz, A.; Van den Abbeele, K.; Mandal, A.K. Dexamethasone treatment may mitigate adverse effects of vitamin D deficiency in hospitalized COVID-19 patients. J. Med. Virol. 2021, 93, 6605–6610. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, A.; Sarfraz, Z.; Razzack, A.A.; Patel, G.; Sarfraz, M. Venous thromboembolism, corticosteroids and COVID-19: A systematic review and meta-analysis. Clin. Appl. Thromb./Hemost. 2021, 27, 1076029621993573. [Google Scholar] [CrossRef]
- Lu, C.; Liu, Y.; Chen, B.; Yang, H.; Hu, H.; Liu, Y.; Zhao, Y. Prognostic value of lymphocyte count in severe COVID-19 patients with corticosteroid treatment. Signal Transduct. Target. Ther. 2021, 6, 106. [Google Scholar] [CrossRef]
- Marrone, A.; Nevola, R.; Sellitto, A.; Cozzolino, D.; Romano, C.; Cuomo, G.; Aprea, C.; Schwartzbaum, M.X.P.; Ricozzi, C.; Imbriani, S. Remdesivir plus dexamethasone versus dexamethasone alone for the treatment of COVID-19 patients requiring supplemental O2 therapy: A prospective controlled non-randomized study. Clin. Infect. Dis. 2022, 75, e403–e409. [Google Scholar] [CrossRef]
- Ip, A.; Berry, D.A.; Hansen, E.; Goy, A.H.; Pecora, A.L.; Sinclaire, B.A.; Bednarz, U.; Marafelias, M.; Berry, S.M.; Berry, N.S. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients—An observational study. PLoS ONE 2020, 15, e0237693. [Google Scholar] [CrossRef] [PubMed]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in Patients Hospitalized with COVID-19 Pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X.; Hermine, O.; Tharaux, P.-L.; Resche-Rigon, M.; Porcher, R.; Ravaud, P.; Bureau, S.; Dougados, M.; Tibi, A.; Azoulay, E. Sarilumab in adults hospitalised with moderate-to-severe COVID-19 pneumonia (CORIMUNO-SARI-1): An open-label randomised controlled trial. Lancet Rheumatol. 2022, 4, e24–e32. [Google Scholar] [CrossRef] [PubMed]
