Artificial Intelligence and Heart-Brain Connections: A Narrative Review on Algorithms Utilization in Clinical Practice
Abstract
:1. Introduction
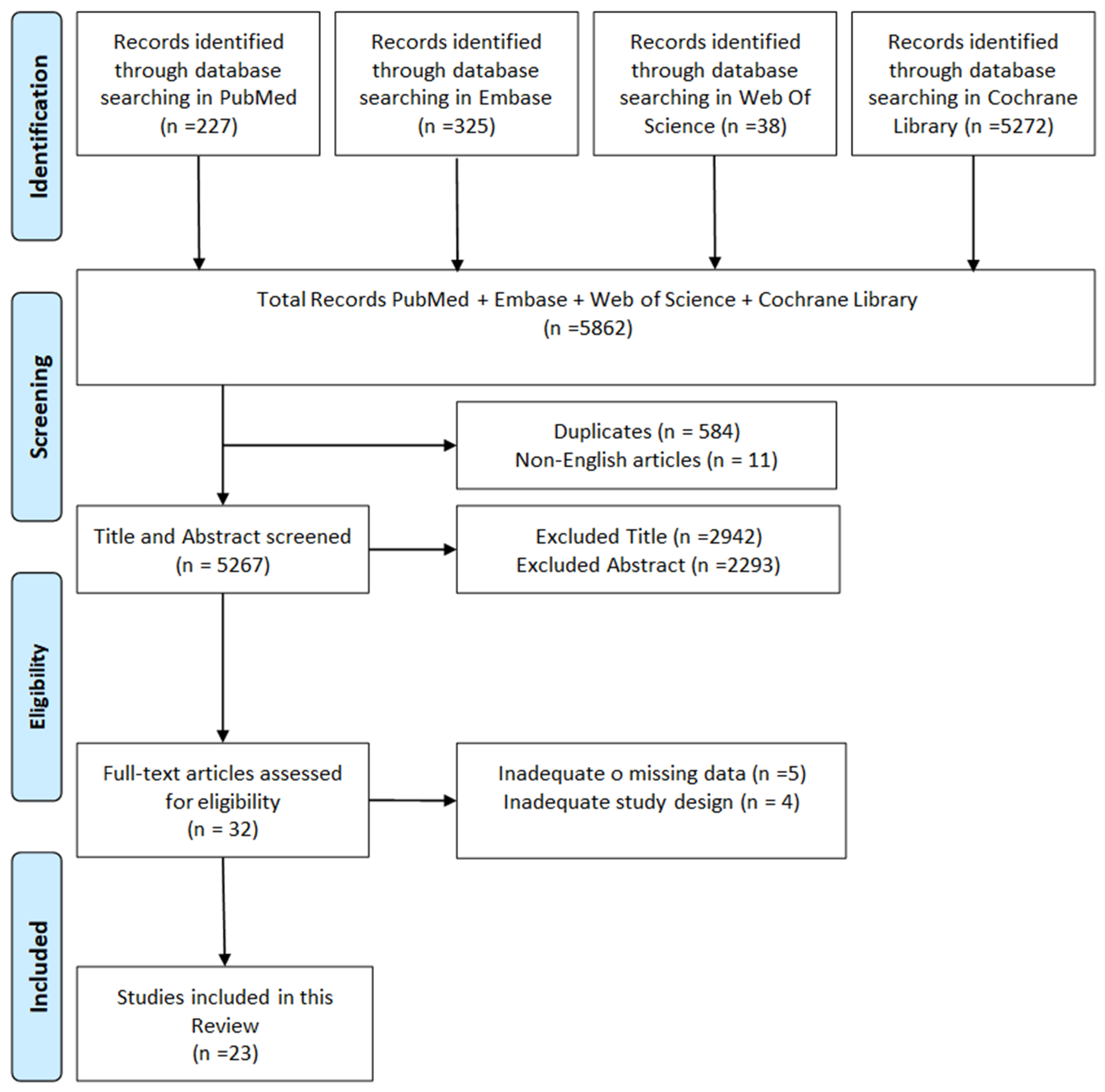
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
- -
- Studies arguing both technical elements of artificial intelligence and clinical elements in cardiology and/or neurology
- -
- Studies written in English;
- -
- No Reviews;
- -
- Studies published within the last 10 years;
- -
- Studies that involved the use of clinical data.
