Bayesian Spatio-Temporal Multilevel Modelling of Patient-Reported Quality of Life following Prostate Cancer Surgery
Abstract
1. Introduction
2. Methods
2.1. Data Source
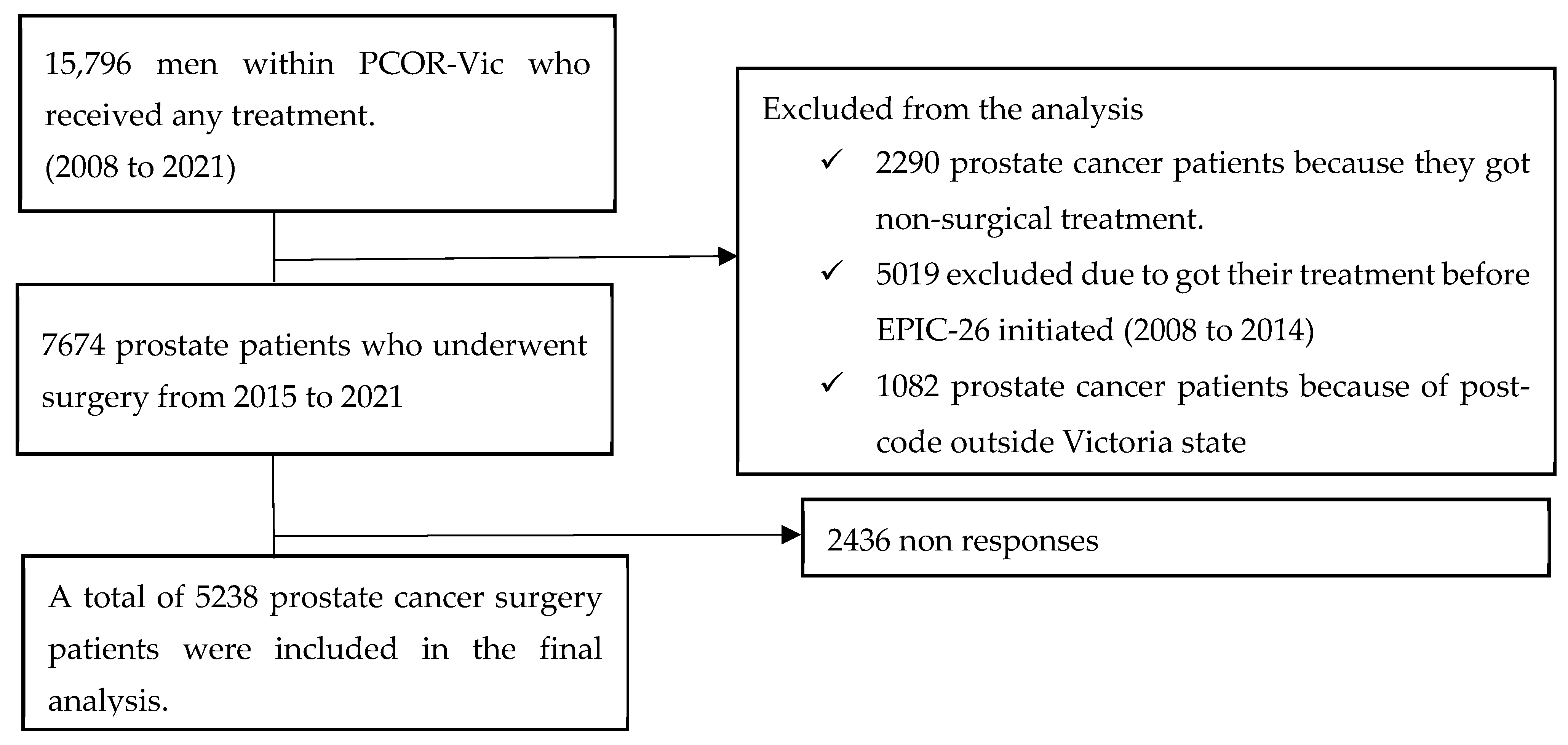
2.2. Inclusion and Exclusion Criteria
2.3. Variables
2.3.1. Outcome Measure (QoL Assessment)
2.3.2. Independent Variables
Patient-Level Variables
Area-Level Variables
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics
3.2. Correlation of QoL with Covariates
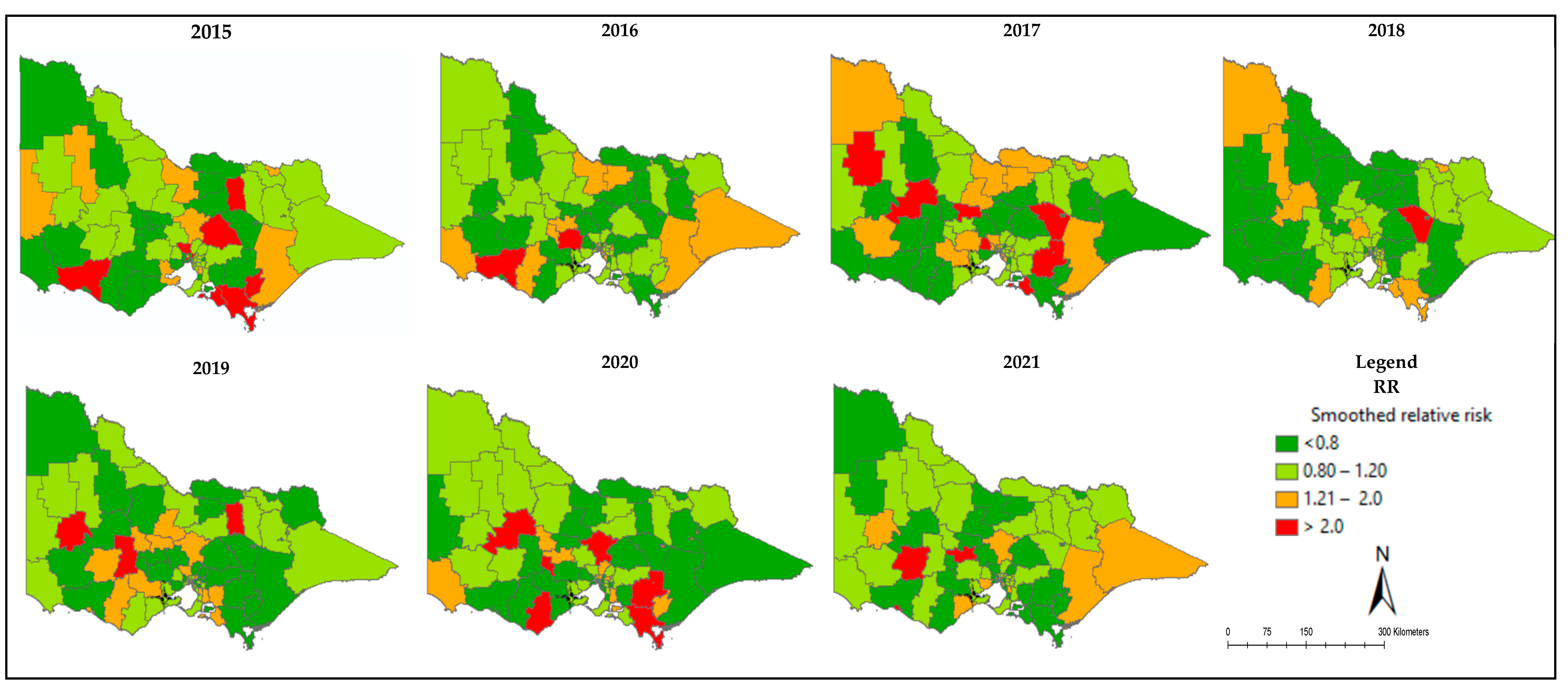
3.3. Mapping of Relative Risk of Poor QoL
3.4. Model Development
3.5. Model Selection and Comparison
3.6. Model Convergence
3.7. Risk Factors of Poor QoL
3.8. Relative Contribution of Spatial Structured and Unstructured Random Effects
3.9. Spatial Weight Matrices
4. Discussion
4.1. Interpretation of the Results
4.2. Strengths and Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOR | Adjusted odds ratio |
| EPIC | Extended Prostate Cancer Index |
| IRSAD | Indexed of Socio-economic Advantage and Disadvantage |
| IUCP | International Society of Urological Pathology |
| LGA | Local government area |
| MCMC | Markov Chain Monte Carlo |
| NCCN | National Comprehensive Cancer Network |
| PCOR | Prostate Cancer Outcome Registry |
| RR | Relative risk |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Feletto, E.; Bang, A.; Cole-Clark, D.; Chalasani, V.; Rasiah, K.; Smith, D. An examination of prostate cancer trends in Australia, England, Canada and USA: Is the Australian death rate too high? World J. Urol. 2015, 33, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.L.; Evans, S.M.; Evans, M.; Tacey, M.; Dodds, L.; Kearns, P.; Milne, R.L.; Foroudi, F.; Millar, J. Trends in conservative management for low-risk prostate cancer in a population-based cohort of Australian men diagnosed between 2009 and 2016. Eur. Urol. Oncol. 2021, 4, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Gwede, C.K.; Pow-Sang, J.; Seigne, J.; Heysek, R.; Helal, M.; Shade, K.; Cantor, A.; Jacobsen, P.B. Treatment decision-making strategies and influences in patients with localized prostate carcinoma. Cancer 2005, 104, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Resnick, M.J.; Koyama, T.; Fan, K.H.; Albertsen, P.C.; Goodman, M.; Hamilton, A.S.; Hoffman, R.M.; Potosky, A.L.; Stanford, J.L.; Stroup, A.M.; et al. Long-term functional outcomes after treatment for localized prostate cancer. N. Engl. J. Med. 2013, 368, 436–445. [Google Scholar] [CrossRef]
