Identifying Predictor Variables for a Composite Risk Prediction Tool for Gestational Diabetes and Hypertensive Disorders of Pregnancy: A Modified Delphi Study
Abstract
1. Introduction
2. Materials and Methods
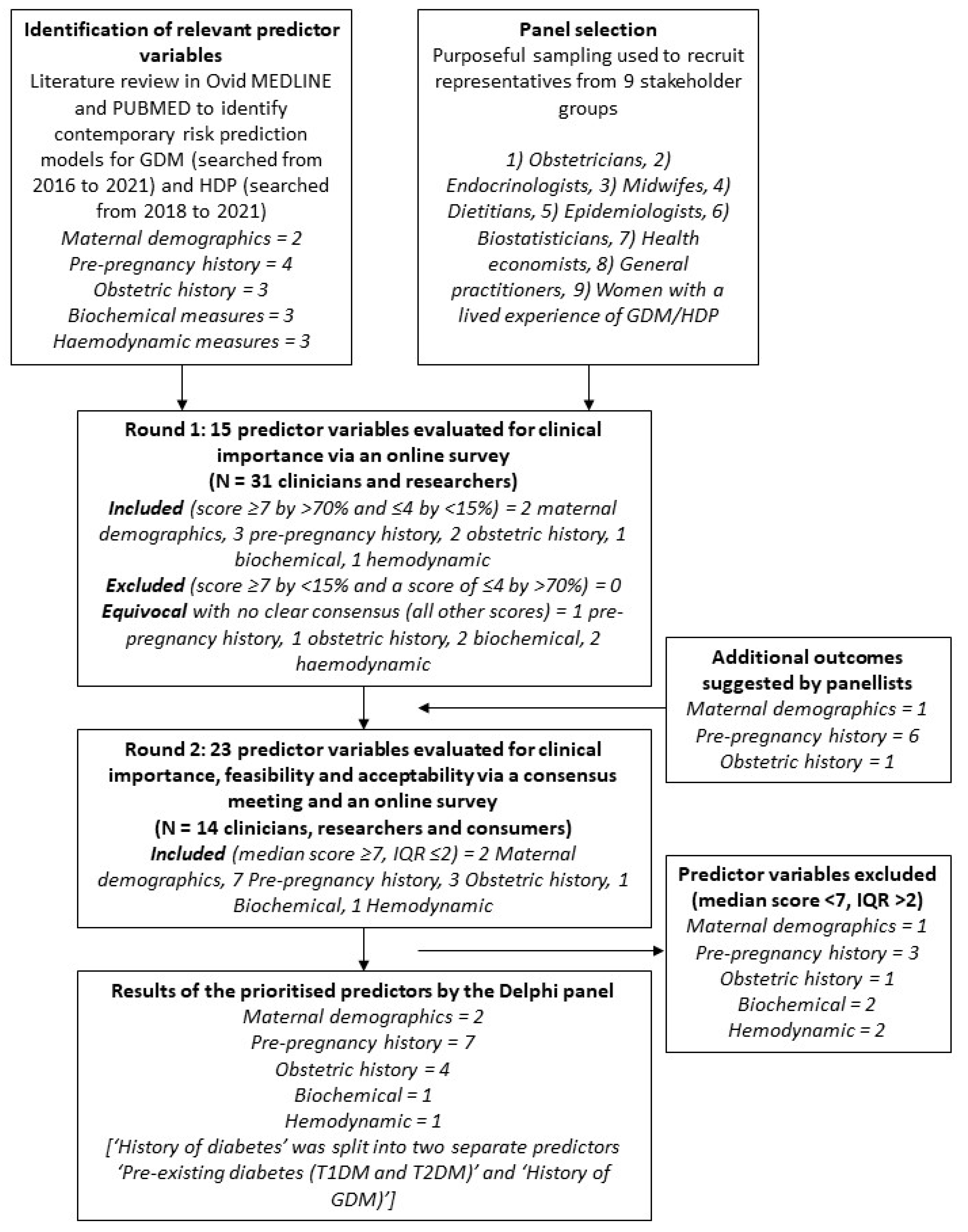
2.1. Identification of Predictor Variables for the Risk Prediction Tool
2.2. Panel Selection
2.3. E-Delphi Round One Online Survey
2.4. Consensus Meeting and Round Two Online Survey
2.5. Data Analysis
2.6. Ethics Statement
3. Results
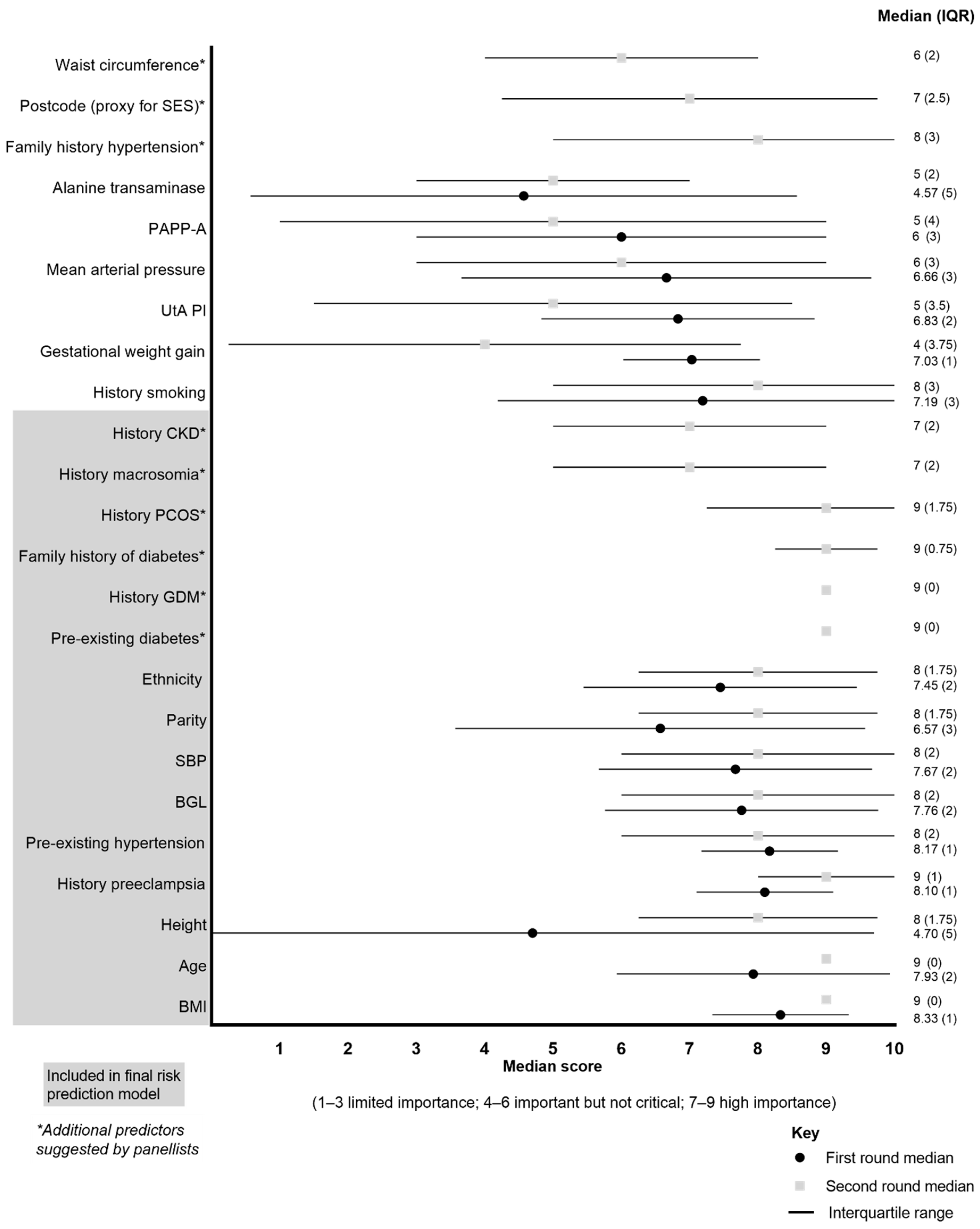
3.1. E-Delphi Round One Online Survey
3.2. Consensus Meeting and Round Two Online Survey
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parikh, N.I.; Gonzalez, J.M.; Anderson, C.A.M.; Judd, S.E.; Rexrode, K.M.; Hlatky, M.A.; Gunderson, E.P.; Stuart, J.J.; Vaidya, D.; American Heart Association Council on Epidemiology and Prevention; et al. Adverse Pregnancy Outcomes and Cardiovascular Disease Risk: Unique Opportunities for Cardiovascular Disease Prevention in Women: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e902–e916. [Google Scholar] [CrossRef]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019, 145 (Suppl. S1), 1. [Google Scholar] [CrossRef] [PubMed]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Cabero Roura, L.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. S3), S173–S211. [Google Scholar] [CrossRef] [PubMed]
- Duley, L.; Meher, S.; Hunter, K.E.; Seidler, A.L.; Askie, L.M. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2019, 2019, CD004659. [Google Scholar] [CrossRef]
- Teede, H.J.; Bailey, C.; Moran, L.J.; Bahri Khomami, M.; Enticott, J.; Ranasinha, S.; Rogozinska, E.; Skouteris, H.; Boyle, J.A.; Thangaratinam, S.; et al. Association of Antenatal Diet and Physical Activity-Based Interventions With Gestational Weight Gain and Pregnancy Outcomes: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2022, 182, 106–114. [Google Scholar] [CrossRef]
- Rossello, X.; Dorresteijn, J.A.N.; Janssen, A.; Lambrinou, E.; Scherrenberg, M.; Bonnefoy-Cudraz, E.; Cobain, M.; Piepoli, M.F.; Visseren, F.L.J.; Dendale, P. Risk prediction tools in cardiovascular disease prevention: A report from the ESC Prevention of CVD Programme led by the European Association of Preventive Cardiology (EAPC) in collaboration with the Acute Cardiovascular Care Association (ACCA) and the Association of Cardiovascular Nursing and Allied Professions (ACNAP). Eur. J. Prev. Cardiol. 2020, 9, 522–532. [Google Scholar] [CrossRef]
