Abstract
Opioid-free anesthesia (OFA) is a heterogeneous group of general anesthesia techniques in which the intraoperative use of opioids is eliminated. This strategy aims to decrease the risk of complications and improve the patient’s safety and comfort. Such potential advantages are particularly beneficial for selected groups of patients, among them obese patients undergoing laparoscopic bariatric surgery. Opioids have been traditionally used as an element of balanced anesthesia, and replacing them requires using a combination of coanalgesics and various types of local and regional anesthesia, which also have their side effects, limitations, and potential disadvantages. Moreover, despite the growing amount of evidence, the empirical data on the superiority of OFA compared to standard anesthesia with multimodal analgesia are contradictory, and potential benefits in many studies are being questioned. Additionally, little is known about the long-term sequelae of such a strategy. Considering the above-mentioned issues, this study aims to present the potential benefits, risks, and difficulties of implementing OFA in bariatric surgery, considering the current state of knowledge and literature.
1. Introduction
Currently, approximately 2.5 billion adults in the world are overweight, of which 890 million are obese, and this number is constantly increasing [1]. Approximately 600,000 patients undergo bariatric surgery annually; a much larger group has indications for it [2]. Markedly, it has been reported that almost 75% of patients undergoing laparoscopic bariatric surgery may experience moderate and severe pain [3]. The most potent group of pharmacological substances used to alleviate it and to surpass the nociception intraoperatively during general anesthesia are opioids, and it is estimated they are administered to approximately 99% of patients in the USA perioperatively [4,5]. Their mechanism of action affects the modulation of pain impulses, mainly in the central nervous system, making them highly effective. Unfortunately, despite their high analgesic potency, opioid use is connected with the risk of adverse reactions in the postoperative period, such as oversedation, respiratory depression, postoperative nausea and vomiting (PONV), as well as opioid hyperalgesia. These side effects attributed to opioids complicate the postoperative period, increase costs, and prolong the length of hospital stays [6].
Considering the specificity of patients with obesity undergoing bariatric surgery and their increased risk of complications in the intra- and postoperative period, new methods of anesthesia and analgesia are constantly being searched for, which would allow for greater safety and comfort. One of the techniques used for this purpose is opioid-free anesthesia (OFA), but despite growing evidence, its use remains controversial, and its benefits are questioned.
2. Purpose
This article aims to critically evaluate published material on opioid-free anesthesia in bariatric surgery, providing an integrated and synthesized overview of the current state of knowledge. In our review, we plan to assess whether the opioid-free anesthesia technique has an advantage over anesthesia with multimodal analgesia, including opioid use, which is currently standard in bariatric surgery.
3. Material and Methods
The literature search was conducted using the PubMed, Web of Science, and SCOPUS databases using the keywords “opioid free anesthesia”, “opioid free analgesia” and “bariatric surgery anesthesia” in the time period from inception to April 2024. A manual search of the reference lists of the selected publications was also performed to identify additional studies for potential inclusion. The initial data search yielded 1318 papers from PubMed, 2847 from Web of Science, and 2457 from SCOPUS. A total of 6622 articles were identified, and after removing duplicates using Rayyan (Johnson & Phillips, 2018, Newport, UK), the remaining 2040 were screened for eligibility. We have included randomized controlled trials referring to bariatric surgery published in English. Through a title and abstract review, 54 studies were further examined, of which 13 studies were retrieved. We excluded 2 studies as they were referring to open bariatric surgery, i.e., laparotomy, which is currently obsolete. The selection process is depicted in Figure 1. After a full text article assessment, 11 relevant randomized controlled trials were included in this review. The relevant studies are depicted in Table 1.
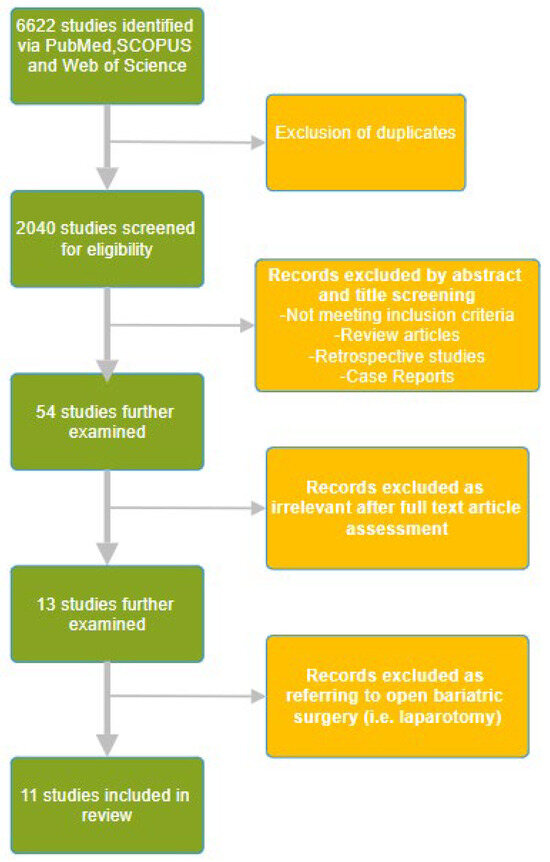
Figure 1.
The randomized controlled trials selection flow diagram.

Table 1.
OFA randomized controlled trials included in the review. LSG—laparoscopic sleeve gastrectomy, LGB—laparoscopic gastric bypass, SADI-S—single anastomosis dueodeno-ileal bypass.
4. Results
4.1. Opioid-Free Anesthesia—Definition and Assumptions
According to most definitions, OFA is a heterogeneous group of techniques that include general anesthesia without systemic or regional opioid administration [18]. By an alternative definition, OFA involves the use of various methods to eliminate opioids and avoid their side effects without negatively affecting the patient’s comfort [19].
Such methods include regional anesthesia (RA) techniques. In the setting of modern laparoscopic bariatric surgery, RA can be used as an element of anesthesia with multimodal analgesia or OFA. The simplest use of RA is the infiltration of the surgical site with local anesthetics [20]. Moreover, interfascial plane blocks, such as Transversus Abdominis Plane (TAP) Block [8,21], Erector Spinae Plane (ESP) Block [22], or the intraperitoneal administration of local anesthetics [23], including a promising blockade of the autonomic innervation of the stomach [24] when used as a part of multimodal analgesia, are also effective in perioperative opioid-sparing. Referring to neuraxial blockades, although epidural and even combined thoracic spinal-epidural anesthesia have been successfully implemented for laparoscopic bariatric procedures [25,26,27], they are deemed to be too invasive and prevent early mobilization, which has a priority in ERAS strategy [28]. On that basis, there is a consensus that this form of RA can be considered nowadays only in rare cases of open bariatric surgery, but not laparoscopic [28]. Additionally, non-opioid analgesics and coanalgesics (Table 2), including drugs to prevent hyperalgesia and non-pharmacological agents, are also used in OFA.

Table 2.
Most frequently used coanalgesics and simple analgesics. NSAID—non-steroidal anti-inflammatory drug.