- Balkhair, A.; Al-Zakwani, I.; Al Busaidi, M.; Al-Khirbash, A.; Al Mubaihsi, S.; BaTaher, H.; Al Aghbari, J.; Al Busaidi, I.; Al Kindi, M.; Baawain, S. Anakinra in hospitalized patients with severe COVID-19 pneumonia requiring oxygen therapy: Results of a prospective, open-label, interventional study. Int. J. Infect. Dis. 2021, 103, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Naveed, Z.; Sarwar, M.; Ali, Z.; Saeed, D.; Choudhry, K.; Sarfraz, A.; Sarfraz, Z.; Felix, M.; Cherrez-Ojeda, I. Anakinra treatment efficacy in reduction of inflammatory biomarkers in COVID-19 patients: A meta-analysis. J. Clin. Lab. Anal. 2022, 36, e24434. [Google Scholar] [CrossRef]
- Khani, E.; Shahrabi, M.; Rezaei, H.; Pourkarim, F.; Afsharirad, H.; Solduzian, M. Current evidence on the use of anakinra in COVID-19. Int. Immunopharmacol. 2022, 111, 109075. [Google Scholar] [CrossRef]
- Ding, J.; Karp, J.E.; Emadi, A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017, 19, 353–363. [Google Scholar] [CrossRef]
- Robertson, S.J.; Ammann, C.G.; Messer, R.J.; Carmody, A.B.; Myers, L.; Dittmer, U.; Nair, S.; Gerlach, N.; Evans, L.H.; Cafruny, W.A. Suppression of acute anti-friend virus CD8+ T-cell responses by coinfection with lactate dehydrogenase-elevating virus. J. Virol. 2008, 82, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Henry, B.M.; Aggarwal, G.; Wong, J.; Benoit, S.; Vikse, J.; Plebani, M.; Lippi, G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am. J. Emerg. Med. 2020, 38, 1722–1726. [Google Scholar] [CrossRef]
- Levine, S.J.; Wu, T.; Shelhamer, J.H. Extracellular release of the type I intracellular IL-1 receptor antagonist from human airway epithelial cells: Differential effects of IL-4, IL-13, IFN-gamma, and corticosteroids. J. Immunol. (Baltim. Md. 1950) 1997, 158, 5949–5957. [Google Scholar] [CrossRef]