2.3. Exclusion Criteria
3. Results
3.1. Studies Conducted in Neurology and Cardiology
3.2. Studies Conducted in the Field of Cardiology
3.3. Studies Conducted in the Field of Neurology
4. Discussion
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuraini, N.Z.A.; Sekar, M.; Wu, Y.S.; Gan, S.H.; Bonam, S.R.; Mat Rani, N.N.I.; Begum, M.Y.; Lum, P.T.; Subramaniyan, V.; Fuloria, N.K.; et al. Promising Nutritional Fruits Against Cardiovascular Diseases: An Overview of Experimental Evidence and Understanding Their Mechanisms of Action. Vasc. Health Risk Manag. 2021, 17, 739–769. [Google Scholar] [CrossRef] [PubMed]
- Gunata, M.; Parlakpinar, H.; Acet, H.A. Melatonin: A review of its potential functions and effects on neurological diseases. Rev. Neurol. 2020, 176, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Y.; Pan, H.; Han, L. Global, regional, and national burden of neurological disorders in 204 countries and territories worldwide. J. Glob. Health 2023, 13, 04160. [Google Scholar] [CrossRef] [PubMed]
- Isath, A.; Koziol, K.J.; Martinez, M.W.; Garber, C.E.; Martinez, M.N.; Emery, M.S.; Baggish, A.L.; Naidu, S.S.; Lavie, C.J.; Arena, R.; et al. Exercise and cardiovascular health: A state-of-the-art review. Prog. Cardiovasc. Dis. 2023, 79, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, J.; Fang, Y.; Yao, D.; Zhang, L.; Zhou, Y.; Wang, Y.; Hu, L.; Lu, Z.; Wang, Y.; et al. Burden of Common Neurologic Diseases in Asian Countries, 1990-2019: An Analysis for the Global Burden of Disease Study 2019. Neurology 2023, 100, e2141–e2154. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Ciumărnean, L.; Milaciu, M.V.; Negrean, V.; Orășan, O.H.; Vesa, S.C.; Sălăgean, O.; Iluţ, S.; Vlaicu, S.I. Cardiovascular Risk Factors and Physical Activity for the Prevention of Cardiovascular Diseases in the Elderly. Int. J. Environ. Res. Public Health 2021, 19, 207. [Google Scholar] [CrossRef] [PubMed]
- Sha, L.; Li, Y.; Zhang, Y.; Tang, Y.; Li, B.; Chen, Y.; Chen, L. Heart-brain axis: Association of congenital heart abnormality and brain diseases. Front. Cardiovasc. Med. 2023, 10, 1071820. [Google Scholar] [CrossRef] [PubMed]
- Gore, J.C. Artificial intelligence in medical imaging. Magn. Reson. Imaging 2020, 68, A1–A4. [Google Scholar] [CrossRef] [PubMed]
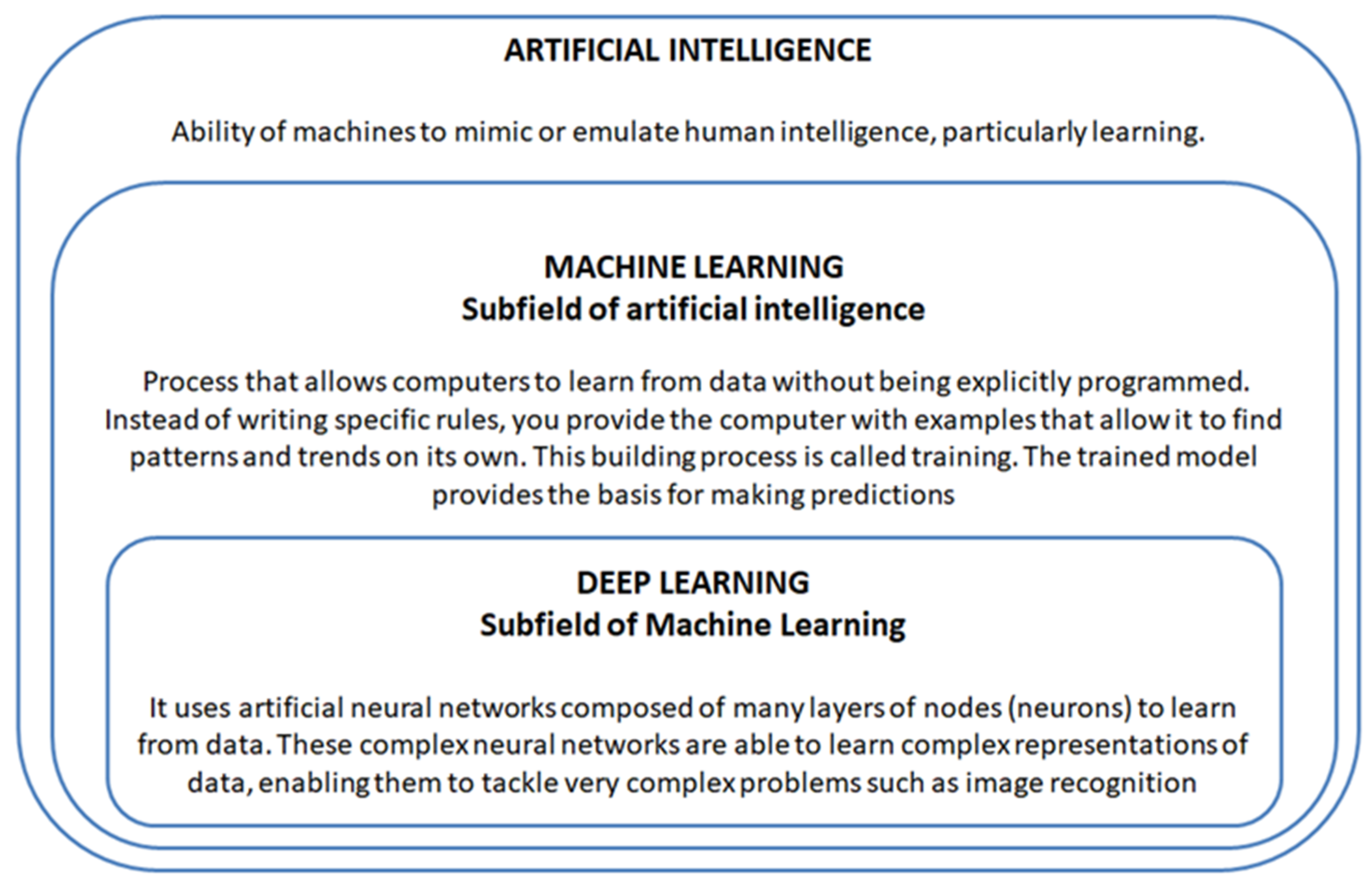
- Erickson, B.J. Basic Artificial Intelligence Techniques: Machine Learning and Deep Learning. Radiol. Clin. N. Am. 2021, 59, 933–940. [Google Scholar] [CrossRef]
- Currie, G.; Hawk, K.E.; Rohren, E.; Vial, A.; Klein, R. Machine Learning and Deep Learning in Medical Imaging: Intelligent Imaging. J. Med. Imaging Radiat. Sci. 2019, 50, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Coyner, A.S.; Kalpathy-Cramer, J.; Chiang, M.F.; Campbell, J.P. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Bini, S.A. Artificial Intelligence, Machine Learning, Deep Learning, and Cognitive Computing: What Do These Terms Mean and How Will They Impact Health Care? J. Arthroplast. 2018, 33, 2358–2361. [Google Scholar] [CrossRef] [PubMed]
- Nazar, M.; Alam, M.M.; Yafi, E.; Su’ud, M.M. A systematic review of human–computer interaction and explainable artificial intelligence in healthcare with artificial intelligence techniques. IEEE Access 2021, 9, 153316–153348. [Google Scholar] [CrossRef]
- Baştanlar, Y.; Özuysal, M. Introduction to machine learning. miRNomics MicroRNA Biol. Comput. Anal. 2014, 1107, 105–128. [Google Scholar] [CrossRef]
- Serre, T. Deep Learning: The Good, the Bad, and the Ugly. Annu. Rev. Vis. Sci. 2019, 5, 399–426. [Google Scholar] [CrossRef] [PubMed]
- Alzubaidi, A.; Tepper, J. Deep Mining from Omics Data. In Data Mining Techniques for the Life Sciences; Springer: New York, NY, USA, 2022; pp. 349–386. [Google Scholar] [CrossRef]
