Effect of Online Clinic on Follow-Up Compliance and Survival Outcomes in Nasopharyngeal Carcinoma: Real-World Cohort Study from Endemic Area
Abstract
:1. Introduction
2. Materials and Methods
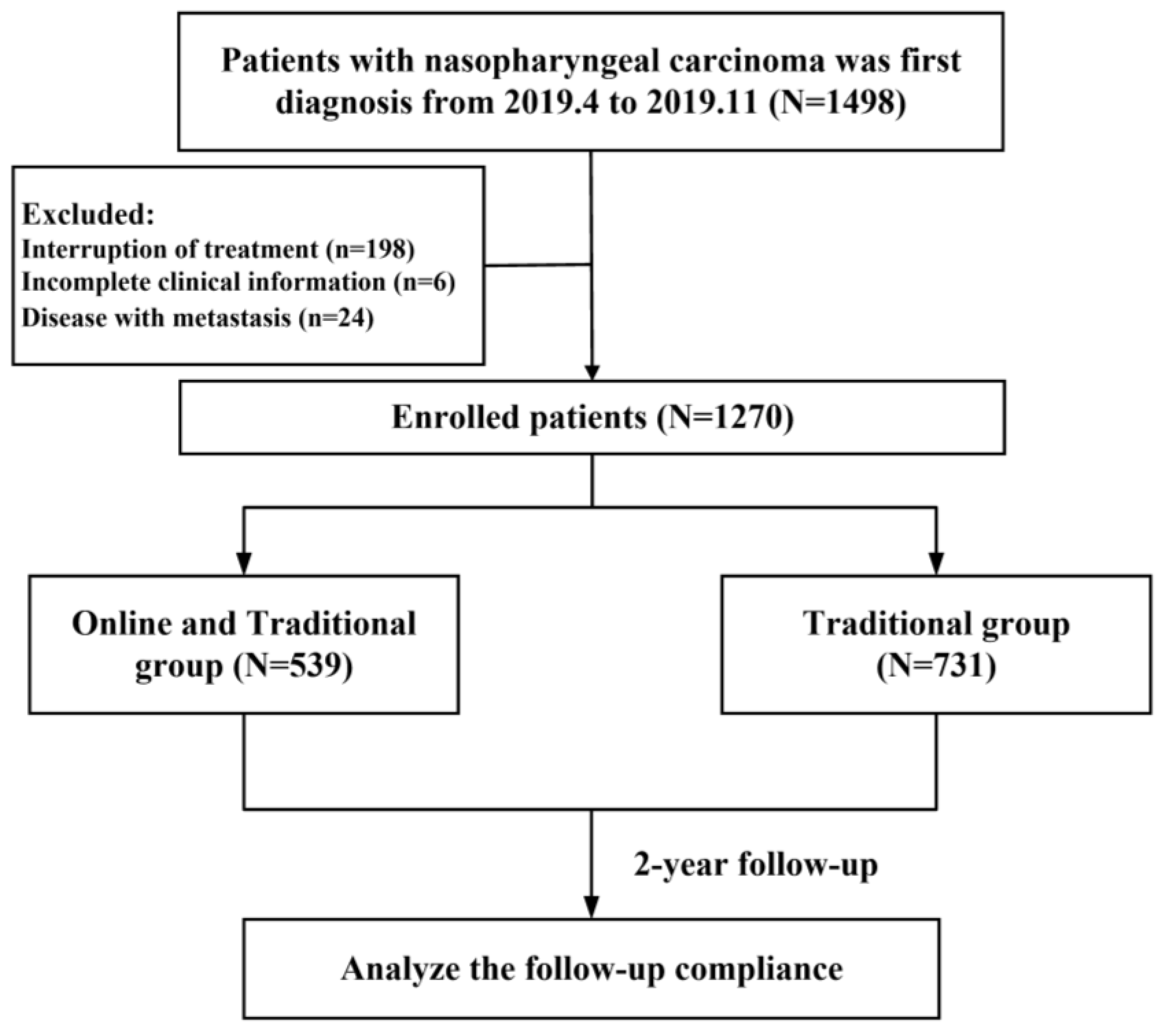
2.1. Study Cohort
2.2. Online Clinic Follow-Up Management System
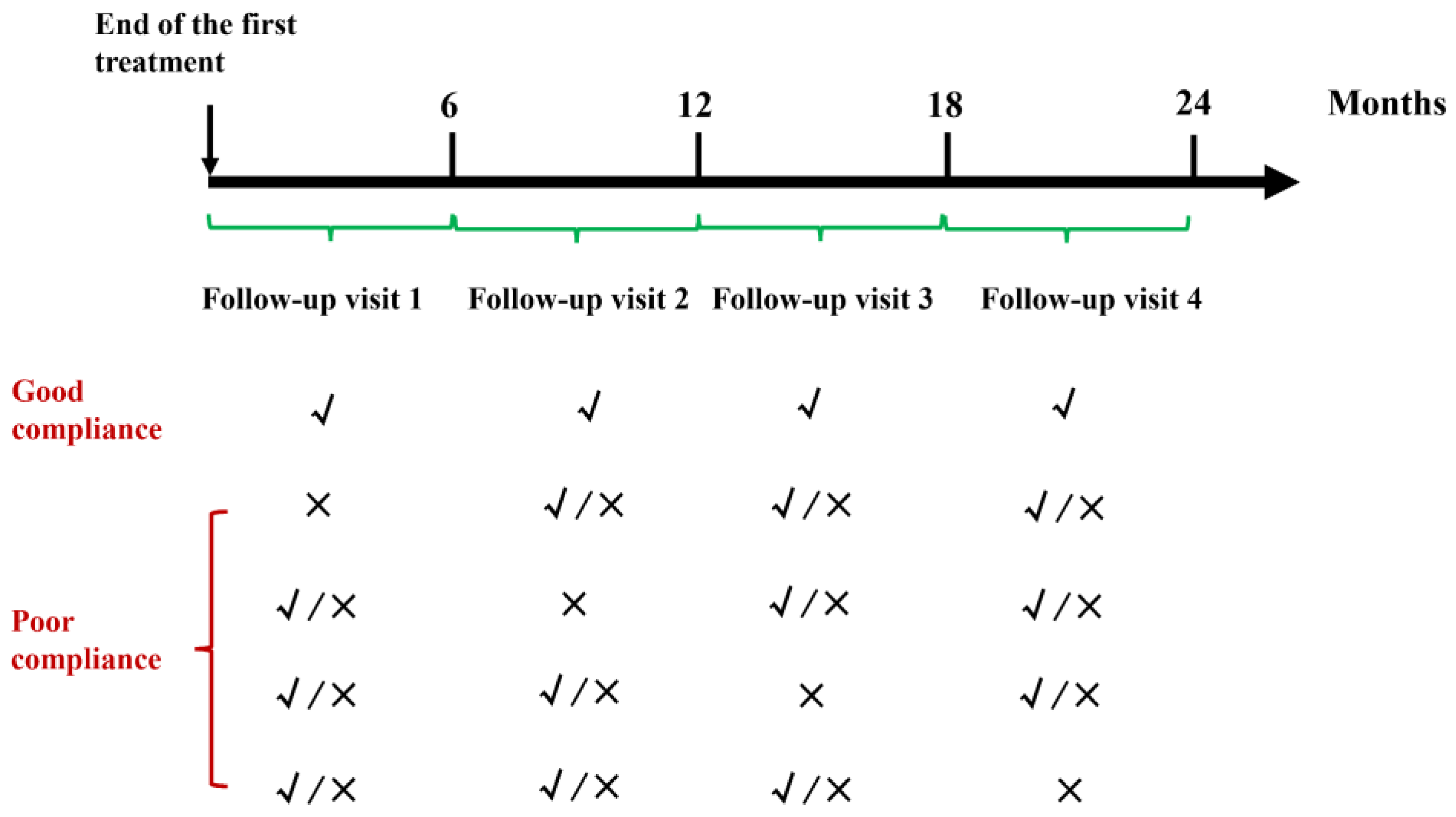
2.3. Follow-Up
2.4. Outcome and Variable Definitions
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Follow-Up Compliance
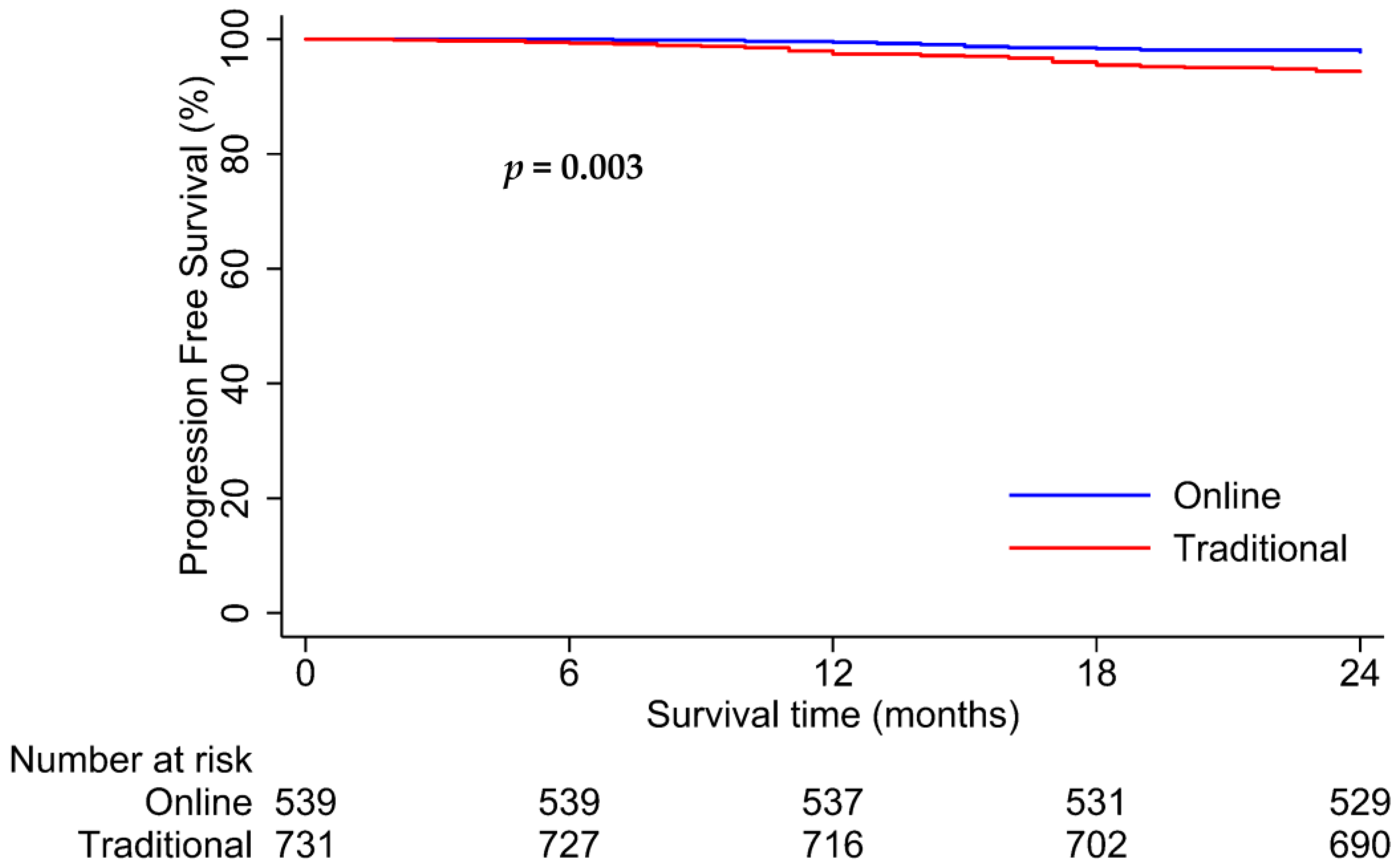
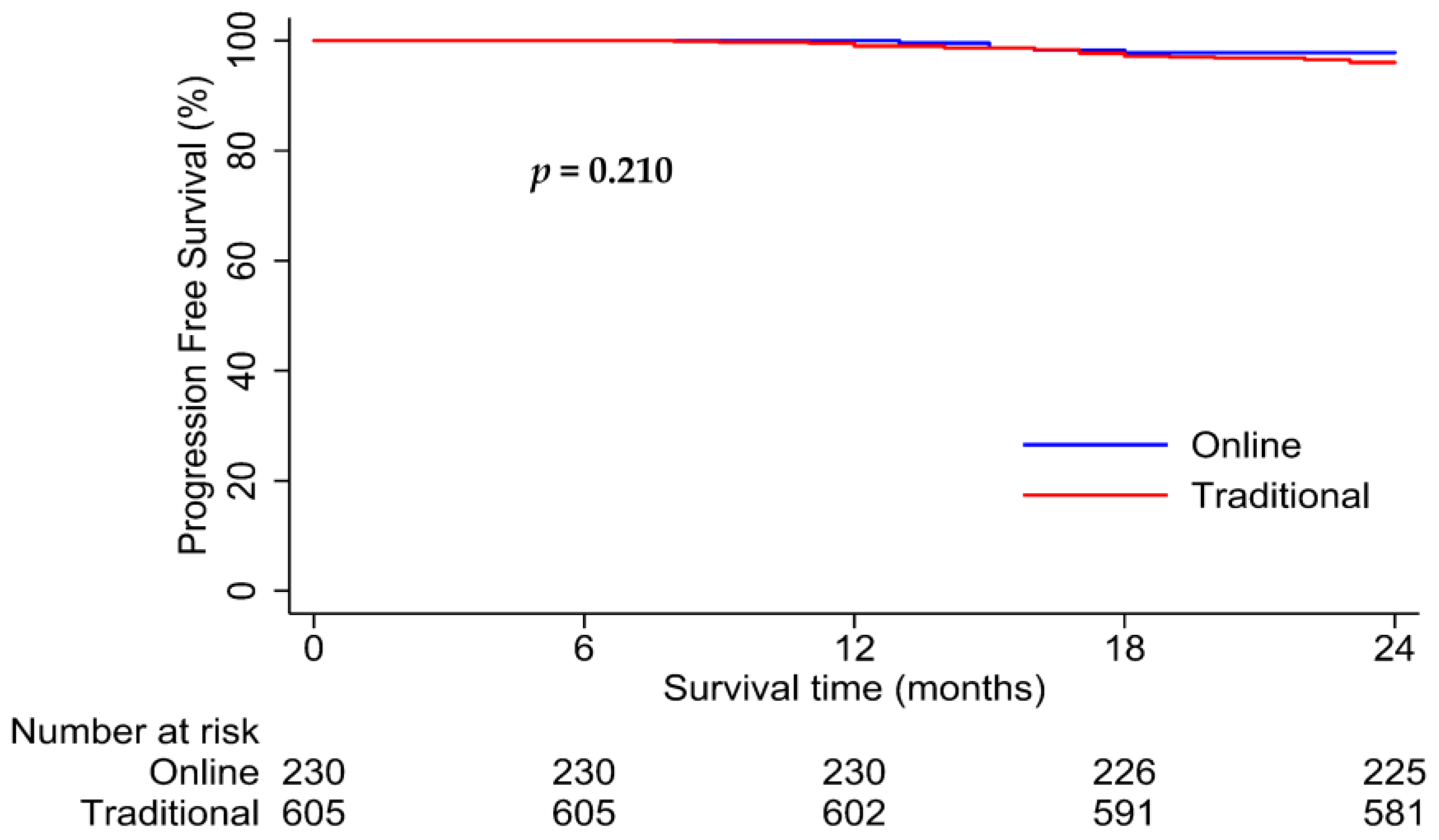
3.3. Survival Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Wong, K.C.W.; Hui, E.P.; Lo, K.W.; Lam, W.K.J.; Johnson, D.; Li, L.; Tao, Q.; Chan, K.C.A.; To, K.F.; King, A.D.; et al. Nasopharyngeal carcinoma: An evolving paradigm. Nat. Rev. Clin. Oncol. 2021, 18, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Balaban, E.P.; Mangu, P.B.; Khorana, A.A.; Shah, M.A.; Mukherjee, S.; Crane, C.H.; Javle, M.M.; Eads, J.R.; Allen, P.; Ko, A.H.; et al. Locally Advanced, Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 2654–2668. [Google Scholar] [CrossRef] [PubMed]
