Exercise Effects on Autonomic Nervous System Activity in Type 2 Diabetes Mellitus Patients over Time: A Meta-Regression Study
Abstract
:1. Introduction
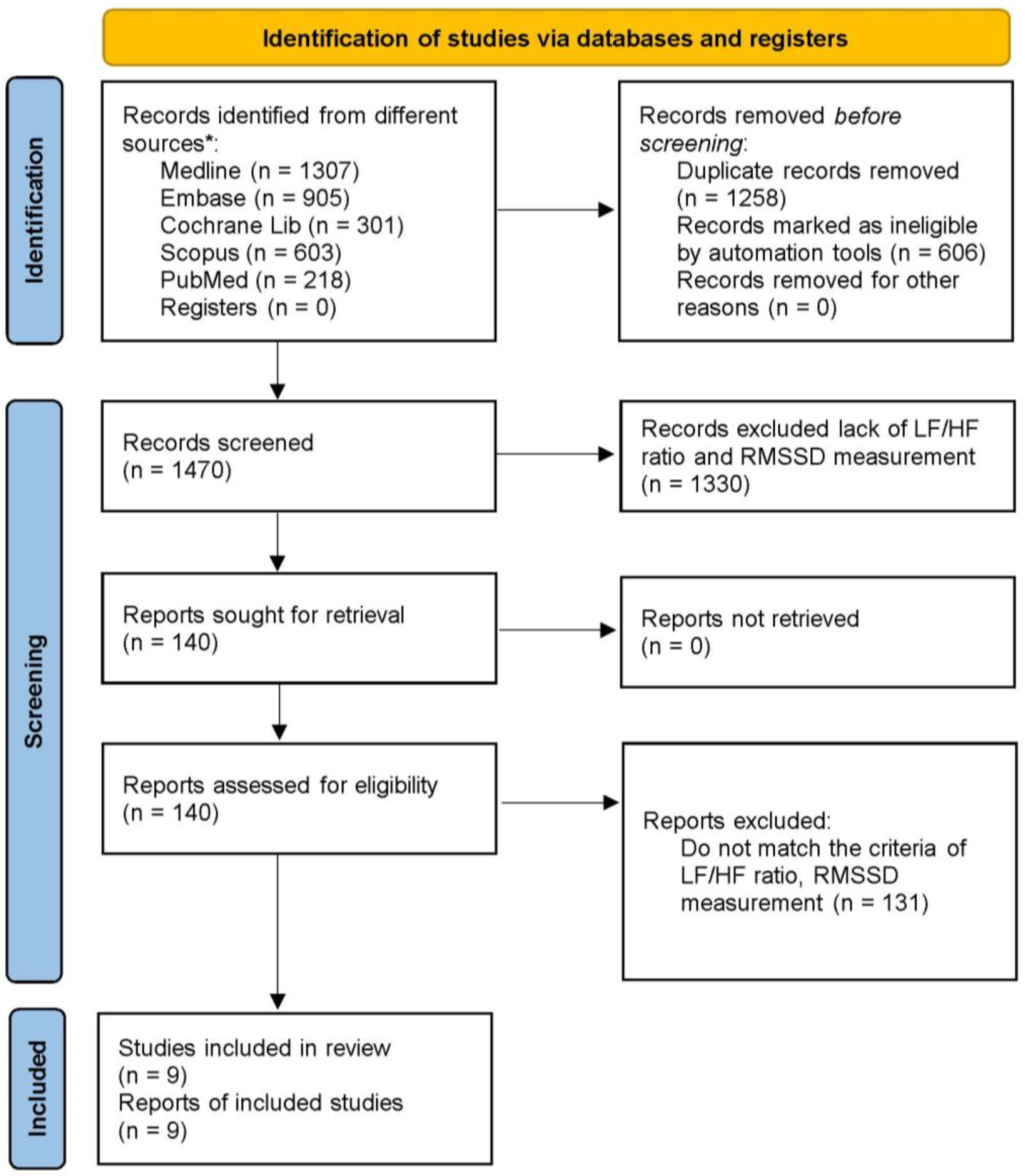
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Methodological Quality of Included Studies
2.6. Data Collection and Analysis
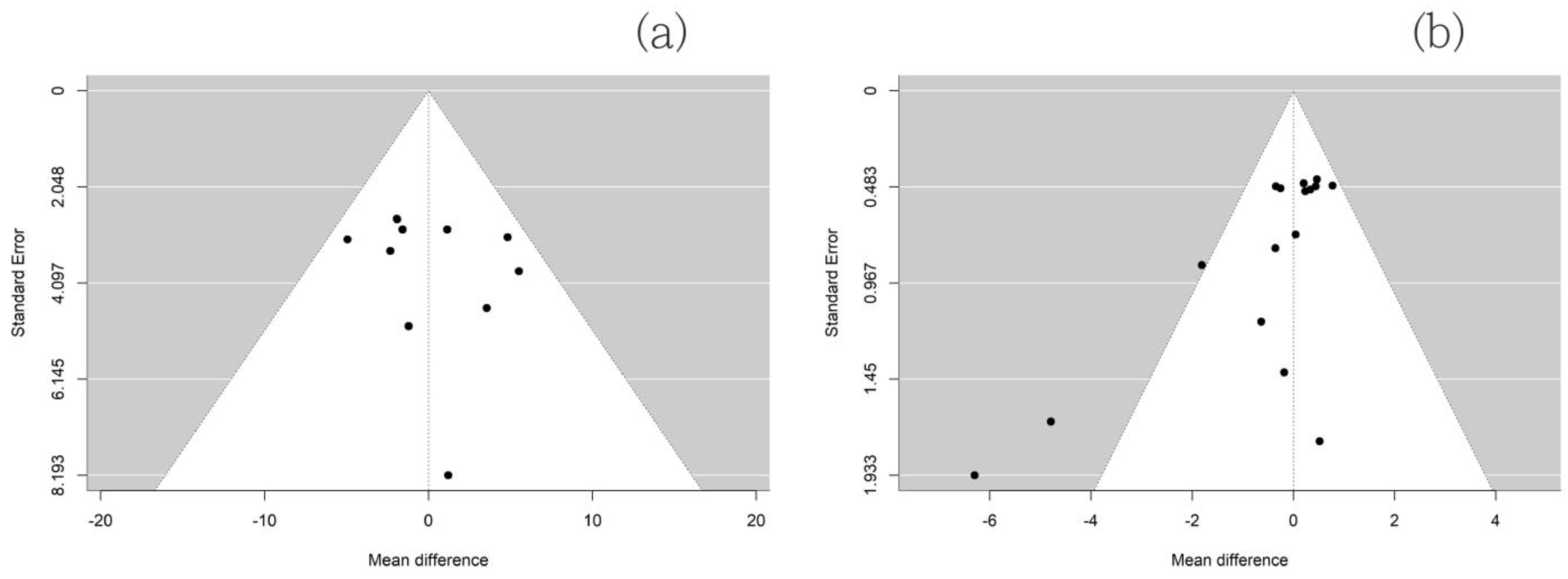
2.7. Data Synthesis and Publication Bias Check
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collaborators, G.B.D.D. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar]
- Bonora, E.; Cigolini, M. DPP-4 inhibitors and cardiovascular disease in type 2 diabetes mellitus. Expectations, observations and perspectives. Nutr. Metab Cardiovasc. Dis. 2016, 26, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Freeman, R.; Erbas, T. Diabetic autonomic neuropathy. Semin. Neurol. 2003, 23, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Kempler, P.; Tesfaye, S.; Chaturvedi, N.; Stevens, L.K.; Webb, D.J.; Eaton, S.; Kerenyi, Z.; Tamas, G.; Ward, J.D.; Fuller, J.H.; et al. Autonomic neuropathy is associated with increased cardiovascular risk factors: The Eurodiab Iddm Complications Study. Diabet. Med. 2002, 19, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Serhiyenko, V.A.; Serhiyenko, A.A. Cardiac autonomic neuropathy: Risk factors, diagnosis and treatment. World J. Diabetes 2018, 9, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ewing, D.J.; Martyn, C.N.; Young, R.J.; Clarke, B.F. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985, 8, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Benichou, T.; Pereira, B.; Mermillod, M.; Tauveron, I.; Pfabigan, D.; Maqdasy, S.; Dutheil, F. Heart rate variability in type 2 diabetes mellitus: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0195166. [Google Scholar] [CrossRef] [PubMed]