For practical reasons, a clear distinction should be made between anesthesia without intraoperative opioids and opioid-free postoperative analgesia. Articles reporting the complete elimination of opioids both during and after bariatric surgery [29,30] are based on single case reports and not on routine, reliable practice. In the OFA concept, it is crucial to distinguish between pain and nociception. Pain, according to the definition of IASP (International Association for the Study of Pain), is an unpleasant sensory and emotional experience that assumes a state of consciousness [31]. Nociception, conversely, refers to a stimulus’s reception and excitation transmission in the nervous system [32]. During general anesthesia, the patient’s pain perception is disabled, and nociception is based on the response of the sympathetic nervous system, which is mainly the easiest to assess the parameters of the circulatory system, such as heart rate (HR) or blood pressure (BP) [33]. OFA assumes the suppression of the sympathetic nervous system in response to a pain stimulus and the modulation of nociception through the use of methods other than the administration of opioids [34]. Such methods include the use of drugs from the alpha 2 agonists group, lidocaine, ketamine, magnesium sulfate, beta-blockers, or gabapentinoids (Table 2) [35]. Despite its potential benefits, especially for obese patients undergoing bariatric surgery, OFA is controversial and is not currently recommended as a standard treatment. This is due to the limited scientific evidence and frequent reliance on expert opinion [36,37]. This article aims to present the potential benefits, risks, and difficulties associated with this technique.
4.2. Potential Benefits of OFA in Bariatric Surgery
Opioid side effects make it difficult to mobilize the patient early, which is a priority in modern bariatric surgery, in which laparoscopic sleeve gastrectomy (LSG) and Roux-en-Y gastric bypass (LGB) are the most frequently performed operations [38]. The use of a comprehensive perioperative care protocol to improve the outcomes of surgical treatment (ERAS, Enhanced Recovery After Surgery), including the reduction or elimination of opioid therapy, is of fundamental importance for patient safety and comfort [28]. Based on its assumptions, the OFA technique should reduce the incidence of respiratory complications, excessive sedation, and postoperative nausea and vomiting (PONV). It should also ensure comparable or better pain control and avoid the risk of opioid-induced hyperalgesia (OIH) [19]. On the other hand, there are concerns about the stability of the circulatory system in patients anesthetized with this technique, as well as whether OFA actually blocks pain conduction or only the stimulation of the sympathetic nervous system in response to a stimulus, and what the long-term consequences of such technique may be [36].
4.3. Respiratory Complications and Oversedation
The avoidance of opioid-induced respiratory depression (OIRD) and oversedation in the postoperative period, and thus an improvement in patient safety, are the main reasons for the interest in OFA in bariatric anesthesia [39,40,41]. The increased susceptibility to respiratory complications in obese patients involves numerous factors such as reduced functional residual capacity (FRC), atelectasis, air leak, co-occurrence of obstructive sleep apnea (OSA), obese hypoventilation syndrome (OHS) and pulmonary hypertension [42,43,44]. An increased risk of respiratory complications is common after the administration of opioids and may be clinically significant even without clear signs of overdose [45,46]. In a meta-analysis devoted to the factors of OIRD, one of the conclusions is the possibility of reducing the risk by using opioid-sparing techniques [45]. In line with this recommendation, the Enhanced Recovery After Bariatric Surgery (ERABS) guidelines suggest the standard use of multimodal analgesia involving coanalgesics, regional anesthesia techniques, and non-opioid analgesics [28]. Despite the potentially improved safety due to the complete elimination of opioids, the amount of evidence for the greater safety of OFA in bariatric surgery compared to general anesthesia with multimodal analgesia is minimal. Such evidence is provided by the study of Mulier et al., in which the rate of desaturation < 94% in the postoperative period in patients in the opioid group was 50%, while in only 2 of 23 in the OFA group [9]. However, the issue remains controversial as other trials do not confirm such an effect [13,14,17]. These discrepancies may be explained by the sedative effect of some drugs used in OFA, especially dexmedetomidine, which may have an ambiguous impact on convalescence and the possibility of early mobilization after surgery, presumably dose-dependent. This effect is caused by a presynaptic effect on alpha 2 receptors in the locus coeruleus [47]. As far as the impact of OFA on recovery is concerned, the trial’s results are inconsistent. In a study dedicated to bariatric surgery, dexmedetomidine was associated with meeting the discharge criteria faster in the recovery room; however, it was not associated with a reduction in the length of the hospital stay [48]. Similar results were obtained by Ulbing et al., in whose study patients in the OFA group achieved a statistically higher result in the subjective assessment of recovery using the QoR-40 form 24 and 48 h after surgery, but this was not associated with a shortened hospital stay [15]. On the contrary, in the trial by Clanet et al., no statistically significant difference between the QoR-40 questionnaire results was obtained 24 and 30 days after the surgery [13]. Furthermore, recovery after surgery can also be affected by hallucinations, especially as ketamine is frequently used in OFA protocols. In one study, they were reported in up to 7% of patients; despite this side effect, the satisfaction level of patients during the perioperative period was not affected [12].
4.4. Postoperative Nausea and Vomiting
PONV has a multifactorial etiology and significantly contributes to the diminished comfort of patients undergoing laparoscopic bariatric surgery [9] as well as being the most frequent cause of the readmission of patients after bariatric surgery [49]. PONV may also hinder the patient’s rapid mobilization and pose a risk of increased blood pressure, wound dehiscence, or bleeding in the perioperative period [50]. Opioid use is one of the few modifiable factors of PONV, especially as its incidence is dose-dependent [51]. There is clear evidence of a reduction in the prevalence of this complication in patients undergoing laparoscopic bariatric surgery, demonstrated in prospective trials [7,9,11,13,14,15] and in a meta-analysis dedicated to this group of patients in comparison to the anesthesia with multimodal analgesia group [52]. Still, there are discrepancies regarding the duration of the beneficial effect. In a prospective, randomized study by Ziemann-Gimmel et al. [11], researchers demonstrated that Total Intravenous Anesthesia (TIVA) OFA allows for a more significant reduction of the risk of PONV than triple antiemetic prophylaxis and the reference point was the frequency and severity of PONV 24 h after surgery [11]. A similar beneficial effect was maintained in the study by Mulier et al. [9], but in other trials, this effect was shown only in the immediate hours after surgery [13,14]. In the latter trial, in the OFA group, significantly fewer patients required antiemetics, but lower PONV incidence persisted until the 4th postoperative hour. In other studies, however, the observed differences did not reach statistical significance [10,17].