- Perregaux, D.; Barberia, J.; Lanzetti, A.J.; Geoghegan, K.F.; Carty, T.; Gabel, C. IL-1 beta maturation: Evidence that mature cytokine formation can be induced specifically by nigericin. J. Immunol. (Baltim. Md. 1950) 1992, 149, 1294–1303. [Google Scholar] [CrossRef]
- Pontali, E.; Volpi, S.; Signori, A.; Antonucci, G.; Castellaneta, M.; Buzzi, D.; Montale, A.; Bustaffa, M.; Angelelli, A.; Caorsi, R. Efficacy of early anti-inflammatory treatment with high doses of intravenous anakinra with or without glucocorticoids in patients with severe COVID-19 pneumonia. J. Allergy Clin. Immunol. 2021, 147, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Pinzón, M.A.; Ortiz, S.; Holguín, H.; Betancur, J.F.; Cardona Arango, D.; Laniado, H.; Arias Arias, C.; Muñoz, B.; Quiceno, J.; Jaramillo, D. Dexamethasone vs methylprednisolone high dose for COVID-19 pneumonia. PLoS ONE 2021, 16, e0252057. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, F.; Erdogan, M.; Mutlu, M.; Icacan, O.; Onar, M.; Bes, C. Efficacy of anticytokine treatments added to corticosteroids in patients with COVID-19-associated pneumonia and hyperinflammation: A single center experience. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7297–7304. [Google Scholar] [PubMed]
- Sarhan, R.M.; Harb, H.S.; Abou Warda, A.E.; Salem-Bekhit, M.M.; Shakeel, F.; Alzahrani, S.A.; Madney, Y.M.; Boshra, M.S. Efficacy of the early treatment with tocilizumab-hydroxychloroquine and tocilizumab-remdesivir in severe COVID-19 Patients. J. Infect. Public Health 2022, 15, 116–122. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; López-Medrano, F.; Pérez-Jacoiste Asín, M.A.; Maestro de la Calle, G.; Bueno, H.; Caro-Teller, J.M.; Catalan, M.; de la Calle, C.; García-García, R.; Gómez, C. Tocilizumab for the treatment of adult patients with severe COVID-19 pneumonia: A single-center cohort study. J. Med. Virol. 2021, 93, 831–842. [Google Scholar] [CrossRef]