- Andersson, P.; Johnsson, J.; Björnsson, O.; Cronberg, T.; Hassager, C.; Zetterberg, H.; Stammet, P.; Undén, J.; Kjaergaard, J.; Friberg, H.; et al. Predicting neurological outcome after out-of-hospital cardiac arrest with cumulative information; development and internal validation of an artificial neural network algorithm. Crit. Care 2021, 25, 83. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, J.; Björnsson, O.; Andersson, P.; Jakobsson, A.; Cronberg, T.; Lilja, G.; Friberg, H.; Hassager, C.; Kjaergard, J.; Wise, M.; et al. Artificial neural networks improve early outcome prediction and risk classification in out-of-hospital cardiac arrest patients admitted to intensive care. Crit. Care 2020, 24, 474. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Chung, S.H.; Lee, C.N.; Joo, H.J. Deep Learning Algorithm of 12-Lead Electrocardiogram for Parkinson Disease Screening. J. Park. Dis. 2023, 13, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.C.; Chhabra, N.; Chao, C.J.; Wang, H.; Zhang, N.; Lim, E.; Baez-Suarez, A.; Attia, Z.I.; Schwedt, T.J.; Dodick, D.W.; et al. Migraine with aura associates with a higher artificial intelligence: ECG atrial fibrillation prediction model output compared to migraine without aura in both women and men. Headache 2022, 62, 939–951. [Google Scholar] [CrossRef]
- Chiu, W.T.; Chung, C.C.; Huang, C.H.; Chien, Y.S.; Hsu, C.H.; Wu, C.H.; Wang, C.H.; Chiu, H.W.; Chan, L. Predicting the survivals and favorable neurologic outcomes after targeted temperature management by artificial neural networks. J. Formos. Med. Assoc. 2022, 121, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, Y.Y.; Wu, B.J.; Huang, P.W.; Cheng, S.E.; Wu, B.F.; Chen, C.C. Contactless facial video recording with deep learning models for the detection of atrial fibrillation. Sci. Rep. 2022, 12, 281. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, F.; Zhang, J.; Cui, X.; Jiang, F.; Chen, N.; Zhou, J.; Chen, J.; Lin, S.; Zou, J. Using machine learning to predict atrial fibrillation diagnosed after ischemic stroke. Int. J. Cardiol. 2022, 347, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Jamthikar, A.; Gupta, D.; Khanna, N.N.; Saba, L.; Araki, T.; Viskovic, K.; Suri, H.S.; Gupta, A.; Mavrogeni, S.; Turk, M.; et al. A low-cost machine learning-based cardiovascular/stroke risk assessment system: Integration of conventional factors with image phenotypes. Cardiovasc. Diagn. Ther. 2019, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Navi, B.B.; Parikh, N.S.; Merkler, A.E.; Okin, P.M.; Devereux, R.B.; Weinsaft, J.W.; Kim, J.; Cheung, J.W.; Kim, L.K.; et al. Machine learning prediction of stroke mechanism in embolic strokes of undetermined source. Stroke 2020, 51, e203–e210. [Google Scholar] [CrossRef] [PubMed]
- Hsiu, H.; Liu, J.C.; Yang, C.J.; Chen, H.S.; Wu, M.S.; Hao, W.R.; Lee, K.Y.; Hu, C.J.; Wang, Y.H.; Fang, Y.A. Discrimination of vascular aging using the arterial pulse spectrum and machine-learning analysis. Microvasc. Res. 2022, 139, 104240. [Google Scholar] [CrossRef] [PubMed]
- Mazza, O.; Shehory, O.; Lev, N. Machine learning techniques in blood pressure management during the acute phase of ischemic stroke. Front. Neurol. 2022, 12, 743728. [Google Scholar] [CrossRef] [PubMed]
- Shelly, S.; Lopez-Jimenez, F.; Chacin-Suarez, A.; Cohen-Shelly, M.; Medina-Inojosa, J.R.; Kapa, S.; Attia, Z.; Chahal, A.A.; Somers, V.K.; Friedman, P.A.; et al. Accelerated Aging in LMNA Mutations Detected by Artificial Intelligence ECG–Derived Age. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2023; Volume 98, pp. 522–532. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Deng, J.; Liu, X.; Shu, T.; Yin, C.; Duan, M.; Fu, L.; Wang, K.; Zeng, S. Interpretable machine learning for predicting 28-day all-cause in-hospital mortality for hypertensive ischemic or hemorrhagic stroke patients in the ICU: A multi-center retrospective cohort study with internal and external cross-validation. Front. Neurol. 2023, 14, 1185447. [Google Scholar] [CrossRef] [PubMed]
- Iakunchykova, O.; Schirmer, H.; Vangberg, T.; Wang, Y.; Benavente, E.D.; van Es, R.; van de Leur, R.R.; Lindekleiv, H.; Attia, Z.I.; Lopez-Jimenez, F.; et al. Machine-learning-derived heart and brain age are independently associated with cognition. Eur. J. Neurol. 2023, 30, 2611–2619. [Google Scholar] [CrossRef]
- Gruwez, H.; Verbrugge, F.H.; Proesmans, T.; Evens, S.; Vanacker, P.; Rutgers, M.P.; Vanhooren, G.; Bertrand, P.; Pison, L.; Haemers, P.; et al. Smartphone-based atrial fibrillation screening in the general population: Feasibility and impact on medical treatment. European heart journal. Digit. Health 2023, 4, 464–472. [Google Scholar] [CrossRef]