- Wolff, R.F.; Moons, K.G.M.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S.; Groupdagger, P. PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann. Intern. Med. 2019, 170, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cowley, L.E.; Farewell, D.M.; Maguire, S.; Kemp, A.M. Methodological standards for the development and evaluation of clinical prediction rules: A review of the literature. Diagn. Progn. Res. 2019, 3, 16. [Google Scholar] [CrossRef]
- Thong, E.P.; Ghelani, D.P.; Manoleehakul, P.; Yesmin, A.; Slater, K.; Taylor, R.; Collins, C.; Hutchesson, M.; Lim, S.S.; Teede, H.J.; et al. Optimising Cardiometabolic Risk Factors in Pregnancy: A Review of Risk Prediction Models Targeting Gestational Diabetes and Hypertensive Disorders. J. Cardiovasc. Dev. Dis. 2022, 9, 55. [Google Scholar] [CrossRef]
- Foster, A.B.; Park, F.; Hyett, J. Do first-trimester screening algorithms for preeclampsia aligned to use of preventative therapies reduce the prevalence of pre-term preeclampsia: A systematic review and meta-analysis. Prenat. Diagn. 2023, 43, 950–958. [Google Scholar] [CrossRef]
- Boulkedid, R.; Abdoul, H.; Loustau, M.; Sibony, O.; Alberti, C. Using and reporting the Delphi method for selecting healthcare quality indicators: A systematic review. PLoS ONE 2011, 6, e20476. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, M.A.; McAuliffe, F.M. Prediction and prevention of Gestational Diabetes: An update of recent literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 202, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Antwi, E.; Amoakoh-Coleman, M.; Vieira, D.L.; Madhavaram, S.; Koram, K.A.; Grobbee, D.E.; Agyepong, I.A.; Klipstein-Grobusch, K. Systematic review of prediction models for gestational hypertension and preeclampsia. PLoS ONE 2020, 15, e0230955. [Google Scholar] [CrossRef]
- Lamain-de Ruiter, M.; Kwee, A.; Naaktgeboren, C.A.; Franx, A.; Moons, K.G.M.; Koster, M.P.H. Prediction models for the risk of gestational diabetes: A systematic review. Diagn. Progn. Res. 2017, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Al Wattar, B.H.; Teede, H.; Garad, R.; Franks, S.; Balen, A.; Bhide, P.; Piltonen, T.; Romualdi, D.; Laven, J.; Thondan, M.; et al. Harmonising research outcomes for polycystic ovary syndrome: An international multi-stakeholder core outcome set. Hum. Reprod. 2020, 35, 404–412. [Google Scholar] [CrossRef]
- Ven, V.; Delbecq, A. Nominal Versus Interacting Group Process For Committee Decision-Making Effectiveness. Acad. Manag. J. 1971, 14, 203–212. [Google Scholar] [CrossRef]