- Venderbos, L.D.; Deschamps, A.; Dowling, J.; Carl, E.G.; Remmers, S.; van Poppel, H.; Roobol, M.J. Europa Uomo Patient Reported Outcome Study (EUPROMS): Descriptive statistics of a prostate cancer survey from patients for patients. Eur. Urol. Focus. 2021, 7, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, V.; Lee, T.; McClintock, T.R.; Lepor, H. Short-, intermediate-, and long-term quality of life outcomes following radical prostatectomy for clinically localized prostate cancer. Rev. Urol. 2013, 15, 161. [Google Scholar]
- King, A.J.; Evans, M.T.H.M.; Moore, T.H.M.; Paterson, C.; Sharp, D.; Persad, R.; Huntley, A.L. Prostate cancer and supportive care: A systematic review and qualitative synthesis of men’s experiences and unmet needs. Eur. J. Cancer Care 2015, 24, 618–634. [Google Scholar] [CrossRef]
- Koo, K.; Papa, N.; Evans, M.; Jefford, M.; IJzerman, M.; White, V.; Evans, S.M.; Ristevski, E.; Emery, J.; Millar, J. Mapping disadvantage: Identifying inequities in functional outcomes for prostate cancer survivors based on geography. BMC Cancer 2022, 22, 283. [Google Scholar] [CrossRef]
- Koo, K.; Papa, N.; Evans, M.; Jefford, M.; IJzerman, M.J.; Millar, J.L. Mapping Geographical Disparities in Population-Level Patient-Reported Quality of Life Following Prostate Cancer Management; Wolters Kluwer Health: Philadelphia, PA, USA, 2021. [Google Scholar]
- Obertova, Z.; Brown, C.; Holmes, M.; Lawrenson, R. Prostate cancer incidence and mortality in rural men–a systematic review of the literature. Rural Remote Health 2012, 12, 247–257. [Google Scholar] [CrossRef]
- Schaake, W.; de Groot, M.; Krijnen, W.P.; Langendijk, J.A.; van den Bergh, A.C. Quality of life among prostate cancer patients: A prospective longitudinal population-based study. Radiother. Oncol. 2013, 108, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Dickey, S.L.; Ogunsanya, M.E. Quality of life among black prostate cancer survivors: An integrative review. Am. J. Men’s Health 2018, 12, 1648–1664. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.M.; Millar, J.L.; Wood, J.M.; Davis, I.D.; Bolton, D.; Giles, G.G.; Frydenberg, M.; Frauman, A.; Costello, A.; McNeil, J.J. The Prostate Cancer Registry: Monitoring patterns and quality of care for men diagnosed with prostate cancer. BJU Int. 2013, 111 Pt B, E158–E166. [Google Scholar] [CrossRef]
- Jackson, C.; Best, A.N.; Richardson, S. Hierarchical related regression for combining aggregate and individual data in studies of socio-economic disease risk factors. J. R. Stat. Soc. Ser. A Stat. Soc. 2008, 171, 159–178. [Google Scholar] [CrossRef]