- Zarębska-Michaluk, D.; Jaroszewicz, J.; Rogalska, M.; Martonik, D.; Pabjan, P.; Berkan-Kawińska, A.; Bolewska, B.; Oczko-Grzesik, B.; Kozielewicz, D.; Tudrujek-Zdunek, M. Effectiveness of tocilizumab with and without dexamethasone in patients with severe COVID-19: A retrospective study. J. Inflamm. Res. 2021, 14, 3359. [Google Scholar] [CrossRef]
- Edalatifard, M.; Akhtari, M.; Salehi, M.; Naderi, Z.; Jamshidi, A.; Mostafaei, S.; Najafizadeh, S.R.; Farhadi, E.; Jalili, N.; Esfahani, M. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: Results from a randomised controlled clinical trial. Eur. Respir. J. 2020, 56, 2002808. [Google Scholar] [CrossRef]
- Ajeganova, S.; De Becker, A.; Schots, R. Efficacy of high-dose anakinra in refractory macrophage activation syndrome in adult-onset Still’s disease: When dosage matters in overcoming secondary therapy resistance. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20974858. [Google Scholar] [CrossRef]
- Kumakura, S.; Murakawa, Y. Clinical characteristics and treatment outcomes of autoimmune-associated hemophagocytic syndrome in adults. Arthritis Rheumatol. 2014, 66, 2297–2307. [Google Scholar] [CrossRef] [Green Version]
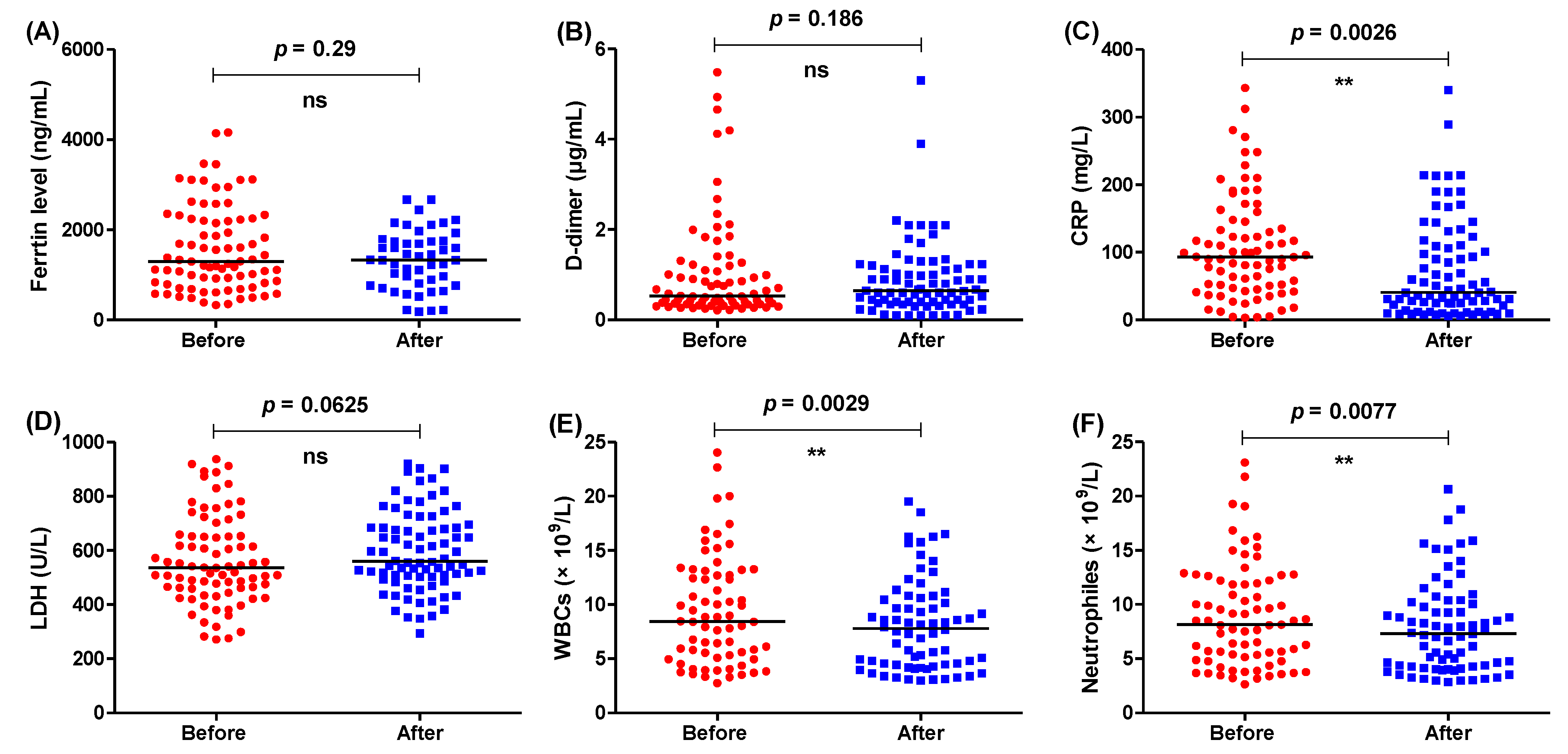
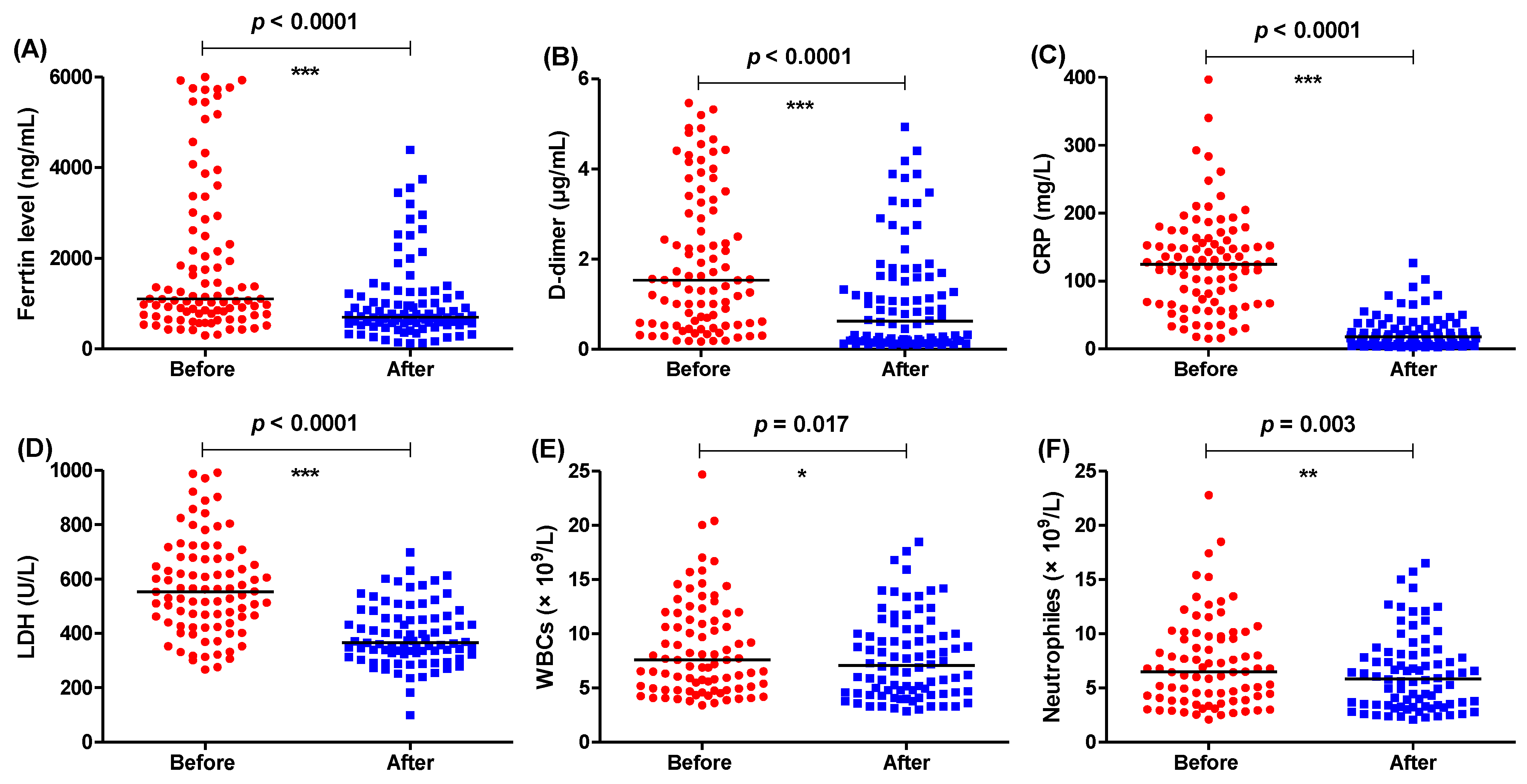
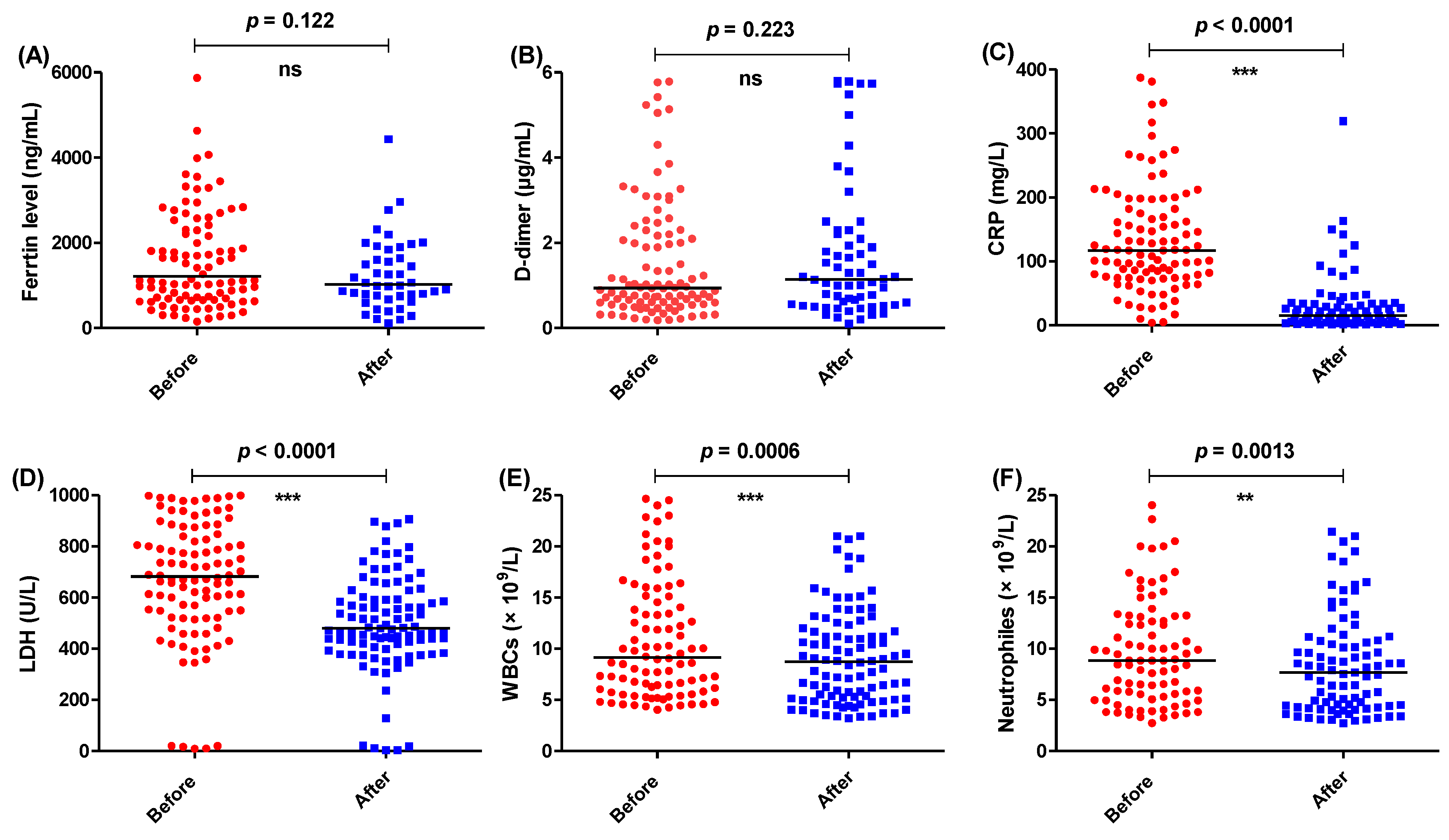


| Usual Treatment Regimen (Control) | Treatment Regimen Including IL-1 Antagonist (IL-1) | Treatment Regimen Including IL-6 Antagonist (IL-6) |
|---|---|---|
|
|
|
| Parameter | Did not Receive IL-Antagonists (Control) | Received IL-1 Antagonist (Anakinra) | Received IL-6 Antagonist (Tocilizumab) |