- Shillinglaw, B.; Viera, A.J.; Edwards, T.; Simpson, R.; Sheridan, S.L. Use of global coronary heart disease risk assessment in practice: A cross-sectional survey of a sample of U.S. physicians. BMC Health Serv. Res. 2012, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Redman, C.W.; Staff, A.C.; Hansson, S.; Wilson, M.L.; Laivuori, H.; Poston, L.; Roberts, J.M.; Global Pregnancy, C. Strategy for standardization of preeclampsia research study design. Hypertension 2014, 63, 1293–1301. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 2022, 27, 148–169. [Google Scholar] [CrossRef]
- Junger, S.; Payne, S.A.; Brine, J.; Radbruch, L.; Brearley, S.G. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: Recommendations based on a methodological systematic review. Palliat. Med. 2017, 31, 684–706. [Google Scholar] [CrossRef] [PubMed]
- Avella, J. Delphi Panels: Research Design, Procedures, Advantages, and Challenges. Int. J. Dr. Stud. 2016, 11, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.; Clark, B.; Brown, V.; Sitzia, J. Good practice in the conduct and reporting of survey research. Int. J. Qual. Health Care J. Int. Soc. Qual. Health Care 2003, 15, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Beiderbeck, D.; Frevel, N.; von der Gracht, H.A.; Schmidt, S.L.; Schweitzer, V.M. Preparing, conducting, and analyzing Delphi surveys: Cross-disciplinary practices, new directions, and advancements. MethodsX 2021, 8, 101401. [Google Scholar] [CrossRef]
- Adam, S.; Rheeder, P. Selective screening strategies for gestational diabetes: A prospective cohort observational study. J. Diabetes Res. 2017, 2017, 2849346. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, K.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; De Block, C.; et al. Estimating the risk of gestational diabetes mellitus based on the 2013 WHO criteria: A prediction model based on clinical and biochemical variables in early pregnancy. Acta Diabetol. 2020, 57, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Capula, C.; Chiefari, E.; Borelli, M.; Oliverio, R.; Vero, A.; Foti, D.; Puccio, L.; Vero, R.; Brunetti, A. A new predictive tool for the early risk assessment of gestational diabetes mellitus. Prim. Care Diabetes 2016, 10, 315–323. [Google Scholar] [CrossRef]
- Donovan, B.M.; Breheny, P.J.; Robinson, J.G.; Baer, R.J.; Saftlas, A.F.; Bao, W.; Greiner, A.L.; Carter, K.D.; Oltman, S.P.; Rand, L.; et al. Development and validation of a clinical model for preconception and early pregnancy risk prediction of gestational diabetes mellitus in nulliparous women. PLoS ONE 2019, 14, e0215173. [Google Scholar] [CrossRef]