- Jonker, M.F.; Donkers, B.; Chaix, B.; van Lenthe, F.J.; Burdorf, A.; Mackenbach, J.P. Estimating the impact of health-related behaviors on geographic variation in cardiovascular mortality: A new approach based on the synthesis of ecological and individual-level data. Epidemiology 2015, 26, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Wah, W.; Evans, M.; Ahern, S.; Earnest, A. A multi-level spatio-temporal analysis on prostate cancer outcomes. Cancer Epidemiol. 2021, 72, 101939. [Google Scholar] [CrossRef] [PubMed]
- Earnest, A.; Morgan, G.; Mengersen, K.; Ryan, L.; Summerhayes, R.; Beard, J. Evaluating the effect of neighbourhood weight matrices on smoothing properties of Conditional Autoregressive (CAR) models. Int. J. Health Geogr. 2007, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Besag, J. Spatial interaction and the statistical analysis of lattice systems. J. R. Stat. Soc. Ser. B 1974, 36, 192–225. [Google Scholar] [CrossRef]
- Assunção, R.; Krainski, E. Neighborhood dependence in Bayesian spatial models. Biom. J. J. Math. Methods Biosci. 2009, 51, 851–869. [Google Scholar] [CrossRef]
- Morrison, K.T.; Nelson, T.A.; Nathoo, F.S.; Ostry, A.S. Application of Bayesian spatial smoothing models to assess agricultural self-sufficiency. Int. J. Geogr. Inf. Sci. 2012, 26, 1213–1229. [Google Scholar] [CrossRef]
- Duncan, E.W.; White, N.M.; Mengersen, K. Spatial smoothing in Bayesian models: A comparison of weights matrix specifications and their impact on inference. Int. J. Health Geogr. 2017, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Statistics, A.B.O. Census of Population and Housing; Australian Government: Canberra, Australia, 2006.
- Szymanski, K.M.; Wei, J.T.; Dunn, R.L.; Sanda, M.G. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology 2010, 76, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- ABS. Census of population and housing: Socio-Economic Indexes for Areas (SEIFA); Australian Bureau of Statistics Australia: Canberra, Australia, 2011.
- Do, H.; Care, A. Measuring remoteness: Accessibility/Remoteness Index of Australia (ARIA), revised ed.; Queensland Government Statistician’s Office: Brisbane, QLD, Australia, 2001.
- Desktop, E.A. Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2011; pp. 437–438. [Google Scholar]
- Besag, J.; York, J.; Mollié, A. Bayesian image restoration, with two applications in spatial statistics. Ann. Inst. Stat. Math. 1991, 43, 1–20. [Google Scholar] [CrossRef]