- Khurshid, S.; Friedman, S.; Reeder, C.; Di Achille, P.; Diamant, N.; Singh, P.; Harrington, L.X.; Wang, X.; Al-Alusi, M.A.; Sarma, G.; et al. ECG-Based Deep Learning and Clinical Risk Factors to Predict Atrial Fibrillation. Circulation 2022, 145, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.P.B.; Armengol de la Hoz, M.; Rangasamy, V.; Subramaniam, B. Machine Learning Models with Preoperative Risk Factors and Intraoperative Hypotension Parameters Predict Mortality After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2021, 35, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Debs, N.; Cho, T.H.; Rousseau, D.; Berthezène, Y.; Buisson, M.; Eker, O.; Mechtouff, L.; Nighoghossian, N.; Ovize, M.; Frindel, C. Impact of the reperfusion status for predicting the final stroke infarct using deep learning. NeuroImage Clin. 2021, 29, 102548. [Google Scholar] [CrossRef] [PubMed]
- Qutrio Baloch, Z.; Raza, S.A.; Pathak, R.; Marone, L.; Ali, A. Machine Learning Confirms Nonlinear Relationship between Severity of Peripheral Arterial Disease, Functional Limitation and Symptom Severity. Diagnostics 2020, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khurshid, S.; Choi, S.H.; Friedman, S.; Weng, L.C.; Reeder, C.; Pirruccello, J.P.; Singh, P.; Lau, E.S.; Venn, R.; et al. Genetic Susceptibility to Atrial Fibrillation Identified via Deep Learning of 12-Lead Electrocardiograms. Circulation. Genom. Precis. Med. 2023, 16, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Orozco, L.E.; Knol, R.J.; Sanchez-Catasus, C.A.; Martinez-Manzanera, O.; van der Zant, F.M.; Knuuti, J. Machine learning in the integration of simple variables for identifying patients with myocardial ischemia. J. Nucl. Cardiol. 2020, 27, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Chang, H.J.; Nam, H.S. Use of Machine Learning Classifiers and Sensor Data to Detect Neurological Deficit in Stroke Patients. J. Med. Internet Res. 2017, 19, e120. [Google Scholar] [CrossRef]
- Amiri, M.; Fisher, P.M.; Raimondo, F.; Sidaros, A.; Cacic Hribljan, M.; Othman, M.H.; Kondziella, D. Multimodal prediction of residual consciousness in the intensive care unit: The CONNECT-ME study. Brain 2023, 146, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Momtahen, S.; Momtahen, M.; Ramaseshan, R.; Golnaraghi, F. An Optical Sensory System for Assessment of Residual Cancer Burden in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. Sensors 2023, 23, 5761. [Google Scholar] [CrossRef]
- Łukasiewicz, P.; Harris, A.B.; Bervell, J.A.; McFarland, E.G. Narrative review of influence of prosthesis lateralization on clinical outcomes in reverse shoulder arthroplasty: Glenoid vs. humerus vs. combined. Ann. Jt. 2023, 8, 24. [Google Scholar] [CrossRef]
- Pinton, P. Impact of artificial intelligence on prognosis, shared decision-making, and precision medicine for patients with inflammatory bowel disease: A perspective and expert opinion. Ann. Med. 2023, 55, 2300670. [Google Scholar] [CrossRef]
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A. Global Burden of Cardiovascular Diseases and Risks Collaborators Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef]
- Chugh, V.; Basu, A.; Kaushik, A.; Bhansali, S.; Basu, A.K. Employing nano-enabled artificial intelligence (AI)-based smart technologies for prediction, screening, and detection of cancer. Nanoscale 2024, 16, 5458–5486. [Google Scholar] [CrossRef]
- Almansouri, N.E.; Awe, M.; Rajavelu, S.; Jahnavi, K.; Shastry, R.; Hasan, A.; AlAbbasi, R.K. Early Diagnosis of Cardiovascular Diseases in the Era of Artificial Intelligence: An In-Depth Review. Cureus 2024, 16, e55869. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, I.; Dizonno, V.; LeComte, K.; Carr, A.; El Kalza, Y.; Shymka, M.; Field, T.S. We have dealt with so much. There’s more coming?: Improving Knowledge About Brain Health in Adults Living With Congenital Heart Disease. CJC Pediatr. Congenit. Heart Dis. 2024, 3, 1–10. [Google Scholar] [CrossRef]
- Rigatti, S.J. Random forest. J. Insur. Med. 2017, 47, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Sousa Júnior, E.; Freitas, A.; Rabelo, R.; Santos, W. Estimation of Radial Basis Function Network Centers via Information Forces. Entropy 2022, 24, 1347. [Google Scholar] [CrossRef] [PubMed]
- Sperandei, S. Understanding logistic regression analysis. Biochem. Medica. 2014, 24, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Noble, W.S. What is a support vector machine? Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef]