- Gao, S.; Leng, J.; Liu, H.; Wang, S.; Li, W.; Wang, Y.; Hu, G.; Chan, J.C.N.; Yu, Z.; Zhu, H.; et al. Development and validation of an early pregnancy risk score for the prediction of gestational diabetes mellitus in Chinese pregnant women. BMJ Open Diabetes Res. Care 2020, 8, e000909. [Google Scholar] [CrossRef]
- Guo, F.; Yang, S.; Zhang, Y.; Yang, X.; Zhang, C.; Fan, J. Nomogram for prediction of gestational diabetes mellitus in urban, Chinese, pregnant women. BMC Pregnancy Childbirth 2020, 20, 43. [Google Scholar] [CrossRef]
- Schaefer, K.K.; Xiao, W.; Chen, Q.; He, J.; Lu, J.; Chan, F.; Chen, N.; Yuan, M.; Xia, H.; Lam, K.B.H.; et al. Prediction of gestational diabetes mellitus in the Born in Guangzhou Cohort Study, China. Int. J. Gynaecol. Obstet. 2018, 143, 164–171. [Google Scholar] [CrossRef]
- Schoenaker, D.A.; Vergouwe, Y.; Soedamah-Muthu, S.S.; Callaway, L.K.; Mishra, G.D. Preconception risk of gestational diabetes: Development of a prediction model in nulliparous Australian women. Diabetes Res. Clin. Pract. 2018, 146, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Snyder, B.M.; Baer, R.J.; Oltman, S.P.; Robinson, J.G.; Breheny, P.J.; Saftlas, A.F.; Bao, W.; Greiner, A.L.; Carter, K.D.; Rand, L.; et al. Early pregnancy prediction of gestational diabetes mellitus risk using prenatal screening biomarkers in nulliparous women. Diabetes Res. Clin. Pract. 2020, 163, 108139. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.N.; Appelblom, H.; Ross, G.P.; Wong, J.; Kouru, H.; Williams, P.F.; Sairanen, M.; Hyett, J.A. First trimester prediction of gestational diabetes mellitus: A clinical model based on maternal demographic parameters. Diabetes Res. Clin. Pract. 2017, 127, 44–50. [Google Scholar] [CrossRef]
- Sweeting, A.N.; Wong, J.; Appelblom, H.; Ross, G.P.; Kouru, H.; Williams, P.F.; Sairanen, M.; Hyett, J.A. A first trimester prediction model for gestational diabetes utilizing aneuploidy and pre-eclampsia screening markers. J. Matern.-Fetal Neonatal Med. 2018, 31, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.N.; Wong, J.; Appelblom, H.; Ross, G.P.; Kouru, H.; Williams, P.F.; Sairanen, M.; Hyett, J.A. A novel early pregnancy risk prediction model for gestational diabetes mellitus. Fetal Diagn. Ther. 2019, 45, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Thériault, S.; Giguère, Y.; Massé, J.; Girouard, J.; Forest, J.-C. Early prediction of gestational diabetes: A practical model combining clinical and biochemical markers. Clin. Chem. Lab. Med. 2016, 54, 509–518. [Google Scholar] [CrossRef]