- Bernardinelli, L.; Clayton, D.; Pascutto, C.; Montomoli, C.; Ghislandi, M.; Songini, M. Bayesian analysis of space—Time variation in disease risk. Stat. Med. 1995, 14, 2433–2443. [Google Scholar] [CrossRef] [PubMed]
- Goudie, R.J.; Turner, R.M.; De Angelis, D.; Thomas, A. MultiBUGS: A parallel implementation of the BUGS modelling framework for faster Bayesian inference. J. Stat. Softw. 2020, 95, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Best, N.; Richardson, S.; Thomson, A. A comparison of Bayesian spatial models for disease mapping. Stat. Methods Med. Res. 2005, 14, 35–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Yu, T.-J.; Lin, V.C.-H.; Yang, M.-S.; Kao, C.-C. Changes in quality of life among prostate cancer patients after surgery. Cancer Nurs. 2012, 35, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Kurian, C.J.; Leader, A.E.; Thong, M.S.; Keith, S.W.; Zeigler-Johnson, C.M. Examining relationships between age at diagnosis and health-related quality of life outcomes in prostate cancer survivors. BMC Public Health 2018, 18, 1060. [Google Scholar] [CrossRef] [PubMed]
- Popiołek, A.; Brzoszczyk, B.; Jarzemski, P.; Piskunowicz, M.; Jarzemski, M.; Borkowska, A.; Bieliński, M. Quality of life of prostate cancer patients undergoing prostatectomy and affective temperament. Cancer Manag. Res. 2022, 14, 1743–1755. [Google Scholar] [CrossRef]
- Chapman, C.H.; Caram, M.E.; Radhakrishnan, A.; Tsodikov, A.; Deville, C.; Burns, J.; Zaslavsky, A.; Chang, M.; Leppert, J.T.; Hofer, T.; et al. Association between PSA values and surveillance quality after prostate cancer surgery. Cancer Med. 2019, 8, 7903–7912. [Google Scholar] [CrossRef]
- Donnelly, D.W.; Vis, L.C.; Kearney, T.; Sharp, L.; Bennett, D.; Wilding, S.; Downing, A.; Wright, P.; Watson, E.; Wagland, R.; et al. Quality of life among symptomatic compared to PSA-detected prostate cancer survivors-results from a UK wide patient-reported outcomes study. BMC Cancer 2019, 19, 947. [Google Scholar] [CrossRef] [PubMed]
- Stenman, U.-H.; Leinonen, J.; Zhang, W.-M.; Finne, P. (Eds.) Prostate-Specific Antigen. Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Pound, C.R.; Brawer, M.K.; Partin, A.W. Evaluation and treatment of men with biochemical prostate-specific antigen recurrence following definitive therapy for clinically localized prostate cancer. Rev. Urol. 2001, 3, 72. [Google Scholar] [PubMed]