- McCraty, R.; Atkinson, M.; Tiller, W.A.; Rein, G.; Watkins, A.D. The effects of emotions on short-term power spectrum analysis of heart rate variability. Am. J. Cardiol. 1995, 76, 1089–1093. [Google Scholar] [CrossRef]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Singer, D.H. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Bigger, J.T., Jr.; Fleiss, J.L.; Steinman, R.C.; Rolnitzky, L.M.; Kleiger, R.E.; Rottman, J.N. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992, 85, 164–171. [Google Scholar] [CrossRef]
- Sequeira, V.C.C.; Bandeira, P.M.; Azevedo, J.C.M. Heart rate variability in adults with obstructive sleep apnea: A systematic review. Sleep Sci. 2019, 12, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Ishibashi, K.; Noguchi, H. Heart rate variability; an index for monitoring and analyzing human autonomic activities. Appl. Human Sci. 1999, 18, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- AlQatari, A.A.; Alturki, J.A.; Abdulali, K.A.; Alhumud, D.A.; Alibrahim, M.A.; Alarab, Y.A.; Salem, A.M.; Yar, T.; Alqurashi, Y.D.; Alsunni, A.A.; et al. Changes in Heart Rate Variability and Baroreflex Sensitivity During Daytime Naps. Nat. Sci. Sleep 2020, 12, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Song, C.; Ikei, H.; Park, B.J.; Lee, J.; Kagawa, T.; Miyazaki, Y. Forest Walking Affects Autonomic Nervous Activity: A Population-Based Study. Front. Public Health 2018, 6, 278. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, H. The Effect of Exercise on Cardiovascular Autonomic Nervous Function in Patients with Diabetes: A Systematic Review. Healthcare 2023, 11, 2668. [Google Scholar] [CrossRef]