|---|---|---|---|
| Number | 80 | 95 | 100 |
| Age-median year (range) | 44 (17–67) | 54 (31–81) | 53 (31–85) |
| Gender-no. (%) | |||
| Male | 65 (81.2) | 56 (58.9) | 84 (84) |
| Female | 15 (18.8) | 39 (41.1) | 16 (16) |
| Liver Functions-median U/L (range) | |||
| AST | 37.8 (20–68) | 33.7 (18–65) | 38.1 (23–71) |
| ALT | 39.2 (18–59) | 40 (20–56) | 45.4 (18–60) |
| Kidney Functions- median mg/dL (range) | |||
| Creatinine | 1.31 (0.86–2.1) | 1.35 (0.5–2.2) | 1.24 (0.64–2) |
| Body Mass Index (BMI)-range | 19–38 | 18–44 | 20–35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al. Kharusi, M.; Al Sheikh, N.; Alhajri, M.; Al. Mandhri, S.A.; Khafagy, E.-S.; Moglad, E.H.; Alotaibi, H.F.; Hegazy, W.A.H. A Prospective Cohort Study of COVID-19: Evaluation of the Early Role of IL-1 and IL-6 Antagonists in Improving the Outcome of the Illness and Reduction in the Risk of Death. Healthcare 2023, 11, 1025. https://doi.org/10.3390/healthcare11071025
Al. Kharusi M, Al Sheikh N, Alhajri M, Al. Mandhri SA, Khafagy E-S, Moglad EH, Alotaibi HF, Hegazy WAH. A Prospective Cohort Study of COVID-19: Evaluation of the Early Role of IL-1 and IL-6 Antagonists in Improving the Outcome of the Illness and Reduction in the Risk of Death. Healthcare. 2023; 11(7):1025. https://doi.org/10.3390/healthcare11071025
Chicago/Turabian StyleAl. Kharusi, Mardheya, Naffesa Al Sheikh, Maiya Alhajri, Seif Ali Al. Mandhri, El-Sayed Khafagy, Ehssan H. Moglad, Hadil Faris Alotaibi, and Wael A. H. Hegazy. 2023. "A Prospective Cohort Study of COVID-19: Evaluation of the Early Role of IL-1 and IL-6 Antagonists in Improving the Outcome of the Illness and Reduction in the Risk of Death" Healthcare 11, no. 7: 1025. https://doi.org/10.3390/healthcare11071025