- van Hoorn, F.; Koster, M.P.; Kwee, A.; Groenendaal, F.; Franx, A.; Bekker, M.N. Implementation of a first-trimester prognostic model to improve screening for gestational diabetes mellitus. BMC Pregnancy Childbirth 2021, 21, 298. [Google Scholar] [CrossRef] [PubMed]
- White, S.L.; Lawlor, D.A.; Briley, A.L.; Godfrey, K.M.; Nelson, S.M.; Oteng-Ntim, E.; Robson, S.C.; Sattar, N.; Seed, P.T.; Vieira, M.C.; et al. Early antenatal prediction of gestational diabetes in obese women: Development of prediction tools for targeted intervention. PLoS ONE 2016, 11, e0167846. [Google Scholar] [CrossRef]
- Ye, Y.; Xiong, Y.; Zhou, Q.; Wu, J.; Li, X.; Xiao, X. Comparison of machine learning methods and conventional logistic regressions for predicting gestational diabetes using routine clinical data: A retrospective cohort study. J. Diabetes Res. 2020, 2020, 4168340. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Huo, L.; Yuan, N.; Sun, J.; Du, J.; Nan, M.; Ji, L. Risk prediction model of gestational diabetes mellitus based on nomogram in a Chinese population cohort study. Sci. Rep. 2020, 10, 21223. [Google Scholar] [CrossRef]
- Zheng, T.; Ye, W.; Wang, X.; Li, X.; Zhang, J.; Little, J.; Zhou, L.; Zhang, L. A simple model to predict risk of gestational diabetes mellitus from 8 to 20 weeks of gestation in Chinese women. BMC Pregnancy Childbirth 2019, 19, 252. [Google Scholar] [CrossRef]
- Nombo, A.P.; Mwanri, A.W.; Brouwer-Brolsma, E.M.; Ramaiya, K.L.; Feskens, E.J. Gestational diabetes mellitus risk score: A practical tool to predict gestational diabetes mellitus risk in Tanzania. Diabetes Res. Clin. Pract. 2018, 145, 130–137. [Google Scholar] [CrossRef]
- Cooray, S.D.; De Silva, K.; Enticott, J.; Dawadi, S.; Boyle, J.A.; Soldatos, G.; Paul, E.; Versace, V.; Teede, H.J. External validation and updating of a prediction model for the diagnosis of gestational diabetes mellitus. medRxiv 2021. [Google Scholar] [CrossRef]
- Teede, H.J.; Harrison, C.L.; Teh, W.T.; Paul, E.; Allan, C.A. Gestational diabetes: Development of an early risk prediction tool to facilitate opportunities for prevention. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 499–504. [Google Scholar] [CrossRef]