- Picard, M.; Tauveron, I.; Magdasy, S.; Benichou, T.; Bagheri, R.; Ugbolue, U.C.; Navel, V.; Dutheil, F. Effect of exercise training on heart rate variability in type 2 diabetes mellitus patients: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251863. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Deeks, J.J.; Dinnes, J.; D’Amico, R.; Sowden, A.J.; Sakarovitch, C.; Song, F.; Petticrew, M.; Altman, D.G. Evaluating non-randomised intervention studies. Health Technol. Assess. 2003, 7, 1–173. [Google Scholar] [CrossRef]
- Maroco, J.L.; Arrais, I.; Silvestre, T.; Pinto, M.; Laranjo, S.; Magalhaes, J.; Santa-Clara, H.; Fernhall, B.; Melo, X. Post-acute exercise cardiovagal modulation in older male adults with and without type 2 diabetes. Eur. J. Appl. Physiol. 2024, 124, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Bhati, P.; Moiz, J.A.; Naqvi, I.H.; Hussain, M.E. Diagnostic performance of resting and post-exercise heart rate variability for detecting cardiac autonomic neuropathy in type 2 diabetes mellitus. Auton. Neurosci. 2019, 219, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Piralaiy, E.; Siahkuhian, M.; Nikookheslat, S.D.; Pescatello, L.S.; Sheikhalizadeh, M.; Khani, M. Cardiac Autonomic Modulation in Response to Three Types of Exercise in Patients with Type 2 Diabetic Neuropathy. J. Diabetes Metab. Disord. 2021, 20, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Ko, K.J.; Baek, U.H. Effects of 12 weeks combined aerobic and resistance exercise on heart rate variability in type 2 diabetes mellitus patients. J. Phys. Ther. Sci. 2016, 28, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Loimaala, A.; Huikuri, H.V.; Koobi, T.; Rinne, M.; Nenonen, A.; Vuori, I. Exercise training improves baroreflex sensitivity in type 2 diabetes. Diabetes 2003, 52, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, M.; Sowden, A.; Petticrew, M.; Arai, L.; Roberts, H.; Britten, N.; Popay, J. Testing methodological guidance on the conduct of narrative synthesis in systematic reviews: Effectiveness of interventions to promote smoke alarm ownership and function. Evaluation 2009, 15, 49–73. [Google Scholar] [CrossRef]