- Muzammil, M.A.; Javid, S.; Afridi, A.K.; Siddineni, R.; Shahabi, M.; Haseeb, M.; Fariha, F.N.U.; Kumar, S.; Zaveri, S.; Nashwan, A.J. Artificial intelligence-enhanced electrocardiography for accurate diagnosis and management of cardiovascular diseases. J. Electrocardiol. 2024, 83, 30–40. [Google Scholar] [CrossRef]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Oreto, G.; Luzza, F.; Satullo, G.; Donato, A.; Carbone, V.; Calabrò, M.P. TachiCardia a QRS larghi: Un problema antico e nuovo [Wide QRS complex tachycardia: An old and new problem]. G. Ital. Cardiol. 2009, 10, 580–595. [Google Scholar]
- Regoli, F.D.; Cattaneo, M.; Kola, F.; Thartori, A.; Bytyci, H.; Saccarello, L.; Amoruso, M.; Di Valentino, M.; Menafoglio, A. Management of hemodynamically stable wide QRS complex tachycardia in patients with implantable cardioverter defibrillators. Front. Cardiovasc. Med. 2023, 9, 1011619. [Google Scholar] [CrossRef] [PubMed]
- Chiatto, L.M.; Corallo, F.; Calabrò, R.S.; Cardile, D.; Pagano, M.; Cappadona, I. A systematic review about the importance of neuropsychological features in heart failure: Is at heart the only failure? Neurol. Sci. 2024, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.T.; Wenner, M.M.; Charkoudian, N. Differential influences of dietary sodium on blood pressure regulation based on race and sex. Auton. Neurosci. Basic Clin. 2021, 236, 102873. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Kadomatsu, S.; Fujisawa, M.; Fukaguchi, K.; Ishizawa, R.; Kanda, N.; Kasugai, D.; Nakajima, M.; Goto, T.; Tsugawa, Y. The Accuracy and Potential Racial and Ethnic Biases of GPT-4 in the Diagnosis and Triage of Health Conditions: Evaluation Study. JMIR Med. Educ. 2023, 9, e47532. [Google Scholar] [CrossRef] [PubMed]
- Serbaya, S.H.; Khan, A.A.; Surbaya, S.H.; Alzahrani, S.M. Knowledge, Attitude and Practice Toward Artificial Intelligence Among Healthcare Workers in Private Polyclinics in Jeddah, Saudi Arabia. Adv. Med. Educ. Pract. 2024, 15, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Bourazana, A.; Xanthopoulos, A.; Briasoulis, A.; Magouliotis, D.; Spiliopoulos, K.; Athanasiou, T.; Vassilopoulos, G.; Skoularigis, J.; Triposkiadis, F. Artificial Intelligence in Heart Failure: Friend or Foe? Life 2024, 14, 145. [Google Scholar] [CrossRef]
- Amiri, Z.; Heidari, A.; Zavvar, M.; Navimipour, N.J.; Esmaeilpour, M. The applications of nature-inspired algorithms in Internet of Things-based healthcare service: A systematic literature review. Trans. Emerg. Telecommun. Technol. 2024, 35, e4969. [Google Scholar] [CrossRef]
- Aminizadeh, S.; Heidari, A.; Dehghan, M.; Toumaj, S.; Rezaei, M.; Navimipour, N.J.; Unal, M. Opportunities and challenges of artificial intelligence and distributed systems to improve the quality of healthcare service. Artif. Intell. Med. 2024, 149, 102779. [Google Scholar] [CrossRef]
- Pagano, M.; Corallo, F.; Anselmo, A.; Giambò, F.M.; Micali, G.; Duca, A.; Cappadona, I. Optimisation of Remote Monitoring Programmes in Heart Failure: Evaluation of Patient Drop-Out Behaviour and Healthcare Professionals’ Perspectives. Healthcare 2024, 12, 1271. [Google Scholar] [CrossRef]
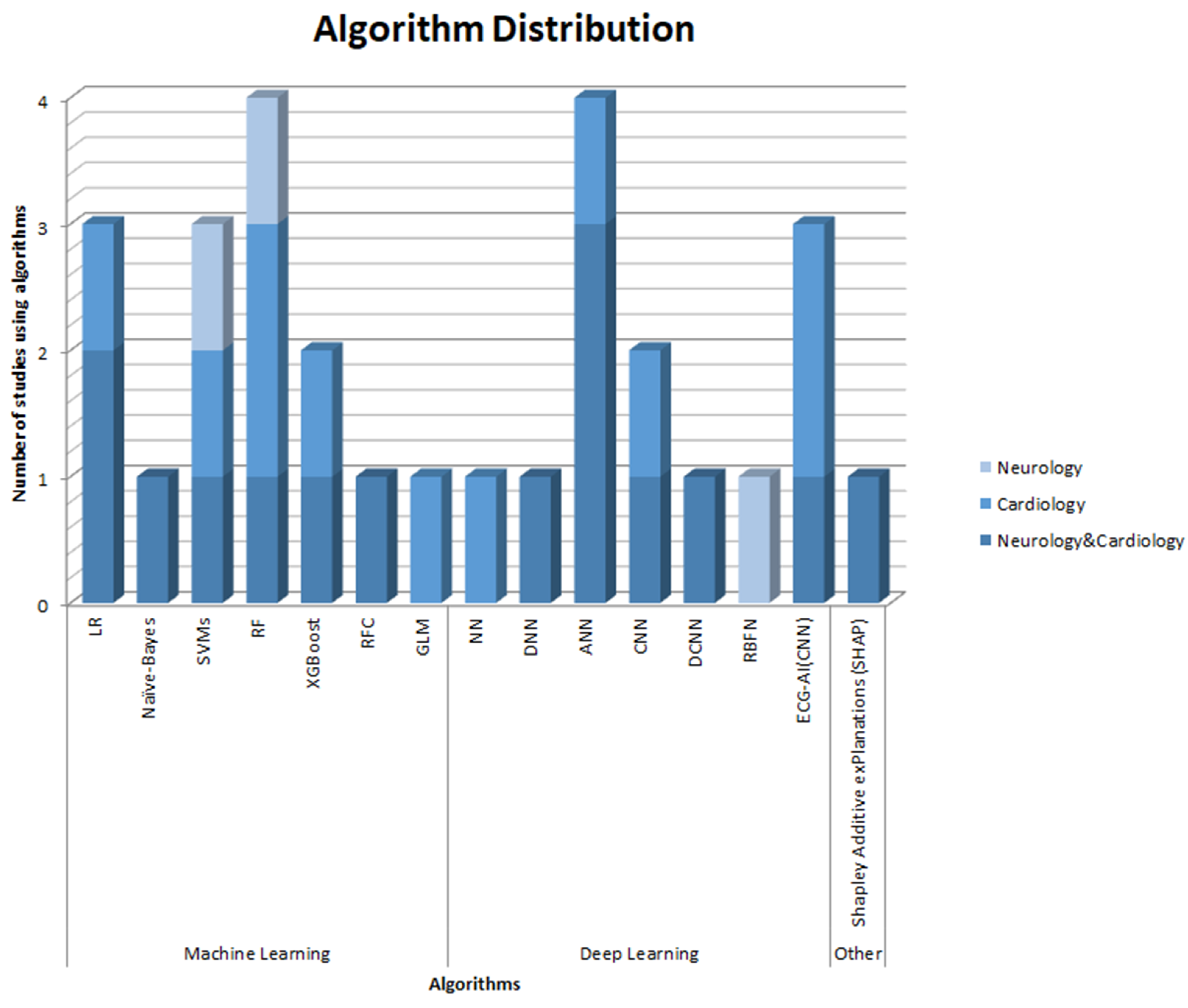

| Area | References | Aim | Sample (N) | Observation Area | Devices Used | AI Algorithm | Results |
|---|---|---|---|---|---|---|---|
| Neurology and Cardiology | Andersson et al., 2021 [18] | Predicting neurological outcome post OHCA using ANN with and without biomarkers | 932 patients By target temperature management study | Prognosis | Missing. | ANN with and without biomarkers; Bayesian algorithms; SHAP algorithms | ANN provide good-to-excellent prognostic accuracy in predicting neurological outcome in comatose patients post OHCA, with an AUROC between 82% and 94% depending on the variables. |
| Johnsson et al., 2020 [19] | Studying the effects of the intervention on the severity classes in patients with cardiac arrest treated with TTM, through a model based on ANN. | 932 patients | Prognosis Predictive | Missing. | Supervised machine learning model based on ANN. | The ANN model has produced better results compared to the logistic regression model. The ANN can be used to stratify a heterogeneous experimental population into risk classes and help determine the effects of intervention among subgroups (AUC 0.852). | |