4.5. Pain Control and Reduction of Postoperative Opioid Consumption
Publications supporting multimodal analgesia in patients undergoing bariatric surgery, as included in the ERAS Society guidelines [28], demonstrate a reduction in the doses of opioids required for pain treatment owing to the use of coanalgesics from the alpha 2 agonists group [53,54,55], lidocaine [56], magnesium sulfate [57] or ketamine [58]. However, there is limited research comparing OFA and anesthesia with multimodal analgesia, including opioid use, for postoperative pain management and opioid dosage, and existing results are inconclusive. In a meta-analysis dedicated to bariatric surgery by Hung et al., a statistically significant reduction in the NRS score was demonstrated in OFA group patients 24 h after surgery; however, considering that this difference did not exceed 1 point on the NRS scale, its clinical significance is questionable [59]. The same study did not reveal a reduction in the total dose of opioids administered postoperatively, only in the initial period in the recovery room [59]. A study by Menck et al. demonstrated no statistically significant differences in both pain scores and opioid requirements at any given time point [17]. Mulier’s 2018 prospective, randomized trial stands out as one of the most notable trials highlighting OFA’s benefits. The study involved 50 patients who were assigned to either the OFA group or the opioid administration group. The OFA group required significantly less opioids in the recovery room, 4.9 to 15.3 mg of morphine (p = 0.04), and had a lower VAS score of 1.7 to 4.9 (p = 0.01) [9]. The study presented some limitations in terms of incomplete information regarding the duration of patients’ stay in the PACU before their transfer to the ward, where there was no significant difference in opioid consumption, and the OFA group presented with only a slightly better VAS score of 2.0 compared to 3.3 in the opioid group (p = 0.016) [9]. Similar results were obtained by Ahmed et al. in their randomized controlled trial, in which lower pain scores were noted 4 and 6 h after the surgery, whereas the total morphine consumption was statistically but not clinically lower: 5.8 in the OFA group vs. 7.2 mg in the opioid group (p = 0.003). [7]. In line with Mulier’s trial, a reduction in the dose of opioids only in the initial postoperative period was also demonstrated by Mieszczański et al. [14]. A possible explanation for this difference may be the fact that drugs used in OFA have a half-life of several hours, and their effects subside shortly after the cessation of their infusion [56,60]. One of the few studies with the continuation of the coanalgesics in the postoperative period is the work of Ulbing et al., which demonstrated a lower opioid consumption and a lower VAS pain score in the OFA group [15]. Maintaining the infusion of these drugs may be critical to achieving the clinical significance of the benefits of OFA in bariatric procedures. This has also been demonstrated in a case report of a patient undergoing LSG as a bridge to eligibility for lung transplantation for interstitial disease [61]. In that case, the infusion of coanalgesics was maintained for 24 h after surgery, which resulted in low total opioid consumption and acceptable NRS scores. In conclusion, there is still a lack of unequivocal evidence that OFA is associated with comparable or better-quality pain management. While the beneficial effects of OFA can be prolonged with the continued administration of coanalgesics in the postoperative period, the assessment of the significance of the clinical benefit of this approach requires further research.
4.6. Tolerance, Opioid-Induced Hyperalgesia (OIH), and Long-Term Sequelae
Intraoperative administration of opioids may spark the development of their acute tolerance, which is associated with an increase in total opioid requirements and increases the frequency of side effects [62,63]. Another coexisting problem is the occurrence of OIH, which entails a lower pain threshold and allodynia [64]. This phenomenon has a separate pathophysiology, but the clinical manifestations are similar and, in practice, difficult to differentiate [64]. OIH and the acute development of tolerance are crucial in bariatric surgery, as frequently used remifentanil has the greatest potential in this respect [65], especially at a high dose [66]. This may have an impact on postoperative pain management [55], and the relationship appears to be dose-dependent [65]. When it comes to proven effects in the prevention and treatment of OIH and the development of tolerance to opioids, ketamine or magnesium sulfate (NMDA receptor antagonists), as well as alpha 2 agonists, often included in OFA protocols, are used [67,68,69,70]. OFA has a beneficial effect on reducing the doses of opioids used and, therefore, on the prevention of OIH and the development of tolerance to opioids. This is of significant importance, as there exist publications, including a meta-analysis, that indicate the lack of any discernible benefits associated with the administration of opioids prior to the pain stimulus [71]. Another question is the impact of OFA on the incidence of Persistent Postoperative Pain, which is estimated to occur in up to 30% of patients [72], with the incidence ranging in the literature from 5 to 54.4% [73,74]. Considering the potential detrimental effects associated with the use of opioids, the implementation of OFA may serve as a viable solution for mitigating such risks. Additional research is necessary considering the limited availability of only one small study that does not indicate significant differences in chronic pain incidence or intensity among patients who underwent hysterectomy over a six-month duration [75].
4.7. Hemodynamic Stability
Laparoscopic bariatric surgery is associated with the risk of hemodynamic instability, which is caused by the insufflation of the peritoneal cavity with carbon dioxide and an increase in intra-abdominal pressure, positioning a patient in a steep anti-Trendelenburg position, or from co-occurring cardiovascular diseases in obese people [76]. In this respect, there are concerns about the use of OFA in this group of patients, which is due to the depressive effect of the drugs used on the circulatory system. Dexmedetomidine, the most commonly used alpha 2 agonist, has a parasympathomimetic effect on the cardiac conduction system and inhibits the sympathetic component of the cardiac plexus, resulting in bradycardia and even sinoatrial block. Moreover, this drug causes the relaxation of the vascular tonus and a decrease in systemic vascular resistance (SVR), leading to a decrease in BP (Blood Pressure). On the other hand, with rapid administration, paradoxical vasoconstriction with an increase in BP may occur due to the non-specific stimulation of alpha 1 receptors [60]. With regard to these dose-related adverse effects of dexmedetomidine, the large multicenter POFA study, which compared OFA anesthesia with dexmedetomidine to anesthesia with remifentanil, demonstrated adverse effects of the first technique, such as hemodynamic instability in the form of bradycardia and in one case, asystole, as well as a more frequent occurrence of hypoxia and greater sedation of patients in the postoperative period [77]. The study was interrupted for safety reasons. The article was criticized because the use of high doses of dexmedetomidine (an average of 1.2 mcg/kg/h) with a long anesthesia time (an average of 268 min) raises doubts, and what is more, the limitation is the high heterogeneity of the anesthetic procedures, of which bariatric surgeries consist only a small portion. Nevertheless, POFA is a significant prospective study that questions the safety and soundness of the OFA technique [61]. Lidocaine also has a hypotensive effect through its negative inotropic effect on cardiomyocytes, similar to magnesium sulfate, an NMDA receptor antagonist, or an antagonist of beta 1 receptors, such as esmolol [78,79,80]. Lidocaine at a dose of 1.5 mg/kg can even be used for controlled hypotension during general anesthesia [78]. The effect of ketamine is ambiguous, although increasing the tension of the sympathetic nervous system causes an increase in HR, BP, and SV (Stroke Volume) while maintaining SVR. Still, in some patients, it may have a negative inotropic effect, causing hypotension and bradycardia [81]. Taking into account the effects mentioned above, hypotension when using OFA may be a problem and require the use of sympathomimetics such as ephedrine or catecholamines more frequently and in higher doses than in conventional anesthesia with multimodal analgesia, as well as more aggressive fluid therapy [14]. This may pose a risk, especially for patients with ischemic heart disease, hypovolemia, or orthostatic hypotension, and OFA is relatively contraindicated in this group [33]. Another problem is the intraoperative, low controllability of some drugs used in OFA, including alpha 2 agonists and lidocaine, the effects of which may be prolonged due to their half-life [60,82]. In the case of hypotension and bradycardia, even after the discontinuation of the administration of these drugs, the disappearance of their effects will be delayed. On the other hand, the data from the clinical trials are inconclusive and contradictory, as in another recent trial, the differences between OFA and remifentanil groups in terms of hemodynamic stability have not reached statistical significance [13], and patients in the opioid group received more fluids than anesthetized without opioids. Similar results, with no significant differences in hemodynamic parameters between OFA and opioid groups, were obtained in a study by Mansour et al. In this particular study, however, ketamine was the only coanalgesic utilized, avoiding the potential hypotension associated with lidocaine, alpha 2 agonists, or magnesium sulfate use [12]. Therefore, further studies are required on the impact of OFA on hemodynamic stability, and the observed differences may result from the heterogeneity of utilized OFA protocols, especially in proportions of particular coanalgesics used.