- Wetterslev, J.; Jakobsen, J.C.; Gluud, C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med. Res. Methodol. 2017, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Grieco, C.R.; Somma, C.T. Exercise effects on postprandial glycemia, mood, and sympathovagal balance in type 2 diabetes. J. Am. Med. Dir. Assoc. 2014, 15, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Grieco, C.R.; Colberg, S.R.; Somma, C.T.; Thompson, A.G.; Vinik, A.I. Acute effect of breathing exercises on heart rate variability in type 2 diabetes: A pilot study. J. Altern. Complement Med. 2014, 20, 642–648. [Google Scholar] [CrossRef]
- Figueroa, A.; Baynard, T.; Fernhall, B.; Carhart, R.; Kanaley, J.A. Endurance training improves post-exercise cardiac autonomic modulation in obese women with and without type 2 diabetes. Eur. J. Appl. Physiol. 2007, 100, 437–444. [Google Scholar] [CrossRef]
- Pagkalos, M.; Koutlianos, N.; Kouidi, E.; Pagkalos, E.; Mandroukas, K.; Deligiannis, A. Heart rate variability modifications following exercise training in type 2 diabetic patients with definite cardiac autonomic neuropathy. Br. J. Sports Med. 2008, 42, 47–54. [Google Scholar] [CrossRef]
- Routledgecurdle, J.A.; Bacon, S.L. Improvements in heart rate variability with exercise therapy. Can J. Cardiol. 2010, 26, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Howorka, K.; Pumprla, J.; Haber, P.; Koller-Strametz, J.; Mondrzyk, J.; Schabmann, A. Effects of physical training on heart rate variability in diabetic patients with various degrees of cardiovascular autonomic neuropathy. Cardiovasc. Res. 1997, 34, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Yamada, T.; Liu, W.J.; Hara, K.; Yanagimoto, S.; Hiraike, Y. Interaction between type 2 diabetes polygenic risk and physical activity on cardiovascular outcomes. Eur. J. Prev. Cardiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, X.; Guo, J.; Roberts, C.K.; McKenzie, S.; Wu, W.C.; Liu, S.; Song, Y. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2015, 4, e002014. [Google Scholar] [CrossRef] [PubMed]
- Kang, H. Trial sequential analysis: Novel approach for meta-analysis. Anesth. Pain Med. 2021, 16, 138–150. [Google Scholar] [CrossRef]
- LeLorier, J.; Gregoire, G.; Benhaddad, A.; Lapierre, J.; Derderian, F. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N. Engl. J. Med. 1997, 337, 536–542. [Google Scholar] [CrossRef]
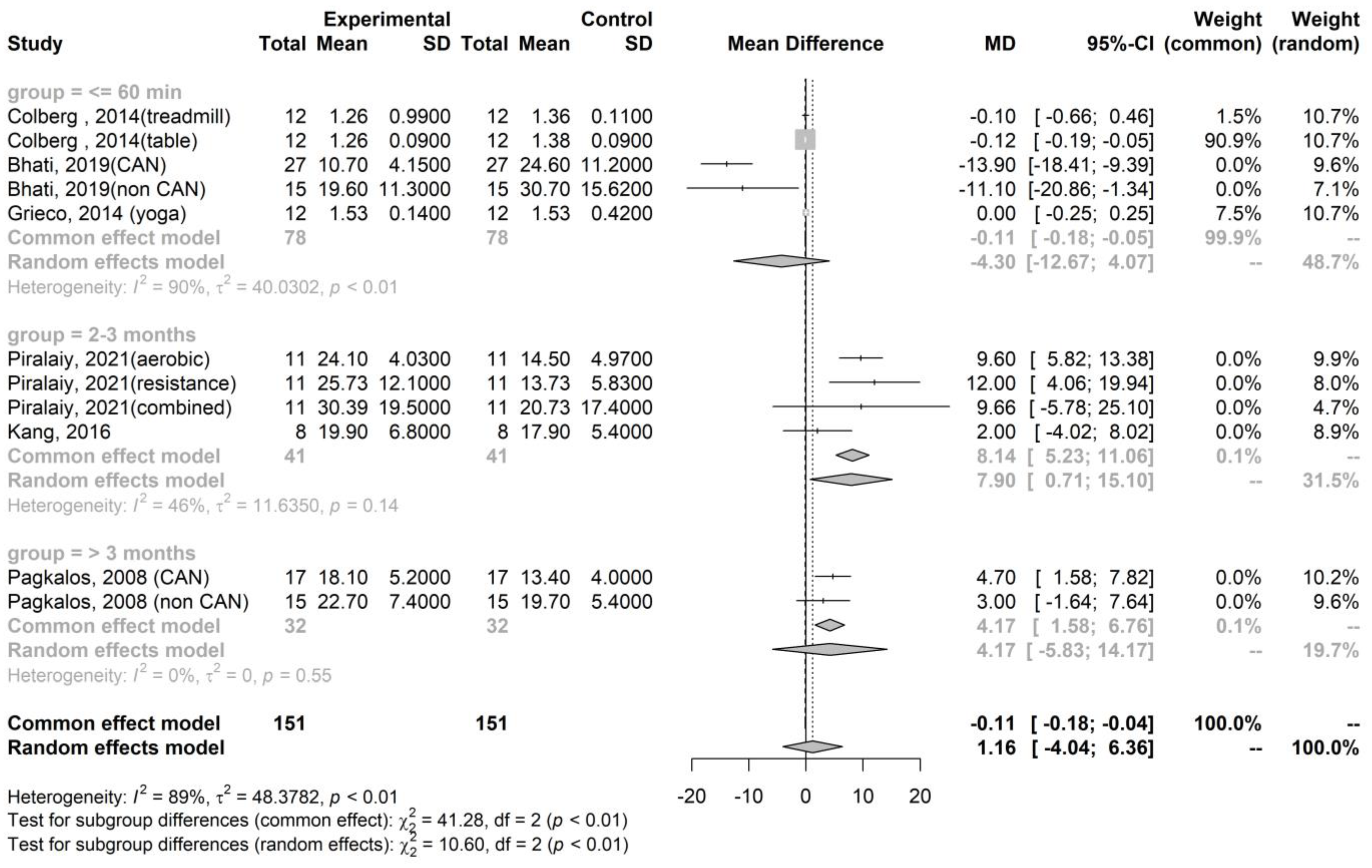
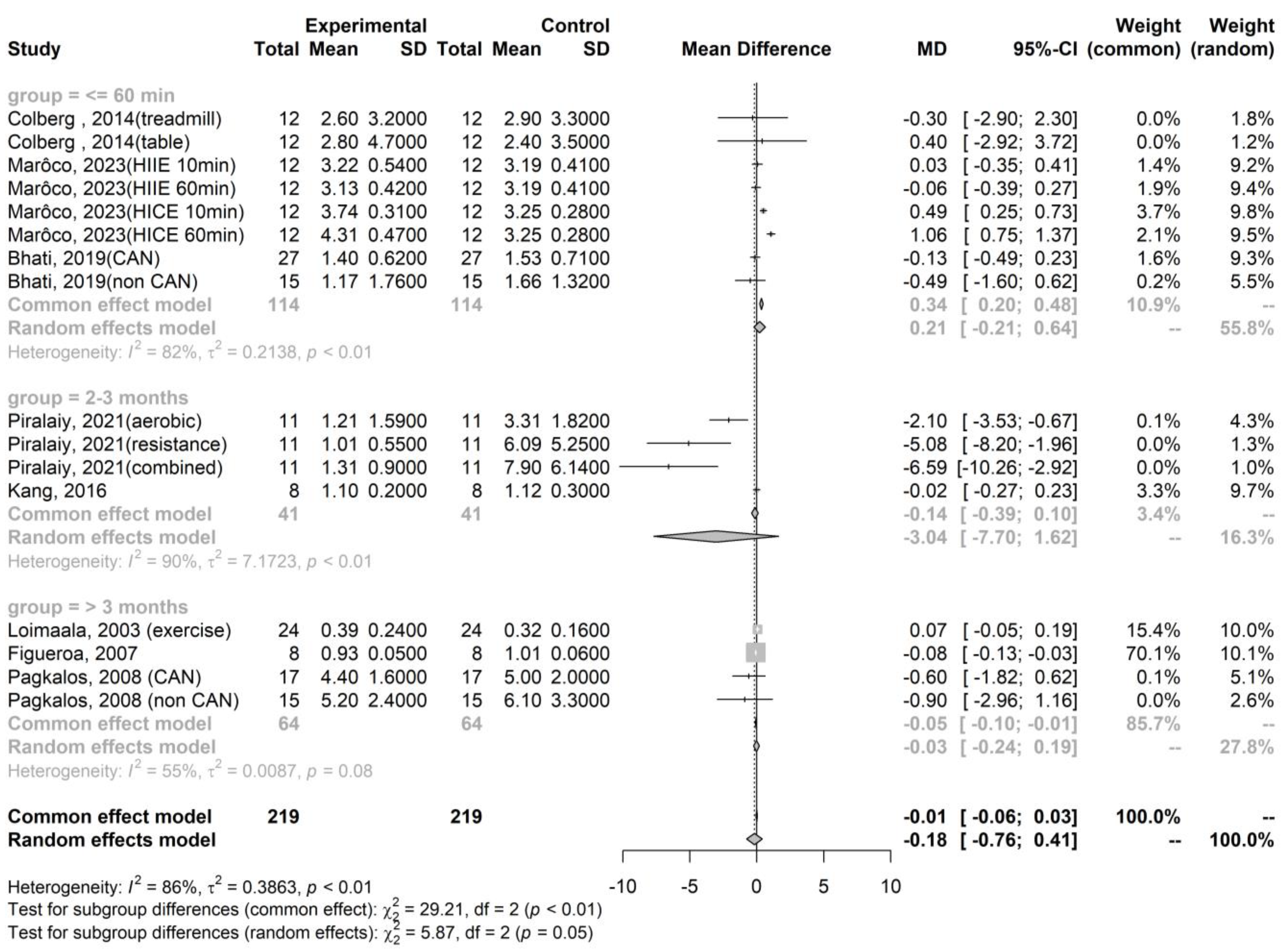
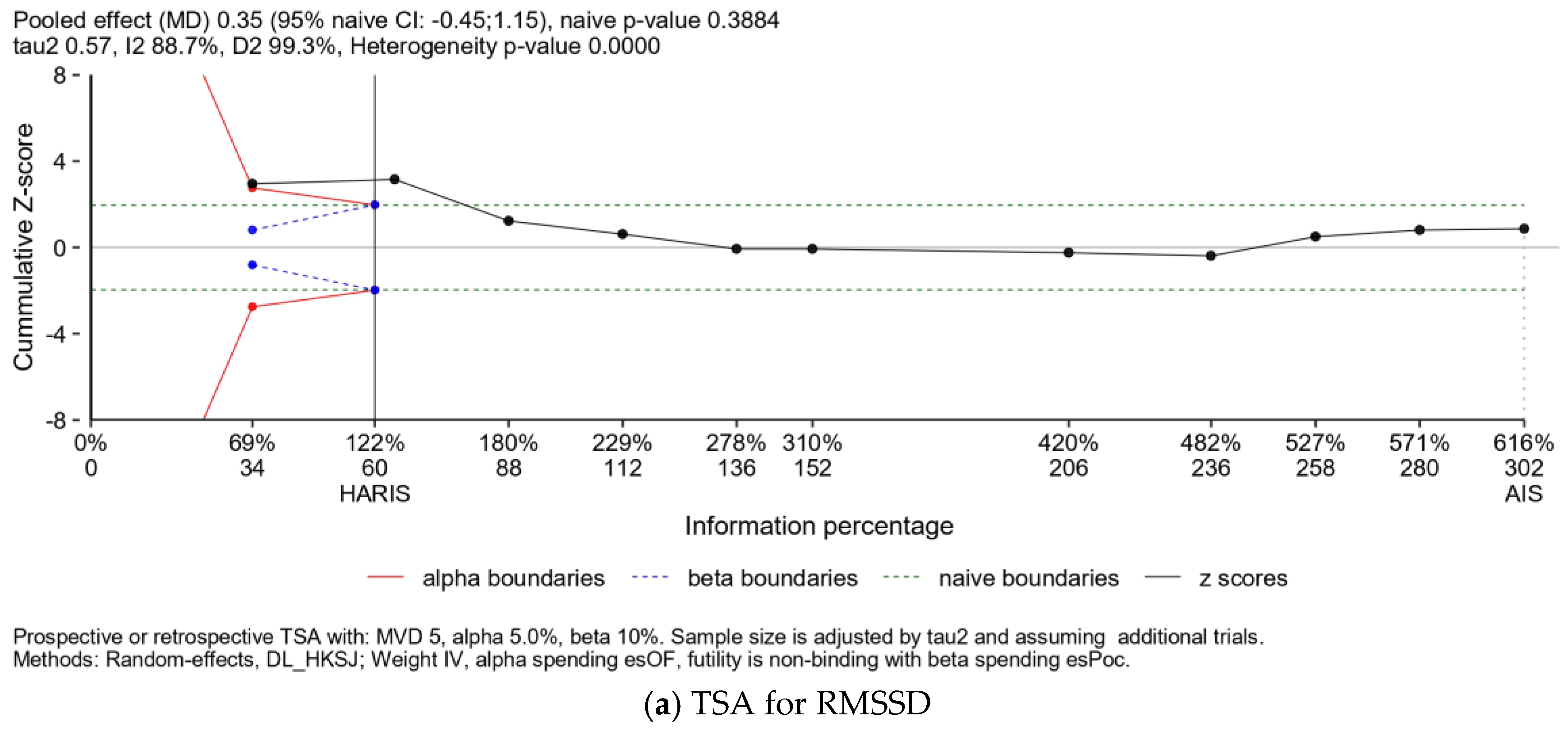
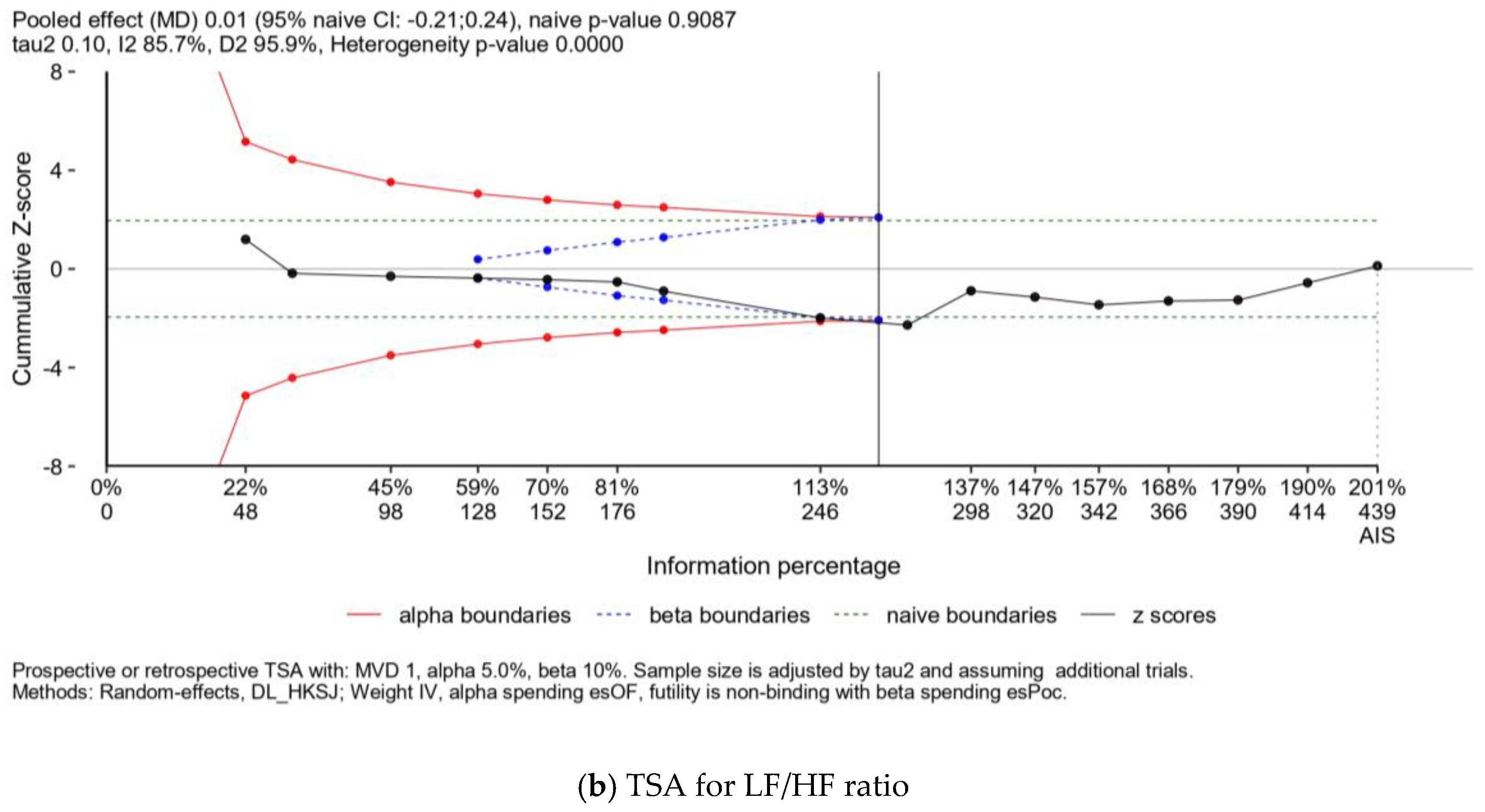
| Publication | Kinds of Exercise | Health Statuses | N, Age (Years Old) | Sympathetic Activity, Measured by the LF/HF Ratio | Parasympathetic Activity, Measured by the RMSSD |
|---|---|---|---|---|---|
| Colberg, 2014 [28] | Walk on a treadmill | Uncomplicated T2DM | N = 12, 58.7 ± 2.4 | Pre-exercise: 2.9 ± 3.3 Immediate post-exercise: 2.6 ± 3.2 | Pre-exercise: 1.4 ± 0.1 Immediate post-exercise: 1.3 ± 1.0 |
| Table tennis | Uncomplicated T2DM | N = 12, 58.7 ± 2.4 | Pre-exercise: 2.4 ± 3.5 Immediate post-exercise: 2.8 ± 4.7 | Pre-exercise: 1.4 ± 0.1 Immediate post-exercise: 1.3 ± 0.1 | |
| Marôco, 2023 [21] | HIIE 10min | T2DM | N = 12, 67 ± 8 | Pre-exercise: 3.2 ± 0.4 Immediate post-exercise: 3.2 ± 0.5 | Not available |
| HIIE 60min | T2DM | N = 12, 67 ± 8 | Pre-exercise: 3.2 ± 0.4 Immediate post-exercise: 3.1 ± 0.4 | Not available | |