| Yoo et al., 2023 [20] | Develop a deep learning algorithm for ECG capable of efficiently screening for IPD | 751 patients IPD (experimental group); 751 patients without IPD (control group); 297 patients with DPD. | Diagnosis | Missing. | Deep Learning algorithm based on CNN. | The CNN-based deep learning model using 12-lead ECG had relatively accurate performance in identifying patients with IPD (AUC 0.85). | |
| Chiang et al., 2022 [21] | Investigating the probability of subclinical AF predicted by an artificial intelligence-enabled algorithm on a single ECG in patients with MwA and MwoA. | 17,840 patients MwA; 22,162 patients MwoA | Predictive | Missing. | AI-ECG based on a CNN | Using a new AI-ECG algorithm, it has been shown that patients with MwA have a significantly higher prediction model output of AF, with an accuracy of 83.3%. | |
| Chiu et al., 2022 [22] | Identifying predictors associated with outcomes for targeted temperature management (TTM) to predict survival and neurological outcomes for patients with ROSC treated with TTM. | 580 patients with cardiac arrest and ROSC treated with TTM. | Predictive | Missing. | ANN (AUC 0.841); LR; RF. | ANN techniques can accurately predict survival and neurological outcomes with higher sensitivity compared to LR and RF (ANN sensitivity 71.6; LR sensitivity 40.5, RF sensitivity 45.3). | |
| Sun et al., 2022 [23] | Estimating the measurement capacity of rPPG with DL models in discriminating AF from non-AF | 105 patients with AF (first group); 116 patients NSR (second group); 232 patients with abnormal ECG but normal AF (third group). | Predictive | Digital camera | DCNN | The algorithm has demonstrated an accuracy of 90% and a positive predictive value of 82%. | |
| Zheng et al., 2022 [24] | Developing a DNN model to select AIS patients at high risk of post-stroke AF for prolonged cardiac monitoring and comparing the model with other Machine Learning models. | 3929 patients affected by AIS | Predictive Monitoring | Missing. | 5 models of Machine Learning: LR; RFC; SVM; XGBoost; DNN. | The DNN model achieved the highest specificity (94%), the highest positive predictive value (51%), and the highest accuracy (93%) compared to other models in identifying patients with AIS at high risk of post-stroke atrial fibrillation. | |
| Jamthikar et al., 2019 [25] | Develop an accurate and cost-effective ML-based system with stenosis such as EEGS that can be used for routine CV/stroke risk assessment of patients | 202 patients | Diagnosis | Missing | RF. | The system demonstrated an 18% improvement in the ML approach integrated with clinical data such as stenosis compared to the conventional ML approach (AUC = 0.68). | |
| Kamel et al., 2020 [26] | Training a machine learning algorithm to distinguish cardioembolic from non-cardioembolic strokes using data from the Cornell Acute Stroke Academic Registry. | 1663 patients: 1083 with known stroke aetiology, 580 with cryptogenic stroke | Diagnosis Predictive | Missing | XGBoost; RF; LR; MARS. | A machine learning algorithm trained on demographic, clinical, echocardiographic, and laboratory data was able to distinguish known cardioembolic strokes from known non-cardioembolic strokes with excellent accuracy (85%). | |
| Hsiu et al., 2022 [27] | To determine the effectiveness of using arterial pulse wave measurements, frequency domain pulse analysis, and machine learning analysis in distinguishing vascular aging. | 100 patients | Diagnosis | Pressure transducer (KFG-2-120-D1-11, Kyowa); Sphygmomanometer (MG150f, Rossmax) | MLP; RF. | This study found significant differences in BPW spectral indices between vascular aging and control subjects. MLP and RF are useful for identifying vascular aging through these indices, with accuracy >80%. | |
| Mazza et al., 2022 [28] | Development of a decision support tool to improve the management of extremely high blood pressure during the first 24 h after acute ischemic stroke using ML tools. | 7265 patients with acute ischemic stroke. | Predictive Monitoring | Missing | Decision tree; RF; MLP; LR. | Antihypertensive treatment in the context of acute ischemic stroke should be adapted to different blood pressure levels and clinical characteristics of the patient, thus providing a better decision-making approach. ML techniques are useful for discovering hidden patterns from data (accuracy 97%) and applying robust queries to datasets, but they pose the risk of overfitting. | |