- Sohal, D.P.S.; Kennedy, E.B.; Khorana, A.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Krishnamurthi, S.; Moravek, C.; O’Reilly, E.M.; Philip, P.A.; et al. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.Q.; Wu, C.F.; Deng, B.; Gao, T.S.; Lv, J.W.; Lin, L.; Chen, F.P.; Kou, J.; Zhang, Z.X.; Huang, X.D.; et al. An optimal posttreatment surveillance strategy for cancer survivors based on an individualized risk-based approach. Nat. Commun. 2020, 11, 3872. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lu, J.; Lin, J.; Xu, B.B.; Xue, Z.; Zheng, H.L.; Lin, G.S.; Huang, J.B.; Shen, L.L.; Zheng, C.H.; et al. An international multi-institution real-world study of the optimal surveillance frequency for stage II/III gastric cancer: The more, the better? Int. J. Surg. 2023, 109, 4101–4112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Y.; Su, Y.; Hu, X.; Peng, X. Nasopharyngeal Carcinoma: Clinical Achievements and Considerations among Treatment Options. Front. Oncol. 2021, 11, 635737. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lei, H.; Liang, Z.; Li, L.; Qu, S.; Zhu, X. Intensity-modulated radiotherapy controls nasopharyngeal carcinoma distant metastasis and improves survival of patients. SpringerPlus 2016, 5, 1459. [Google Scholar] [CrossRef]