| MICE 10min | T2DM | N = 12, 67 ± 8 | Pre-exercise: 3.3 ± 0.3 Immediate post-exercise: 3.7 ± 0.3 | Not available | |
| MICE 60min | T2DM | N = 12, 67 ± 8 | Pre-exercise: 3.3 ± 0.3 Immediate post-exercise: 4.3 ± 0.5 | Not available | |
| Bhati, 2019 [23] | A maximal exercise test | T2DM with CAN | N = 27, 51.7 ± 6.1 | Pre-exercise: 1.5 ± 0.7 Immediate post-exercise: 1.4 ± 0.6 | Pre-exercise: 24.6 ± 11.2 Immediate post-exercise: 10.7 ± 4.2 |
| A maximal exercise test | T2DM without CAN | N = 15, 50.2 ± 6.4 | Pre-exercise: 1.7 ± 1.3 Immediate post-exercise: 1.2 ± 1.8 | Pre-exercise: 30.7 ± 15.6 Immediate post-exercise: 19.6 ± 11.3 | |
| Grieco, 2014 [29] | Yoga | T2DM | N = 12, 54.9 ± 7.4 | Not available | Pre-exercise: 1.5 ± 0.4 Immediate post-exercise: 1.5 ± 0.1 |
| Piralaiy, 2021 [23] | Aerobic training (25–45 min per day, 3–5 times per week for 12 weeks) | T2DM | N = 11, 55.2 ± 8.2 | Pre-exercise: 3.3 ± 1.8 Short-term post-exercise: 1.2 ± 1.6 | Pre-exercise: 14.5 ± 5.0 Short-term post-exercise: 24.1 ± 4.0 |
| Resistant training (25–45 min per day, 3–5 times per week for 12 weeks) | T2DM | N = 11, 55.2 ± 8.2 | Pre-exercise: 6.1 ± 5.3 Short-term post-exercise: 1.0 ± 0.6 | Pre-exercise: 13.7 ± 5.8 Short-term post-exercise: 25.7 ± 12.1 | |
| Combination aerobic and resistance training (25–45 min per day, 3–5 times per week for 12 weeks) | T2DM | N = 11, 55.2 ± 8.2 | Pre-exercise: 7.9 ± 6.1 Short-term post-exercise: 1.3 ± 0.9 | Pre-exercise: 20.7 ± 17.4 Short-term post-exercise: 30.4 ± 15.9 | |
| Kang, 2016 [24] | Combination aerobic and resistance training for 60 min per day, three times per week for 12 weeks | T2DM | N = 8, 56.0 ± 7.4 | Pre-exercise: 1.12 ± 0.3 Short-term post-exercise: 1.1 ± 0.2 | Pre-exercise: 17.9 ± 5.4 Short-term post-exercise: 19.9 ± 6.8 |