- Krupski, T.L.; Bergman, J.; Kwan, L.; Litwin, M.S. Quality of prostate carcinoma care in a statewide public assistance program. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2005, 104, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Shahjalal, M.; Sultana, M.; Gow, J.; Hoque, M.E.; Mistry, S.K.; Hossain, A.; Mahumud, R.A. Assessing health-related quality of life among cancer survivors during systemic and radiation therapy in Bangladesh: A cancer-specific exploration. BMC Cancer 2023, 23, 1208. [Google Scholar] [CrossRef] [PubMed]
- Mosadeghrad, A.M. Factors affecting medical service quality. Iran. J. Public Health 2014, 43, 210. [Google Scholar] [PubMed]
- Wah, W.; Ahern, S.; Evans, S.; Millar, J.; Evans, M.; Earnest, A. Geospatial and temporal variation of prostate cancer incidence. Public Health 2021, 190, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, H.R.; Wireko, A.A.; Adebusoye, F.T.; Ferreira, T.; Pacheco-Barrios, N.; Abdul-Rahman, T.; Mykolayivna, N.I. Challenges and opportunities in prostate cancer surgery in South America: Insights into robot-assisted radical prostatectomies—A perspective. Health Sci. Rep. 2023, 6, e1519. [Google Scholar] [CrossRef] [PubMed]
- Queenan, J.; Feldman-Stewart, D.; Brundage, M.; Groome, P. Social support and quality of life of prostate cancer patients after radiotherapy treatment. Eur. J. Cancer Care 2010, 19, 251–259. [Google Scholar] [CrossRef]
- Dasgupta, P.; Baade, P.D.; Aitken, J.F.; Ralph, N.; Chambers, S.K.; Dunn, J. Geographical variations in prostate cancer outcomes: A systematic review of international evidence. Front. Oncol. 2019, 9, 238. [Google Scholar] [CrossRef]
- Tessema, Z.T.; Tesema, G.A.; Ahern, S.; Earnest, A. A Systematic Review of Areal Units and Adjacency Used in Bayesian Spatial and Spatio-Temporal Conditional Autoregressive Models in Health Research. Int. J. Environ. Res. Public Health 2023, 20, 6277. [Google Scholar] [CrossRef]
| Variables | Category | n (%) |
|---|---|---|
| Age | Median (IQR) | 66(9) |
| Age group | ≤55 | 590 (11.26%) |
| 56–65 | 2110 (40.28%) | |
| 66–75 | 2376 (45.36%) | |
| 76–85 | 162 (3.09%) | |
| Year of surgery | 2015 | 749 (14.30%) |
| 2016 | 860 (16.42%) | |
| 2017 | 914 (17.45%) | |
| 2018 | 872 (16.60%) | |
| 2019 | 805 (15.37%) | |
| 2020 | 726 (13.86%) | |
| 2021 | 312 (5.96%) | |
| Treating hospital location | Metro | 3696 (70.56%) |
| Regional | 1542 (29.44%) | |
| Treating institution | Public | 1423 (27.21%) |
| Private | 3813 (72.79%) | |