4.8. Intraoperative Nociception and Monitoring
Repeated nociceptive stimulation reaching higher levels of the nervous system causes central sensitization, defined by IASP as increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold input [31]. This process contributes to the development of acute and persistent postoperative pain [34,83], which, inadequately treated, apart from numerous other unfavorable effects, is one of the main factors in its chronification. The adopted intraoperative opioid dosage is, in most cases, based on the features of sympathetic nervous system stimulation and is considered an indicator of nociception, i.e., based on the assessment of hemodynamic parameters [35]. Drugs used in OFA are weak analgesics (alpha 2 agonists, ketamine, lidocaine) or have no direct analgesic effect. Therefore, it is unclear whether these drugs provide hemodynamic stability by effectively attenuating nociception or simply blocking the effector, namely the sympathetic nervous system, and what the consequences of this might be. Monitoring intraoperative nociception is challenging and includes the assessment of the vegetative system based on heart rate variation (HRV), the Analgesia Nociception Index (ANI), the NoL Index [84] or the High-Frequency Variability Index (HFVI) [85] as well as the measurement of pupil width [86], and indirectly by determining stress hormones before and after surgery (e.g., cortisol) [9]. Parameters derived from the EEG recording (e.g., bispectral index BIS, entropy) are not suitable for strictly assessing nociception, and their assessment after ketamine administration is unreliable [87]. The amount of research on obese patients anesthetized using the OFA technique is scarce. The literature describes two case reports of such patients with class III obesity and nociception monitoring by ANI assessment [29,88]. In both articles, this technique allowed for maintaining the ANI in the desired range of 50–70, which allows for reasonable control of nociception and minimizing the risk of the patient feeling pain after waking up [29,88]. However, this method, like other techniques based on HRV assessment, is subject to significant limitations, including the use of atropine, sympathomimetics, or other drugs affecting HR used in OFA [89,90]. In the previously cited work, Mulier demonstrated lower cortisol concentration in patients anesthetized for laparoscopic bariatric surgery in the OFA group compared to anesthesia with sufentanil administration. The cortisol concentration was measured before the induction of anesthesia and then in the postoperative department; the increase was statistically significantly lower in the OFA group, which may indirectly indicate lower perioperative stress in this group of subjects [9]. Moreover, a beneficial effect of OFA on reducing immunologically mediated stress response was demonstrated in a study by Campos-Perez et al. [16], in which patients in the OFA group undergoing LGB had lower interleukin 6 (IL6) serum concentrations postoperatively 13 pg/mL (5.43–22) vs. 49.58 pg/mL (18.50–112.20), respectively, p = 0.019. IL-6 is considered to be one of the most important pro-inflammatory interleukins and biomarkers of inflammation and immune activation. On the other hand, no differences in other primary outcomes, TNF-α and IL-1β serum concentration, were detected. In this study, no clinical or statistical differences in parameters such as PONV incidence or NRS scale result were observed. Due to observation time being limited to 24 h after the surgery, no conclusions on the clinical significance of IL-6 reduction sequelae can be made [16].
Considering the limited data on blocking nociception and stress response using the OFA technique and the long-term consequences of this method of anesthesia, more research is warranted in this area.
5. Discussion
The literature proves that OFA is feasible and can be successfully implemented as a strategy for bariatric surgery [19]. As far as certainties are concerned, there is unequivocal evidence that OFA decreases the incidence and severity of PONV compared not only to opioid-liberal anesthesia but also to opioid-sparing [11,52]. In other fields, in which OFA is expected to be superior, there is conflicting or scarce evidence, or the evidence is not allowed to be adapted as a standard, and this evidence refers to improving postoperative pain management and decreasing the postoperative opioid requirements [7,9,10,12,13,14,15,17], the incidence of opioid-induced respiratory depression or oversedation [9,13,14,17], maintaining hemodynamical stability [12,13,14] or finally, improving the recovery [8,9,10,13,15] and long-term outcomes in terms of postoperative chronic pain incidence. One of the main factors contributing to this fact is the vast heterogeneity of OFA protocols and coanalgesics used, and even the adapted dosing regimens—ideal body weight, lean body weight, or adjusted body weight—as well as if and what type of RA techniques were used.
In our experience, OFA is associated with more interventions of the anesthetist intraoperatively, may pose a risk of hemodynamic instability, and does not shorten the length of hospital stays as compared with anesthesia with multimodal analgesia [14]. In our center, we use it in patients with the paramount risk of respiratory complications, for example, on chronic oxygen treatment [61,91] or with super-obesity (BMI > 50) in an individual risk assessment, and also in cases of severe PONV history. In such cases, to maximize the potential benefits, we maintain coanalgesic administration throughout up to 12–16 h after the surgery.
Future research concerning OFA should compare it with multimodal, low-opioid strategies (not just “opioid-based” anesthesia), assess the optimal dosing regimen and if prolonging coanalgesic administration postoperatively would improve the results, and finally, the long-term impact on the recovery and the postoperative pain chronification.
6. Conclusions
The elimination of opioids during anesthesia is possible, but it poses many difficulties and must be considered in the context of the entire perioperative period, not just the operating room. While, currently, adapting opioid-sparing strategies as an element of anesthesia with multimodal analgesia to minimize the use of opioids is considered a standard in bariatric perioperative care, there are indications that a more radical approach, such as OFA, may have advantages. Based on these assumptions, more and more centers are introducing their use. On the other hand, there is growing evidence that the benefits may be limited, and issues related to safety, long-term effects, and the place of OFA in the Fast Track Surgery doctrine are not resolved. Therefore, considering the meager amount of literature, OFA should not be used as a standard and only as an anesthetic technique in bariatric surgery. The question of the broader application of this method requires further research.
Author Contributions
Study conception: P.M. and J.T.; design: P.M. and M.K.; acquisition of the data: P.M. and M.K.; analysis and interpretation of the data: P.M. and M.K.; drafting of the manuscript: P.M.; critical revision: M.K. and J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support this study are available within the reference or available from the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organisation 2024 Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 April 2024).