| Loimaala, 2003 [25] | Endurance training twice a week and supervised muscle strength training twice a week for 12 months | T2DM | N = 24, 53.6 ± 6.2 | Pre-exercise: 0.32 ± 0.16 Long-term post-exercise: 0.39 ± 0.24 | Not available |
| Figueroa, 2007 [30] | 16 weeks of walking training | T2DM | N = 8, 50.0 ± 1.0 | Pre-exercise: 1.01 ± 0.06 Long-term post-exercise: 0.93 ± 0.05 | Not available |
| Pagkalos, 2008 [31] | An aerobic exercise training program three times a week for 6 months | T2DM with CAN | N = 17, 56.2 ± 5.8 | Pre-exercise: 5.0 ± 2.0 Long-term post-exercise: 4.4 ± 1.6 | Pre-exercise: 13.4 ± 4.0 Long-term post-exercise: 18.1 ± 5.2 |
| An aerobic exercise training program three times a week for 6 months | T2DM without CAN | N = 15, 55.8 ± 5.6 | Pre-exercise: 6.1 ± 3.3 Long-term post-exercise: 5.2 ± 2.4 | Pre-exercise: 19.7 ± 5.4 Long-term post-exercise: 22.7 ± 7.4 |
| Outcome | Variables | Estimate (95% C.I.) | S.E. | z Value | p Value |
|---|---|---|---|---|---|
| RMSSD | |||||
| Mean age in years | 2.36 (1.01–3.72) | 0.68 | 3.43 | 0.001 | |
| Male (%) | 13.76 (3.59–23.94) | 5.19 | 2.65 | 0.008 | |
| The duration of exercise in months | 1.50 (0.41–2.59) | 0.56 | 2.69 | 0.007 | |
| Intercept | −140.5 (−218.6–62.3) | 39.89 | −3.52 | <0.001 | |
| LF/HF ratio | |||||
| Mean age in years | 0.05 (0.001–0.098) | 0.02 | 1.98 | 0.048 | |
| Intercept | −2.99 (−5.86–−0.119) | 1.47 | −2.04 | 0.041 |
| The Groups | Study | Types of Exercises | HRV Measurement | Influence of Autonomic Nervous System Activity after Exercise |
|---|---|---|---|---|
| Immediate (exercise within 60 min) | ||||
| Marôco, 2023 [21] | MICE and HIIE | LF/HF ratio | Mild increase in the LF/HF ratio in MICE | |
| Bhati, 2019 [22] | A maximal exercise test | RMSSD and LF/HF ratio | Decrease in both RMSSD and LF/HF ratio | |