- Sepúlveda-Martínez, A.; Rencoret, G.; Silva, M.C.; Ahumada, P.; Pedraza, D.; Muñoz, H.; Valdés, E.; Parra-Cordero, M. First trimester screening for preterm and term pre-eclampsia by maternal characteristics and biophysical markers in a low-risk population. J. Obstet. Gynaecol. Res. 2019, 45, 104–112. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B.; Sajdak, S.; Lubiński, J. Pre-pregnancy obesity vs. other risk factors in probability models of preeclampsia and gestational hypertension. Nutrients 2020, 12, 2681. [Google Scholar] [CrossRef]
- Mula, R.; Meler, E.; Albaiges, G.; Rodriguez, I. Strategies for the prediction of late preeclampsia. J. Matern. Fetal Neonatal Med. 2019, 32, 3729–3733. [Google Scholar] [CrossRef]
- Yang, L.; Sun, G.; Wang, A.; Jiang, H.; Zhang, S.; Yang, Y.; Li, X.; Hao, D.; Xu, M.; Shao, J. Predictive models of hypertensive disorders in pregnancy based on support vector machine algorithm. Technol. Health Care 2020, 28 (Suppl. S1), 181–186. [Google Scholar] [CrossRef]
- Serra, B.; Mendoza, M.; Scazzocchio, E.; Meler, E.; Nolla, M.; Sabrià, E.; Rodríguez, I.; Carreras, E. A new model for screening for early-onset preeclampsia. Am. J. Obstet. Gynecol. 2020, 222, 608.e1–608.e18. [Google Scholar] [CrossRef] [PubMed]
- Jhee, J.H.; Lee, S.; Park, Y.; Lee, S.E.; Kim, Y.A.; Kang, S.-W.; Kwon, J.-Y.; Park, J.T. Prediction model development of late-onset preeclampsia using machine learning-based methods. PLoS ONE 2019, 14, e0221202. [Google Scholar] [CrossRef] [PubMed]
- Sovio, U.; Smith, G. Evaluation of a simple risk score to predict preterm pre-eclampsia using maternal characteristics: A prospective cohort study. BJOG 2019, 126, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yun, L.; Zhang, L.; Lin, J.; Xu, R. A risk factor-based predictive model for new-onset hypertension during pregnancy in Chinese Han women. BMC Cardiovasc. Disord. 2020, 20, 155. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.; Aquilina, J. Prospective observational study to determine the accuracy of first-trimester serum biomarkers and uterine artery Dopplers in combination with maternal characteristics and arteriography for the prediction of women at risk of preeclampsia and other adverse pregnancy outcomes. J. Matern. Fetal Neonatal Med. 2018, 31, 2789–2806. [Google Scholar] [CrossRef]
- Schaller, S.; Knippel, A.J.; Verde, P.E.; Kozlowski, P. Concordance-analysis and evaluation of different diagnostic algorithms used in first trimester screening for late-onset preeclampsia. Hypertens. Pregnancy 2020, 39, 172–185. [Google Scholar] [CrossRef]