- Angrisani, L.; Santonicola, A.; Iovino, P.; Palma, R.; Kow, L.; Prager, G.; Ramos, A.; Shikora, S. Collaborative Study Group for the IFSO Worldwide Survey. IFSO Worldwide Survey 2020–2021: Current Trends for Bariatric and Metabolic Procedures. Obes. Surg. 2024, 34, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Iamaroon, A.; Tangwiwat, S.; Nivatpumin, P.; Lertwacha, T.; Rungmongkolsab, P.; Pangthipampai, P. Risk Factors for Moderate to Severe Pain during the First 24 Hours after Laparoscopic Bariatric Surgery While Receiving Intravenous Patient-Controlled Analgesia. Anesthesiol. Res. Pract. 2019, 2019, 6593736. [Google Scholar] [CrossRef] [PubMed]
- Kessler, E.R.; Shah, M.; Gruschkus, S.K.; Raju, A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: Opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy 2013, 33, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Koepke, E.J.; Manning, E.L.; Miller, T.; Ganesh, A.; Williams, D.G.A.; Manning, M.W. The rising tide of opioid use and abuse: The role of the anesthesiologist. Perioper. Med. 2018, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Oderda, G.M.; Said, Q.; Evans, R.S.; Stoddard, G.J.; Lloyd, J.; Jackson, K.; Rublee, D.; Samore, M.H. Opioid-Related Adverse Drug Events in Surgical Hospitalizations: Impact on Costs and Length of Stay. Ann. Pharmacother. 2007, 41, 400–407. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Abdelghany, M.S.; Afandy, M.E. The effect of opioid-free anesthesia on the post-operative opioid consumption in laparoscopic bariatric surgeries: A randomized controlled double-blind study. J. Opioid Manag. 2022, 18, 47–56. [Google Scholar] [CrossRef]
- Ibrahim, M.; Elnabtity, A.M.; Hegab, A.; Alnujaidi, O.A.; El Sanea, O. Combined Opioid Free and Loco-Regional Anaesthesia Enhances the Quality of Recovery in Sleeve Gastrectomy Done under Eras Protocol: A Randomized Controlled Trial. BMC Anesthesiol. 2022, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Mulier, J.P.; Wouters, R.; Dillemans, B.; De Kock, M. A randomized controlled, double-blind trial evaluating the effect of opioid-free versus opioid general anaesthesia on postoperative pain and discomfort measured by the QoR-40. J. Clin. Anesth. Pain Med. 2018, 2, 15. [Google Scholar]
- Soudi, A.M.; Hammad, R.A.; ElShafie, M.A.; Ahmed, I.M.; Alhadidy, M.A. Comparing Opioid Free General Anesthesia to Traditional Balanced General Anesthesia Regarding Achievement of Enhanced Recovery in Laparoscopic Bariatric Surgeries. Ain Shams J. Anesthesiol. 2022, 14, 24. [Google Scholar] [CrossRef]
- Ziemann-Gimmel, P.; Goldfarb, A.A.; Koppman, J.; Marema, R.T. Opioid-Free Total Intravenous Anaesthesia Reduces Postoperative Nausea and Vomiting in Bariatric Surgery beyond Triple Prophylaxis. Br. J. Anaesth. 2014, 112, 906–911. [Google Scholar] [CrossRef]
- Mansour, M.; Mahmoud, A.A.; Geddawy, M. Nonopioid versus Opioid Based General Anesthesia Technique for Bariatric Surgery: A Randomized Double-Blind Study. Saudi J. Anaesth. 2013, 7, 387. [Google Scholar] [CrossRef] [PubMed]
- Clanet, M.; Touihri, K.; El Haddad, C.; Goldsztejn, N.; Himpens, J.; Fils, J.F.; Gricourt, Y.; Van der Linden, P.; Coeckelenbergh, S.; Joosten, A.; et al. Effect of Opioid-Free versus Opioid-Based Strategies during Multimodal Anaesthesia on Postoperative Morphine Consumption after Bariatric Surgery: A Randomised Double-Blind Clinical Trial. BJA Open 2024, 9, 100263. [Google Scholar] [CrossRef]
- Mieszczański, P.; Górniewski, G.; Ziemiański, P.; Cylke, R.; Lisik, W.; Trzebicki, J. Comparison between Multimodal and Intraoperative Opioid Free Anesthesia for Laparoscopic Sleeve Gastrectomy: A Prospective, Randomized Study. Sci. Rep. 2023, 13, 12677. [Google Scholar] [CrossRef]
- Ulbing, S.; Infanger, L.; Fleischmann, E.; Prager, G.; Hamp, T. The Performance of Opioid-Free Anesthesia for Bariatric Surgery in Clinical Practice. Obes. Surg. 2023, 33, 1687–1693. [Google Scholar] [CrossRef]
- Campos-Pérez, W.; Ramírez-Plascencia, L.; Pérez-Robles, M.; Rivera-Valdés, J.J.; Sánchez-Muñoz, P.; Pérez-Vargas, L.; González-Landeros, D.; Cuevas, J.H.M.; Martínez-López, E.A. Comparison of opioid-containing anesthesia versus opioid-free anesthesia using the Cortínez-Sepúlveda model on differential cytokine responses in obese patients undergoing gastric bypass surgery: A randomized controlled trial. BMC Anesthesiol. 2022, 22, 294. [Google Scholar] [CrossRef] [PubMed]
- Menck, J.T.; Tenório, S.B.; de Oliveira, R.M.; Strobel, R.; dos Santos, B.B.; Junior, A.F.; de Cesaro, M.P. Opioid-Free Anesthesia for Laparoscopic Gastroplasty. A Prospective and Randomized Trial. Open Anesth. J. 2022, 16, e258964582208110. [Google Scholar] [CrossRef]
- Sultana, A.; Torres, D.; Schumann, R. Special Indications for Opioid Free Anaesthesia and Analgesia, Patient and Procedure Related: Including Obesity, Sleep Apnoea, Chronic Obstructive Pulmonary Disease, Complex Regional Pain Syndromes, Opioid Addiction and Cancer Surgery. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Forget, P. Opioid-Free Anaesthesia. Why and How? A Contextual Analysis. Anaesth. Crit. Care Pain Med. 2019, 38, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Boerboom, S.L.; de Haes, A.; vd Wetering, L.; Aarts, E.O.; Janssen, I.M.C.; Geurts, J.W.; Kamphuis, E.T. Preperitoneal Bupivacaine Infiltration Reduces Postoperative Opioid Consumption, Acute Pain, and Chronic Postsurgical Pain after Bariatric Surgery: A Randomized Controlled Trial. Obes. Surg. 2018, 28, 3102–3110. [Google Scholar] [CrossRef]