| Colberg, 2014 [28] | Walk on a treadmill and play table tennis | RMSSD and LF/HF ratio | The RMSSD and LF/HF ratio decrease during treadmill walking, while the LF/HF ratio increases and the RMSSD decreases during table tennis | |
| Grieco, 2014 [29] | Yoga | RMSSD | No change | |
| Short-term (exercise between 2–3 months) | ||||
| Piralaiy, 2021 [23] | Aerobic training, resistance training, and combination | RMSSD and LF/HF ratio | Increase in RMSSD and decrease in LF/HF ratio | |
| Kang, 2016 [24] | Combination aerobic and resistance training | RMSSD and LF/HF ratio | Increase in RMSSD and no change in LF/HF ratio | |
| Long-term (exercise exceeding 4 months) | ||||
| Loimaala, 2003 [25] | Endurance training | LF/HF ratio | Increase in LF/HF ratio | |
| Figueroa, 2007 [30] | Walking training | LF/HF ratio | Decrease in LF/HF ratio | |
| Pagkalos, 2008 [31] | An aerobic exercise training program | RMSSD and LF/HF ratio | Increase in RMSSD and decrease in LF/HF ratio |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, J.-K.; Chiang, P.-C.; Kao, H.-H.; You, W.-C.; Kao, Y.-H. Exercise Effects on Autonomic Nervous System Activity in Type 2 Diabetes Mellitus Patients over Time: A Meta-Regression Study. Healthcare 2024, 12, 1236. https://doi.org/10.3390/healthcare12121236
Chiang J-K, Chiang P-C, Kao H-H, You W-C, Kao Y-H. Exercise Effects on Autonomic Nervous System Activity in Type 2 Diabetes Mellitus Patients over Time: A Meta-Regression Study. Healthcare. 2024; 12(12):1236. https://doi.org/10.3390/healthcare12121236
Chicago/Turabian StyleChiang, Jui-Kun, Po-Chen Chiang, Hsueh-Hsin Kao, Weir-Chiang You, and Yee-Hsin Kao. 2024. "Exercise Effects on Autonomic Nervous System Activity in Type 2 Diabetes Mellitus Patients over Time: A Meta-Regression Study" Healthcare 12, no. 12: 1236. https://doi.org/10.3390/healthcare12121236