| Gleason score at diagnosis | ≤6 | 481 (9.31%) |
| 7 | 3700 (71.58%) | |
| 8 | 554 (10.72%) | |
| 9 | 424 (8.20%) | |
| 10 | 9 (0.17%) | |
| ISUP * | 1 | 457 (8.72%) |
| 2 | 2483 (47.40%) | |
| 3 | 1151 (21.97%) | |
| 4 | 721 (13.76%) | |
| 5 | 426 (8.13%) | |
| PSA * at diagnosis | ≤10 | 4058 (77.47%) |
| 10.1–20.0 | 719 (13.73%) | |
| >20 | 461 (8.80%) | |
| National Comprehensive Cancer Network (NCCN) risk group | Low risk | 294 (5.61%) |
| Intermediate risk | 3491 (66.65%) | |
| High risk | 1035 (19.76%) | |
| Very high risk/Metastatic | 418 (7.98%) | |
| Clinical T stage | T1 | 2167 (41.37%) |
| T2 | 1279 (24.42%) | |
| T3/T4 | 200 (69.61%) | |
| Unknown | 1592 (30.39%) | |
| Area-level variables | ||
| IRSD * | Quartile 1 | 20 (25.32%) |
| Quartile 2 | 22 (27.85%) | |
| Quartile 3 | 18 (22.78%) | |
| Quartile 4 | 19 (24.05%) | |
| Remoteness | Accessible | 18 (22.78%) |
| Moderately accessible | 7 (8.86%) | |
| Highly accessible | 54 (68.35%) | |
| Variables | QoL | Total | p-Value | |
|---|---|---|---|---|
| Poor Quality of Life | Good Quality of Life | |||
| Quality of life | 1906 (36.39%) | 3332 (63.61%) | 5238 | |
| Age group | ||||
| ≤55 | 138 (23.39%) | 452 (76.61%) | 590 | <0.001 |
| 56–65 | 675 (31.39%) | 1435 (68.01%) | 2110 | |
| 66–75 | 1005 (42.30%) | 1371 (57.70%) | 2376 | |
| 76–85 | 88 (54.32%) | 74 (45.68%) | 162 | |
| Treating hospital location | ||||
| Metro | 1309 (35.42%) | 2378 (64.58%) | 3696 | 0.024 |
| Regional | 597 (38.72%) | 954 (61.28%) | 1542 | |
| Treating institution | ||||
| Public | 626 (43.93%) | 799 (56.07%) | 1425 | <0.001 |
| Private | 1280 (33.57%) | 2533 (66.43%) | 3813 | |
| Gleason score at diagnosis | ||||
| 6 or less | 134 (29.32%) | 323 (70.68%) | 454 | <0.001 |
| 7 | 1236 (34.01%) | 2398 (65.99%) | 3634 | |
| 8 | 246 (45.39%) | 296 (54.61%) | 542 | |
| 9 | 221 (58.87%) | 197 (47.13%) | 418 | |
| 10 | 5 (62.05%) | 3 (37.50%) | 8 | |
| Gleason risk group | ||||
| ISUP1 | 134 (29.32%) | 323 (70.68%) | 457 | <0.001 |
| ISUP2 | 809 (32.58%) | 1674 (67.42%) | 2483 | |
| ISUP3 | 427 (37.10%) | 724 (62.90%) | 1151 | |
| ISUP4/ISUP5 | 536 (46.73%) | 611 (46.73%) | 1147 | |
| PSA * at diagnosis | ||||
| ≤10 | 1384 (34.11%) | 2674 (65.89%) | 4058 | <0.001 |
| 10.1–20.0 | 333 (46.31%) | 386 (53.69%) | 719 | |
| >20 | 189 (41.00%) | 272 (59.00%) | 461 | |
| NCCN * group | ||||
| Low risk | 74 (25.17%) | 220 (74.83%) | 294 | <0.001 |
| Intermediate risk | 1165 (33.37%) | 2326 (66.63%) | 3491 | |