- Tang, L.L.; Chen, Y.P.; Chen, C.B.; Chen, M.Y.; Chen, N.Y.; Chen, X.Z.; Du, X.J.; Fang, W.F.; Feng, M.; Gao, J.; et al. The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun. 2021, 41, 1195–1227. [Google Scholar] [CrossRef]
- Bossi, P.; Chan, A.T.; Licitra, L.; Trama, A.; Orlandi, E.; Hui, E.P.; Halámková, J.; Mattheis, S.; Baujat, B.; Hardillo, J.; et al. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 452–465. [Google Scholar] [CrossRef]
- Lu, T.; Xu, H.; Huang, W.; Zong, J.; Pan, C.; Huang, C.; Xiao, Y.; Chen, B.; Li, J.; Pan, J.; et al. Constructing an individualized surveillance framework for nasopharyngeal carcinoma based on a dynamic risk-adapted approach. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2023, 185, 109716. [Google Scholar] [CrossRef]
- Nathan, P.C.; Greenberg, M.L.; Ness, K.K.; Hudson, M.M.; Mertens, A.C.; Mahoney, M.C.; Gurney, J.G.; Donaldson, S.S.; Leisenring, W.M.; Robison, L.L.; et al. Medical care in long-term survivors of childhood cancer: A report from the childhood cancer survivor study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 4401–4409. [Google Scholar] [CrossRef] [PubMed]