- Földi, M.; Soós, A.; Hegyi, P.; Kiss, S.; Szakács, Z.; Solymár, M.; Pétervári, E.; Balaskó, M.; Kusza, K.; Molnár, Z. Transversus Abdominis Plane Block Appears to Be Effective and Safe as a Part of Multimodal Analgesia in Bariatric Surgery: A Meta-Analysis and Systematic Review of Randomized Controlled Trials. Obes. Surg. 2020, 31, 531–543. [Google Scholar] [CrossRef]
- Nair, A.; Rangaiah, M.; Dudhedia, U.; Borkar, N. Analgesic Efficacy and Outcomes of Ultrasound-Guided Erector Spinae Plane Block in Patients Undergoing Bariatric and Metabolic Surgeries: A Systematic Review. J. Med. Ultrasound 2023, 31, 178. [Google Scholar] [CrossRef] [PubMed]
- Omar, I.; Abualsel, A. Efficacy of Intraperitoneal Instillation of Bupivacaine after Bariatric Surgery: Randomized Controlled Trial. Obes. Surg. 2019, 29, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Daes, J.; Morrell, D.J.; Hanssen, A.; Caballero, M.; Luque, E.; Pantoja, R.; Luquetta, J.; Pauli, E.M. Paragastric Autonomic Neural Blockade to Prevent Early Visceral Pain and Associated Symptoms after Laparoscopic Sleeve Gastrectomy: A Randomized Clinical Trial. Obes. Surg. 2022, 32, 3551–3560. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; Muñoz, J.L.; Gonzalez, J.; Zubiaga, L.; García, A.; Jimenez, M.; Ferrigni, C.; Durán, M. Postoperative pain after laparoscopic sleeve gastrectomy: Comparison of three analgesic schemes (isolated intravenous analgesia, epidural analgesia associated with intravenous analgesia and port-sites infiltration with bupivacaine associated with intravenous analgesia). Surg. Endosc. 2017, 31, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-C.; Chen, W.-H.; Shih, Y.-H.; Hung, K.-C. Epidural anesthesia for laparoscopic bariatric surgery: A case report. Springerplus 2015, 4, 363. [Google Scholar] [CrossRef] [PubMed]
- Soltan, W.A.; Fathy, E.; Khattab, M.; Mostafa, M.S.; Hasan, H.; Refaat, A.; Eltantawy, M.A.M.; Ziada, H.F.M.; Sarhan, M.D. Combined Thoracic Spinal-Epidural Anesthesia for Laparoscopic Sleeve Gastrectomy; One Hundred Case Experience. Obes. Surg. 2022, 32, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, E.; dos Reis Falcão, L.F.; O’Kane, M.; Liem, R.; Pournaras, D.J.; Salminen, P.; Urman, R.D.; Wadhwa, A.; Gustafsson, U.O.; Thorell, A. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery after Surgery (ERAS) Society Recommendations: A 2021 Update. World J. Surg. 2022, 46, 729–751. [Google Scholar] [CrossRef] [PubMed]
- Veiga de Sá, A.; Cavaleiro, C.; Campos, M. Haemodynamic and Analgesic Control in a Perioperative Opioid-Free Approach to Bariatric Surgery—A Case Report. Indian J. Anaesth. 2020, 64, 141. [Google Scholar] [CrossRef] [PubMed]
- Gaszynski, T.; Gaszynska, E.; Szewczyk, T. Dexmedetomidine for Awake Intubation and an Opioid-Free General Anesthesia in a Superobese Patient with Suspected Difficult Intubation. Drug Des. Dev. Ther. 2014, 8, 909–912. [Google Scholar] [CrossRef]
- Internation Association for The Study of Pain. Available online: https://www.iasp-pain.org/resources/terminology/ (accessed on 10 April 2024).
- Sneddon, L.U. Evolution of Nociception and Pain: Evidence from Fish Models. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190290. [Google Scholar] [CrossRef]
- Mulier, J. Opioid Free General Anesthesia: A Paradigm Shift? Rev. Española Anestesiol. Reanim. 2017, 64, 427–430. [Google Scholar] [CrossRef]
- Cividjian, A.; Petitjeans, F.; Liu, N.; Ghignone, M.; de Kock, M.; Quintin, L. Do We Feel Pain during Anesthesia? A Critical Review on Surgery-Evoked Circulatory Changes and Pain Perception. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 445–467. [Google Scholar] [CrossRef] [PubMed]
- Mulier, J.; Dekock, M. Opioid Free General Anesthesia, a New Paradigm? Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Lirk, P.; Rathmell, J.P. Opioid-Free Anaesthesia: Con: It Is Too Early to Adopt Opioid-Free Anaesthesia Today. Eur. J. Anaesthesiol. 2019, 36, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Macfater, H.; Xia, W.; Srinivasa, S.; Hill, A.G.; Van De Velde, M.; Joshi, G.P.; Beloeil, H.; Bonnet, F.; Hill, A.; Joshi, G.P.; et al. Evidence-based Management of Postoperative Pain in Adults Undergoing Laparoscopic Sleeve Gastrectomy. World J. Surg. 2019, 43, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO). 8th IFSO Global Registry Report 2023. Available online: https://www.ifso.com/pdf/8th-ifso-registry-report-2023.pdf (accessed on 10 April 2024).
- Hardt, K.; Wappler, F. Anesthesia for morbidly obese patients. Dtsch. Arztebl. Int. 2023, 120, 779–785. [Google Scholar] [CrossRef]
- Gupta, K.; Nagappa, M.; Prasad, A.; Abrahamyan, L.; Wong, J.; Weingarten, T.N.; Chung, F. Risk Factors for Opioid-Induced Respiratory Depression in Surgical Patients: A Systematic Review and Meta-Analyses. BMJ Open 2018, 8, e024086. [Google Scholar] [CrossRef] [PubMed]
- Mulier, J.P. Perioperative Opioids Aggravate Obstructive Breathing in Sleep Apnea Syndrome. Curr. Opin. Anaesthesiol. 2016, 29, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Croci, M.; Ravagnan, I.; Tredici, S.; Pedoto, A.; Lissoni, A.; Gattinoni, L. The Effects of Body Mass on Lung Volumes, Respiratory Mechanics, and Gas Exchange during General Anesthesia. Anesth. Analg. 1998, 87, 654–660. [Google Scholar] [CrossRef]
- Mutter, T.C.; Chateau, D.; Moffatt, M.; Ramsey, C.; Roos, L.L.; Kryger, M. A Matched Cohort Study of Postoperative Outcomes in Obstructive Sleep Apnea. Anesthesiology 2014, 121, 707–718. [Google Scholar] [CrossRef]
- Eichenberger, A.-S.; Proietti, S.; Wicky, S.; Frascarolo, P.; Suter, M.; Spahn, D.R.; Magnusson, L. Morbid Obesity and Postoperative Pulmonary Atelectasis: An Underestimated Problem. Anesth. Analg. 2002, 95, 1788–1792. [Google Scholar] [CrossRef]