| High risk | 476 (45.99%) | 559 (54.01%) | 1035 | |
| Very high risk/Metastatic | 191 (45.69%) | 227 (54.31%) | 418 | |
| T stage | ||||
| T1 | 700 (32.30%) | 1467 (58.23%) | 2167 | <0.001 |
| T2 | 518 (40.50%) | 761 (59.50%) | 1279 | |
| T3/T4 | 96 (46.50%) | 107 (53.50%) | 200 | |
| Unknown | 595 (37.37%) | 997 (62.63%) | 1592 | |
| Model | WAIC |
|---|---|
| +β1age group | 11,740 |
| + β1age group+ β2nccn | 11,731 |
| + β1age group+ β2nccn+ β3PSA | 11,721 |
| + β1age group+ β2nccn+ β3PSA +β4institution | 11,709 |
| + β1age group+ β2nccn+ β3PSA +β4institution + β5accessibility | 11,658 |
| Full model WAIC | 11,658 |
| Model | WAIC |
|---|---|
| Adjacency-based weight matrix (Queen-1) | 13,110 |
| Distance-based weight matrix | 11,658 |
| Variables | Adjacency-Based Weight Matrix (Queen-1) | Distance-Based Weight Matrix | Estimated % Change from Adjacency-Based Weight Matrices | ||
|---|---|---|---|---|---|
| OR (95% CrI) | AOR (95% CrI) | OR with 95% CrI | AOR 95% CrI | ||
| Age group | |||||
| ≤55 | 1 | 1 | 1 | 1 | |
| 56–65 | 0.70 (0.62, 1.01) | 0.68 (0.59, 1.13) | 1.09 (0.90, 1.34) | 1.08 (0.89, 1.33) | 1.1 |
| 66–75 | 1.12 (1.01, 1.26) * | 1.10 (1.07, 1.12) * | 1.71 (1.40, 2.09) * | 1.70 (1.39, 2.08) * | 4.5 |
| 76–85 | 2.06 (1.48, 2.88) * | 2.04 (1.46, 2.86) * | 2.91 (2.01, 4.26) * | 2.90 (2.00, 4.25) * | 0.49 |
| National Comprehensive Cancer Network (NCCN) Risk Group | |||||
| Low risk | 1 | 1 | 1 | 1 | |
| Intermediate risk | 0.64 (0.55, 1.01) | 0.62 (0.53, 1.01) | 0.69 (0.47, 1.02) | 0.68 (0.46, 1.02) | 0.19 |
| High risk | 1.07 (0.90, 1.27) | 1.05 (0.88, 1.25) | 0.99 (0.63, 1.58) | 0.97 (0.61, 1.57) | 1.62 |
| Very high risk/Metastatic | 1.20 (1.02, 1.54) * | 1.18 (1.00, 1.52) * | 1.23 (1.01, 2.07) * | 1.22 (0.72, 2.06) | 0.20 |
| Gleason Risk Group | |||||
| ISUP1 | 1 | 1 | |||
| ISUP2 | 0.94 (0.68, 1.30) | 0.98 (0.71, 1.34) | |||
| ISUP3 | 1.03 (0.73, 1.44) | 1.06 (0.76, 1.47) | |||
| ISUP4/ISUP5 | 1.00 (0.65, 1.55) | 1.07 (0.71, 1.56) | |||
| Clinical T Stage | |||||
| T1 | 1 | 1 | |||
| T2 | 0.84 (0.69, 1.00) | 1.13 (0.99, 1.32) | |||
| T3/T4 | 1.24 (0.90, 1.69) | 1.05 (0.75, 1.47) | |||
| Unknown | 1.01 (0.87, 1.18) | 1.06 (0.91, 1.22) | |||
| Prostate-Specific Antigen (PSA) | |||||
| ≤10 | 1 | 1 | 1 | 1 | |
| 10.1–20.0 | 1.07 (1.02, 1.45) * | 1.05 (1.01, 1.43) * | 1.34 (1.13, 1.59) * | 1.33 (1.12, 1.58) * | 4.8 |
| >20 | 1.01 (0.69, 1.51) | 0.99 (0.67, 1.49) | 0.88 (0.67, 1.15) | 0.87 (0.65, 1.13) | 1.28 |
| Treating Institution | |||||
| Private | 1 | 1 | 1 | 1 | |