| Shelly et al., 2023 [29] | Demonstrate premature aging in patients with lamin A/C (LMNA) gene mutations after hypothesizing that they have a biological age greater than chronological age | 31 LMNA patients | Predictive | Missing | AI-ECG | AI-ECG predicted that patients with LMNA have a biological age greater than the chronological age and accelerated aging even in the absence of cardiac abnormalities by traditional methods. Raw ECG signals can identify chronological age and, importantly, differential aging rates in a genetic disease caused by mutations in LMNA. | |
| Huang et al., 2023 [30] | Development of an IML model that can accurately predict 28-day all-cause mortality in hypertensive patients with ischemic or haemorrhagic stroke. | 2526 hypertensive patients with ischemic or haemorrhagic stroke admitted to intensive care. | Predictive | Missing | ANN; GBM; XGBoost; LR; SVM. | The IML model developed in this study has potential in clinical practice, as it can help personalize prevention and strengthen therapeutic strategies (mean AUC value 75%). | |
| Iakunchykova et al., 2023 [31] | To study the effect of HDA on cognitive performance and estimation of BDA for a subgroup of participants through MRI imaging to study the relationship between the two ML-based age estimators and its possible role as a mediator of the relationship between HDA and cognitive function. | 7779 participants by a Norwegian cohort: of which 1694 had T1-weighted structural MRI available. | Prognosis Predictive | Schiller AT104 PC [ECG] | DNN | Delta cardiac age, which represents the cumulative effects of lifetime exposures, was associated with brain age. HDA was associated with cognitive function that was minimally explained through BDA. | |
| Cardiology | Gruwez et al., 2023 [32] | To determine the feasibility, detection rate, and therapeutic implications of a large-scale screening in AF patients through smartphones. | 60,629 patients | Diagnosis Monitoring | Smartphone. | Smartphone application based on PPG technology (FibriCheck©, Qompium, Hasselt, Belgium). | AF screening based on smartphones is feasible on a large scale. Indeed, the quality of the photoplethysmography signal was sufficient for the analysis of 88% of the measurements. Furthermore, the algorithm classified the heart rate as normal in 80% of the measurements (without taking further actions), and as suspected AF in 10,231 (1.7%) measurements (confirmed after offline validation in 2998 measurements). |
| Khurshid et al., 2022 [33] | Training of a convolutional neural network (“ECG-AI”) to explicitly predict the time to the onset of atrial fibrillation. | 45,770 patients | Predictive | Missing. | ECG-AI. | The ECG-AI can allow for an efficient quantification of future risk of atrial fibrillation. Indeed, the algorithm has achieved a reported positive predictive value of 92% for AF. | |
| Fernandes et al., 2021 [34] | Improvement in mortality prediction accuracy after cardiac surgery by machine learning models incorporating intraoperative risk factors. | 5015 adult patients undergoing cardiac surgery | Predictive | Missing. | LR; RF; ANN; SVM; XGBoost. | Machine learning models that incorporate intraoperative adverse factors have the potential to provide better discriminative ability for risk stratification and patient triage after cardiac surgery. Indeed, all five models demonstrated good predictive ability (AUROC values ≥ 0.7). In particular, the XGB model showed better predictive ability, PPV, specificity, and sensitivity compared to the other models. | |
| Debs et al., 2021 [35] | Impact of reperfusion state integration on the performance of deep learning models in predicting final infarct in patients with proximal intracranial occlusions treated with thrombectomy, and comparison of results with current clinical prediction methods. | 74 patients with reperfusion; 35 patients without reperfusion | Prognosis Predictive | Missing. | CNN. | CNN-based models achieved higher DSC and AUC values compared to those of the perfusion-diffusion mismatch models. The CNN models achieved good accuracy, measured with an average DSC of 78%. | |