- Drummond, G.B.; Bates, A.; Mann, J.; Arvind, D.K. Characterization of Breathing Patterns during Patient-Controlled Opioid Analgesia. Br. J. Anaesth. 2013, 111, 971–978. [Google Scholar] [CrossRef]
- Overdyk, F.; Dahan, A.; Roozekrans, M.; der van Schrier, R.; Aarts, L.; Niesters, M. Opioid-Induced Respiratory Depression in the Acute Care Setting: A Compendium of Case Reports. Pain Manag. 2014, 4, 317–325. [Google Scholar] [CrossRef]
- Zhang, Z.; Ferretti, V.; Güntan, İ.; Moro, A.; Steinberg, E.A.; Ye, Z.; Zecharia, A.Y.; Yu, X.; Vyssotski, A.L.; Brickley, S.G.; et al. Neuronal Ensembles Sufficient for Recovery Sleep and the Sedative Actions of A2 Adrenergic Agonists. Nat. Neurosci. 2015, 18, 553–561. [Google Scholar] [CrossRef]
- Tufanogullari, B.; White, P.F.; Peixoto, M.P.; Kianpour, D.; Lacour, T.; Griffin, J.; Skrivanek, G.; Macaluso, A.; Shah, M.; Provost, D.A. Dexmedetomidine Infusion during Laparoscopic Bariatric Surgery: The Effect on Recovery Outcome Variables. Anesth. Analg. 2008, 106, 1741–1748. [Google Scholar] [CrossRef]
- Weingarten, T.N.; Hawkins, N.M.; Beam, W.B.; Brandt, H.A.; Koepp, D.J.; Kellogg, T.A.; Sprung, J. Factors associated with prolonged anesthesia recovery following laparoscopic bariatric surgery: A retrospective analysis. Obes. Surg. 2015, 25, 1024–1030. [Google Scholar] [CrossRef]
- Frauenknecht, J.; Kirkham, K.R.; Jacot-Guillarmod, A.; Albrecht, E. Analgesic Impact of Intra-operative Opioids vs. Opioid-free Anaesthesia: A Systematic Review and Meta-analysis. Anaesthesia 2019, 74, 651–662. [Google Scholar] [CrossRef]
- Roberts, G.W.; Bekker, T.B.; Carlsen, H.H.; Moffatt, C.H.; Slattery, P.J.; McClure, A.F. Postoperative Nausea and Vomiting Are Strongly Influenced by Postoperative Opioid Use in a Dose-Related Manner. Anesth. Analg. 2005, 101, 1343–1348. [Google Scholar] [CrossRef]
- Ao, Y.; Ma, J.; Zheng, X.; Zeng, J.; Wei, K. Opioid-Sparing Anesthesia Versus Opioid-Free Anesthesia for the Prevention of Postoperative Nausea and Vomiting after Laparoscopic Bariatric Surgery: A Systematic Review and Network Meta-Analysis. Anesth. Analg. 2024. Published online. [Google Scholar] [CrossRef]
- Singh, P.M.; Panwar, R.; Borle, A.; Mulier, J.P.; Sinha, A.; Goudra, B. Perioperative Analgesic Profile of Dexmedetomidine Infusions in Morbidly Obese Undergoing Bariatric Surgery: A Meta-Analysis and Trial Sequential Analysis. Surg. Obes. Relat. Dis. 2017, 13, 1434–1446. [Google Scholar] [CrossRef]
- Blaudszun, G.; Lysakowski, C.; Elia, N.; Tramèr, M.R. Effect of Perioperative Systemic A2 Agonists on Postoperative Morphine Consumption and Pain Intensity. Anesthesiology 2012, 116, 1312–1322. [Google Scholar] [CrossRef]
- Grape, S.; Kirkham, K.R.; Frauenknecht, J.; Albrecht, E. Intra-operative Analgesia with Remifentanil vs. Dexmedetomidine: A Systematic Review and Meta-analysis with Trial Sequential Analysis. Anaesthesia 2019, 74, 793–800. [Google Scholar] [CrossRef]
- Sakata, R.K.; de Lima, R.C.; Valadão, J.A.; Leal, P.C.; Moura, E.C.; Cruz, V.P.; de Oliveira, C.M. Randomized, Double-Blind Study of the Effect of Intraoperative Intravenous Lidocaine on the Opioid Consumption and Criteria for Hospital Discharge after Bariatric Surgery. Obes. Surg. 2019, 30, 1189–1193. [Google Scholar] [CrossRef]
- Kizilcik, N.; Koner, O. Magnesium Sulfate Reduced Opioid Consumption in Obese Patients Undergoing Sleeve Gastrectomy: A Prospective, Randomized Clinical Trial. Obes. Surg. 2018, 28, 2783–2788. [Google Scholar] [CrossRef]
- Kasputytė, G.; Karbonskienė, A.; Macas, A.; Maleckas, A. Role of Ketamine in Multimodal Analgesia Protocol for Bariatric Surgery. Medicina 2020, 56, 96. [Google Scholar] [CrossRef]
- Hung, K.-C.; Chiu, C.-C.; Hsu, C.-W.; Lin, C.-M.; Liao, S.-W.; Teng, I.-C.; Chen, I.-W.; Sun, C.-K. Impact of Opioid-Free Anesthesia on Analgesia and Recovery Following Bariatric Surgery: A Meta-Analysis of Randomized Controlled Studies. Obes. Surg. 2022, 32, 3113–3124. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, P. Current Role of Dexmedetomidine in Clinical Anesthesia and Intensive Care. Anesth. Essays Res. 2011, 5, 128. [Google Scholar] [CrossRef]
- Mieszczański, P.; Janiak, M.; Ziemiański, P.; Cylke, R.; Lisik, W.; Trzebicki, J. Successful Anesthetic Management for Obese Patients with Interstitial Lung Disease Undergoing Laparoscopic Sleeve Gastrectomy: A Bridge to Improved Lung Transplant Eligibility. Am. J. Case Rep. 2024, 25, e942736-1–e942736-6. [Google Scholar] [CrossRef]
- Guignard, B.; Bossard, A.E.; Coste, C.; Sessler, D.I.; Lebrault, C.; Alfonsi, P.; Fletcher, D.; Chauvin, M. Acute Opioid Tolerance. Anesthesiology 2000, 93, 409–417. [Google Scholar] [CrossRef]
- Chia, Y.-Y.; Liu, K.; Wang, J.-J.; Kuo, M.-C.; Ho, S.-T. Intraoperative High Dose Fentanyl Induces Postoperative Fentanyl Tolerance. Can. J. Anesth. 1999, 46, 872–877. [Google Scholar] [CrossRef]
- Angst, M.S.; Clark, J.D. Opioid-Induced Hyperalgesia. Anesthesiology 2006, 104, 570–587. [Google Scholar] [CrossRef]
- Albrecht, E.; Grape, S.; Frauenknecht, J.; Kilchoer, L.; Kirkham, K.R. Low- versus High-dose Intraoperative Opioids: A Systematic Review with Meta-analyses and Trial Sequential Analyses. Acta Anaesthesiol. Scand. 2019, 64, 6–22. [Google Scholar] [CrossRef]
- Huang, X.; Cai, J.; Lv, Z.; Zhou, Z.; Zhou, X.; Zhao, Q.; Sun, J.; Chen, L. Postoperative Pain after Different Doses of Remifentanil Infusion during Anaesthesia: A Meta-Analysis. BMC Anesthesiol. 2024, 24, 25. [Google Scholar] [CrossRef]