| Public | 1.35 (1.18, 1.57) * | 1.33 (1.16, 1.55) * | 1.36 (1.18, 1.56) * | 1.35 (1.17, 1.53) * | 0.05 |
| Treating Hospital Location | |||||
| Regional | 1 | 1 | |||
| Metro | 0.81 (0.58, 1.01) | 0.92 (0.75, 1.10) | |||
| Index of Relative Socio-economic Disadvantage (IRSD) | |||||
| 1st quartile | 1 | 1 | |||
| 2nd quartile | 0.35 (0.10, 1.12) | 1.16 (0.81, 1.66) | |||
| 3rd quartile | 0.47 (0.12, 1.63) | 1.06 (0.55, 1.76) | |||
| 4th quartile | 0.93 (0.28, 2.93) | 1.08 (0.51, 3.45) | |||
| Accessibility/Remoteness Index of Areas Plus (ARIA+) | |||||
| Accessible | 1 | 1 | 1 | 1 | |
| Moderately accessible | 0.64 (0.41, 1.13) | 0.62 (0.39, 1.11) | 0.54 (0.35, 0.97) * | 0.53 (0.34, 1.01) | 0.32 |
| Highly accessible | 0.49 (0.38, 0.67) * | 0.47 (0.36, 0.65) * | 0.61 (0.39, 0.96) * | 0.60 (0.38, 0.94) * | 0.32 |
| Parameters | Posterior Mean with 95% Credible Interval | |
|---|---|---|
| Distance-Based Weight Matrices | Adjacency-Based Weight Matrices | |
| var(u) | 0.807 | 0.701 |
| var(v) | 0.223 | 0.213 |
| ∅ | 0.78 | 0.76 |
| Neighbourhood Type | Mean | Median | Min | Max | SD |
|---|---|---|---|---|---|
| Adjacency-based weight matrix (Queen-I) | 5.11 | 5 | 1 | 9 | 2 |
| Distance-based weight matrix | 30 | 40 | 1 | 48 | 21.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessema, Z.T.; Tesema, G.A.; Wah, W.; Ahern, S.; Papa, N.; Millar, J.L.; Earnest, A. Bayesian Spatio-Temporal Multilevel Modelling of Patient-Reported Quality of Life following Prostate Cancer Surgery. Healthcare 2024, 12, 1093. https://doi.org/10.3390/healthcare12111093
Tessema ZT, Tesema GA, Wah W, Ahern S, Papa N, Millar JL, Earnest A. Bayesian Spatio-Temporal Multilevel Modelling of Patient-Reported Quality of Life following Prostate Cancer Surgery. Healthcare. 2024; 12(11):1093. https://doi.org/10.3390/healthcare12111093
Chicago/Turabian StyleTessema, Zemenu Tadesse, Getayeneh Antehunegn Tesema, Win Wah, Susannah Ahern, Nathan Papa, Jeremy Laurence Millar, and Arul Earnest. 2024. "Bayesian Spatio-Temporal Multilevel Modelling of Patient-Reported Quality of Life following Prostate Cancer Surgery" Healthcare 12, no. 11: 1093. https://doi.org/10.3390/healthcare12111093
APA StyleTessema, Z. T., Tesema, G. A., Wah, W., Ahern, S., Papa, N., Millar, J. L., & Earnest, A. (2024). Bayesian Spatio-Temporal Multilevel Modelling of Patient-Reported Quality of Life following Prostate Cancer Surgery. Healthcare, 12(11), 1093. https://doi.org/10.3390/healthcare12111093