- Murtoniemi, K.; Villa, P.M.; Matomäki, J.; Keikkala, E.; Vuorela, P.; Hämäläinen, E.; Kajantie, E.; Pesonen, A.-K.; Räikkönen, K.; Taipale, P.; et al. Prediction of pre-eclampsia and its subtypes in high-risk cohort: Hyperglycosylated human chorionic gonadotropin in multivariate models. BMC Pregnancy Childbirth 2018, 18, 279. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubaie, Z.T.A.; Hudson, H.M.; Jenkins, G.; Mahmoud, I.; Ray, J.G.; Askie, L.M.; Lord, S.J. Prediction of pre-eclampsia in nulliparous women using routinely collected maternal characteristics: A model development and validation study. BMC Pregnancy Childbirth 2020, 20, 23. [Google Scholar] [CrossRef]
- Antwi, E.; Klipstein-Grobusch, K.; Browne, J.L.; Schielen, P.C.; Koram, K.A.; Agyepong, I.A.; Grobbee, D.E. Improved prediction of gestational hypertension by inclusion of placental growth factor and pregnancy associated plasma protein-a in a sample of Ghanaian women. Reprod. Health 2018, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Pihl, K.; Sørensen, S.; Jørgensen, F.S. Prediction of preeclampsia in nulliparous women according to first trimester maternal factors and serum markers. Fetal Diagn. Ther. 2020, 47, 277–283. [Google Scholar] [CrossRef]
- Sandström, A.; Snowden, J.M.; Höijer, J.; Bottai, M.; Wikström, A.-K. Clinical risk assessment in early pregnancy for preeclampsia in nulliparous women: A population based cohort study. PLoS ONE 2019, 14, e0225716. [Google Scholar] [CrossRef]
- Allotey, J.; Laivuori, H.; Snell, K.I.; Smuk, M.; Hooper, R.; Chan, C.L.; Ahmed, A.; Chappell, L.C.; von Dadelszen, P.; Dodds, J.; et al. Validation and development of models using clinical, biochemical and ultrasound markers for predicting pre-eclampsia: An individual participant data meta-analysis. Health Technol. Assess. 2020, 24, 1. [Google Scholar] [CrossRef]
- O’gorman, N.; Wright, D.; Syngelaki, A.; Akolekar, R.; Wright, A.; Poon, L.C.; Nicolaides, K.H. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am. J. Obstet. Gynecol. 2016, 214, 103.e1–103.e12. [Google Scholar] [CrossRef]
| Participant Characteristic a | Round One (n = 31) | Round Two (n = 14) |
|---|---|---|
| Age, mean ± SD | 42 ± 10 | 43 ± 10 |
| Gender (Female) | 77 (24) | 86 (12) |
| Profession | ||
| Obstetrics and gynecology | 23 (7) | 7 (1) |
| Endocrinologist | 19 (6) | 29 (4) |
| Midwife | 16 (5) | 7 (1) |
| Dietitian | 16 (5) | 14 (2) |
| Epidemiologist | 10 (3) | 14 (2) |
| Biostatistician | 6 (2) | 7 (1) |
| Health economist | 3 (1) | 0 |
| General practitioner | 3 (1) | 7 (1) |
| Consumer with a lived experience of gestational diabetes mellitus | 0 | 14 (2) |