- Wilson, S.H.; Hellman, K.M.; James, D.; Adler, A.C.; Chandrakantan, A. Mechanisms, Diagnosis, Prevention and Management of Perioperative Opioid-Induced Hyperalgesia. Pain Manag. 2021, 11, 405–417. [Google Scholar] [CrossRef]
- Loftus, R.W.; Yeager, M.P.; Clark, J.A.; Brown, J.R.; Abdu, W.A.; Sengupta, D.K.; Beach, M.L. Intraoperative Ketamine Reduces Perioperative Opiate Consumption in Opiate-Dependent Patients with Chronic Back Pain Undergoing Back Surgery. Anesthesiology 2010, 113, 639–646. [Google Scholar] [CrossRef]
- Koppert, W.; Sittl, R.; Scheuber, K.; Alsheimer, M.; Schmelz, M.; Schüttler, J. Differential Modulation of Remifentanil-Induced Analgesia and Postinfusion Hyperalgesia by S -Ketamine and Clonidine in Humans. Anesthesiology 2003, 99, 152–159. [Google Scholar] [CrossRef]
- Song, J.W.; Lee, Y.-W.; Yoon, K.B.; Park, S.J.; Shim, Y.H. Magnesium Sulfate Prevents Remifentanil-Induced Postoperative Hyperalgesia in Patients Undergoing Thyroidectomy. Anesth. Analg. 2011, 113, 390–397. [Google Scholar] [CrossRef]
- Doleman, B.; Leonardi-Bee, J.; Heinink, T.P.; Boyd-Carson, H.; Carrick, L.; Mandalia, R.; Lund, J.N.; Williams, J.P. Pre-Emptive and Preventive Nsaids for Postoperative Pain in Adults Undergoing All Types of Surgery. Cochrane Database Syst. Rev. 2021, 2021, CD012978. [Google Scholar] [CrossRef]
- Vogelaerts, R.; Van Pachtenbeke, L.; Raudsepp, M.; Morlion, B. Chronic Abdominal Pain after Bariatric Surgery: A Narrative Review. Acta Anaesthesiol. Belg. 2022, 73, 249–258. [Google Scholar] [CrossRef]
- Gribsholt, S.B.; Pedersen, A.M.; Svensson, E.; Thomsen, R.W.; Richelsen, B. Prevalence of Self-Reported Symptoms after Gastric Bypass Surgery for Obesity. JAMA Surg. 2016, 151, 504. [Google Scholar] [CrossRef]
- Pierik, A.S.; Coblijn, U.K.; de Raaff, C.A.L.; van Veen, R.N.; van Tets, W.F.; van Wagensveld, B.A. Unexplained Abdominal Pain in Morbidly Obese Patients after Bariatric Surgery. Surg. Obes. Relat. Dis. 2017, 13, 1743–1751. [Google Scholar] [CrossRef]
- Katz, J.; Clairoux, M.; Redahan, C.; Kavanagh, B.P.; Carroll, S.; Nierenberg, H.; Jackson, M.; Beattie, J.; Taddio, A.; Sandler, A.N. High Dose Alfentanil Pre-Empts Pain after Abdominal Hysterectomy. Pain 1996, 68, 109–118. [Google Scholar] [CrossRef]
- El-Dawlatly, A.A. Hemodynamic profile during laparoscopic cholecystectomy versus laparoscopic bariatric surgery: The impact of morbid obesity. Middle East J. Anaesthesiol. 2007, 19, 51–60. [Google Scholar]
- Beloeil, H.; Garot, M.; Lebuffe, G.; Gerbaud, A.; Bila, J.; Cuvillon, P.; Dubout, E.; Oger, S.; Nadaud, J.; Becret, A.; et al. Balanced Opioid-Free Anesthesia with Dexmedetomidine versus Balanced Anesthesia with Remifentanil for Major or Intermediate Noncardiac Surgery. Anesthesiology 2021, 134, 541–551. [Google Scholar] [CrossRef]
- Omar, A.M. Can Systemic Lidocaine Be Used in Controlled Hypotension? A Double-Blinded Randomized Controlled Study in Patients Undergoing Functional Endoscopic Sinus Surgery. Egypt. J. Anaesth. 2013, 29, 295–300. [Google Scholar] [CrossRef]
- Elsharnouby, N.M.; Elsharnouby, M.M. Magnesium Sulphate as a Technique of Hypotensive Anaesthesia. Br. J. Anaesth. 2006, 96, 727–731. [Google Scholar] [CrossRef]
- Deegan, R.; Wood, A.J. Β-Receptor Antagonism Does Not Fully Explain Esmolol-Induced Hypotension. Clin. Pharmacol. Ther. 1994, 56, 223–228. [Google Scholar] [CrossRef]
- Sheth, M.K.; Brand, A.; Halterman, J. Ketamine-Induced Changes in Blood Pressure and Heart Rate in Pre-Hospital Intubated Patients. Adv. J. Grad. Res. 2017, 3, 20–33. [Google Scholar] [CrossRef]
- Abernethy, D.R.; Greenblatt, D.J. Lidocaine Disposition in Obesity. Am. J. Cardiol. 1984, 53, 1183–1186. [Google Scholar] [CrossRef]
- Searle, R.; Simpson, K. Chronic Post-Surgical Pain. Contin. Educ. Anaesth. Crit. Care Pain 2010, 10, 12–14. [Google Scholar] [CrossRef]
- Dai, J.; Wu, D.; Cui, X.; Li, S.; Xu, F. Application of Surgical Pleth Index in the Opioid-Free Anesthesia: A Randomized Controlled Trial. Medicine 2023, 102, e3517. [Google Scholar] [CrossRef]
- Yoshida, K.; Obara, S.; Inoue, S. Analgesia Nociception Index and High Frequency Variability Index: Promising Indicators of Relative Parasympathetic Tone. J. Anesth. 2022, 37, 130–137. [Google Scholar] [CrossRef]
- Sabourdin, N.; Barrois, J.; Louvet, N.; Rigouzzo, A.; Guye, M.-L.; Dadure, C.; Constant, I. Pupillometry-Guided Intraoperative Remifentanil Administration versus Standard Practice Influences Opioid Use: A Randomized Study. Anesthesiology 2017, 127, 284–292. [Google Scholar] [CrossRef]
- Hans, P.; Dewandre, P.-Y.; Brichant, J.F.; Bonhomme, V. Comparative Effects of Ketamine on BISPECTRAL Index and Spectral Entropy of the Electroencephalogram under Sevoflurane Anaesthesia. Br. J. Anaesth. 2005, 94, 336–340. [Google Scholar] [CrossRef]
- Coeckelenbergh, S.; Estebe, J.-P. Guiding Opioid-Free Intravenous Antinociception with the Analgesia Nociception Index: A Case Report. Braz. J. Anesthesiol. 2020, 70, 678–681. [Google Scholar] [CrossRef]
- Graça, R.; Lobo, F.A. Analgesia Nociception Index (ANI) and Ephedrine: A Dangerous Liasion. J. Clin. Monit. Comput. 2021, 35, 953–954. [Google Scholar] [CrossRef]
- Höcker, J.; Broch, O.; Gräsner, J.T.; Gruenewald, M.; Ilies, C.; Steinfath, M.; Bein, B. Surgical Stress Index in Response to Pacemaker Stimulation or Atropine. Br. J. Anaesth. 2010, 105, 150–154. [Google Scholar] [CrossRef]
- Afraz, S.; Dang, J.T.; Modasi, A.; Switzer, N.; Birch, D.W.; Karmali, S. Bariatric surgery outcomes in oxygen-dependent patients: Analysis of the MBSAQIP database. Surg. Obes. Relat. Dis. 2019, 15, 1571–1580. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).