| Qutrio Baloch et al., 2020 [36] | Using machine learning to explore the relationship between functional limitation and symptom severity and severity of PAD. | 703 patients with confirmed or suspected PAD | Predictive | Missing. | Supervised ML composed of RF, NN, and GLM | ML has shown that the severity of symptoms evaluated by the quality of life questionnaire is a highly important variable among patients with PAD. The algorithm achieved a positive predictive value of 66%, a specificity of 92%, and a sensitivity of 26%. | |
| Wang et al., 2023 [37] | Study on genetic association through atrial fibrillation risk estimates generated by the ECG-AI model to evaluate the genetic basis reflected by the output. | 39,986 patients | Predictive | Missing | ECG-AI | ECG-AI models can identify individuals at risk of disease through specific biological pathways. Indeed, by estimating the genetic correlation between the risks of AF predicted by ECG-AI and CHARGE-AF, a significant correlation of 39.3% was found. | |
| Juarez-Orozco et al., 2020 [38] | To evaluate the feasibility and performance of ML in identifying patients with ischemia or high risk of MACE determined through quantitative myocardial perfusion reserve (MPR) PET | 1234 patients underwent PET with nitrogen-13 ammonia | Diagnosis | Missing | LogitBoost algorithms; Naïve Bayes algorithms; RF; SVM. | ML is feasible and applicable to identify patients who will have myocardial ischemia and an elevated risk of MACE through simple predictors and quantitative myocardial perfusion PET imaging (AUC 0.71). | |
| Neurology | Park et al., 2017 [39] | Development of a PDT tool with machine learning classifiers to detect stroke symptoms | 16 patients with Stroke; 10 healthy patients | Diagnosis | Smartphone; 2 detection devices (positioned on each subject’s wrists) | SVM; RBFN; RF. | Sensors and machine learning methods can reliably detect signs of stroke and quantify proximal arm weakness. Indeed, machine learning-based classifiers have been shown to correctly classify up to 92.3% of PDT cases. |
| Amiri et al., 2023 [40] | To evaluate the accuracy of fMRI and EEG to identify residual consciousness in acute DoC in the ICU. | 87 DoC patients: 51 with UWS and 36 with MCS. | Predictive | Missing | RF; SVM. | By combining individual EEG- and fMRI-based models, an optimal combination of overall model performance, positive predictive value, and sensitivity can be achieved (AUC 79%). |
| Algorithms | Strengths | Weaknesses |
|---|---|---|
| Random Forest (RF) |
|
|
| Logistic Regression (LR) |
|
|
| Support-Vector Machines (SVMs) |
|
|
| XGBoost |
|
|
| Artificial Neural Networks (ANN) |
|
|
| Multi-Layer Perceptron (MLP) |
|
|
| Convolutional Neural Network (CNN) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micali, G.; Corallo, F.; Pagano, M.; Giambò, F.M.; Duca, A.; D’Aleo, P.; Anselmo, A.; Bramanti, A.; Garofano, M.; Mazzon, E.; et al. Artificial Intelligence and Heart-Brain Connections: A Narrative Review on Algorithms Utilization in Clinical Practice. Healthcare 2024, 12, 1380. https://doi.org/10.3390/healthcare12141380
Micali G, Corallo F, Pagano M, Giambò FM, Duca A, D’Aleo P, Anselmo A, Bramanti A, Garofano M, Mazzon E, et al. Artificial Intelligence and Heart-Brain Connections: A Narrative Review on Algorithms Utilization in Clinical Practice. Healthcare. 2024; 12(14):1380. https://doi.org/10.3390/healthcare12141380
Chicago/Turabian StyleMicali, Giuseppe, Francesco Corallo, Maria Pagano, Fabio Mauro Giambò, Antonio Duca, Piercataldo D’Aleo, Anna Anselmo, Alessia Bramanti, Marina Garofano, Emanuela Mazzon, and et al. 2024. "Artificial Intelligence and Heart-Brain Connections: A Narrative Review on Algorithms Utilization in Clinical Practice" Healthcare 12, no. 14: 1380. https://doi.org/10.3390/healthcare12141380
APA StyleMicali, G., Corallo, F., Pagano, M., Giambò, F. M., Duca, A., D’Aleo, P., Anselmo, A., Bramanti, A., Garofano, M., Mazzon, E., Bramanti, P., & Cappadona, I. (2024). Artificial Intelligence and Heart-Brain Connections: A Narrative Review on Algorithms Utilization in Clinical Practice. Healthcare, 12(14), 1380. https://doi.org/10.3390/healthcare12141380