| Did not disclose profession | 3 (1) | 0 |
| Outcome | % of Panelists Ranking Each Outcome as Important (Score > 7/10) |
|---|---|
| Outcomes with consensus in a | |
| Body mass index | 90 |
| Age | 84 |
| Pre-existing (chronic) hypertension | 84 |
| Ethnicity | 81 |
| History pre-eclampsia | 81 |
| Blood glucose level | 81 |
| Systolic blood pressure | 81 |
| History of smoking | 74 |
| Gestational weight gain | 74 |
| Outcomes with consensus out b | |
| Nil | - |
| Outcomes with no consensus c | |
| Mean arterial pressure | 55 |
| Parity | 45 |
| Uterine artery pulsatility index | 39 |
| Pregnancy-associated plasma protein A | 32 |
| Height | 26 |
| Alanine transaminase | 19 |
| Additional variables nominated for consideration | |
| |
| |
| |
| |
| |
| |
| |
| |
| Variable | Reason for Exclusion |
|---|---|
| Gestational weight gain |
|
| Uterine artery pulsatility index |
|
| Alanine transaminase and pregnancy-associated plasma protein A |
|
| Postcode |
|
| Waist circumference and mean arterial pressure |
|
| History of smoking |
|
| Family history of hypertension (including chronic hypertension and HDP) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cowan, S.; Lang, S.; Goldstein, R.; Enticott, J.; Taylor, F.; Teede, H.; Moran, L.J. Identifying Predictor Variables for a Composite Risk Prediction Tool for Gestational Diabetes and Hypertensive Disorders of Pregnancy: A Modified Delphi Study. Healthcare 2024, 12, 1361. https://doi.org/10.3390/healthcare12131361
Cowan S, Lang S, Goldstein R, Enticott J, Taylor F, Teede H, Moran LJ. Identifying Predictor Variables for a Composite Risk Prediction Tool for Gestational Diabetes and Hypertensive Disorders of Pregnancy: A Modified Delphi Study. Healthcare. 2024; 12(13):1361. https://doi.org/10.3390/healthcare12131361
Chicago/Turabian StyleCowan, Stephanie, Sarah Lang, Rebecca Goldstein, Joanne Enticott, Frances Taylor, Helena Teede, and Lisa J. Moran. 2024. "Identifying Predictor Variables for a Composite Risk Prediction Tool for Gestational Diabetes and Hypertensive Disorders of Pregnancy: A Modified Delphi Study" Healthcare 12, no. 13: 1361. https://doi.org/10.3390/healthcare12131361
APA StyleCowan, S., Lang, S., Goldstein, R., Enticott, J., Taylor, F., Teede, H., & Moran, L. J. (2024). Identifying Predictor Variables for a Composite Risk Prediction Tool for Gestational Diabetes and Hypertensive Disorders of Pregnancy: A Modified Delphi Study. Healthcare, 12(13), 1361. https://doi.org/10.3390/healthcare12131361