- May, L.; Schwartz, D.D.; Frugé, E.; Laufman, L.; Holm, S.; Kamdar, K.; Harris, L.; Brackett, J.; Unal, S.; Tanyildiz, G.; et al. Predictors of Suboptimal Follow-up in Pediatric Cancer Survivors. J. Pediatr. Hematol./Oncol. 2017, 39, e143–e149. [Google Scholar] [CrossRef] [PubMed]
- Dabkeviciene, D.; Vincerzevskiene, I.; Urbonas, V.; Venius, J.; Dulskas, A.; Brasiuniene, B.; Janulionis, E.; Burneckis, A.; Zileviciene, A.; Tiskevicius, S.; et al. The Impact of the COVID-19 Pandemic on Cancer Patient’s Management-Lithuanian Cancer Center Experience. Healthcare 2021, 9, 1522. [Google Scholar] [CrossRef] [PubMed]
- de Lusignan, S.; Mold, F.; Sheikh, A.; Majeed, A.; Wyatt, J.C.; Quinn, T.; Cavill, M.; Gronlund, T.A.; Franco, C.; Chauhan, U.; et al. Patients’ online access to their electronic health records and linked online services: A systematic interpretative review. BMJ Open 2014, 4, e006021. [Google Scholar] [CrossRef]
- Dicuonzo, G.; Galeone, G.; Shini, M.; Massari, A. Towards the Use of Big Data in Healthcare: A Literature Review. Healthcare 2022, 10, 1232. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Torkamani, A.; Butte, A.J.; Glicksberg, B.S.; Schuller, B.; Rodriguez, B.; Ting, D.S.W.; Bates, D.; Schaden, E.; Peng, H.; et al. The promise of digital healthcare technologies. Front. Public Health 2023, 11, 1196596. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Chen, S.; Li, M.; Ung, C.O.L.; Hu, H. Policy Interventions, Development Trends, and Service Innovations of Internet Hospitals in China: Documentary Analysis and Qualitative Interview Study. J. Med. Internet Res. 2021, 23, e22330. [Google Scholar] [CrossRef] [PubMed]
- Quer, G.; Radin, J.M.; Gadaleta, M.; Baca-Motes, K.; Ariniello, L.; Ramos, E.; Kheterpal, V.; Topol, E.J.; Steinhubl, S.R. Wearable sensor data and self-reported symptoms for COVID-19 detection. Nat. Med. 2021, 27, 73–77. [Google Scholar] [CrossRef]
- Basch, E.; Schrag, D.; Henson, S.; Jansen, J.; Ginos, B.; Stover, A.M.; Carr, P.; Spears, P.A.; Jonsson, M.; Deal, A.M.; et al. Effect of Electronic Symptom Monitoring on Patient-Reported Outcomes Among Patients with Metastatic Cancer: A Randomized Clinical Trial. JAMA 2022, 327, 2413–2422. [Google Scholar] [CrossRef]
- Car, J.; Huckvale, K.; Hermens, H. Telehealth for long term conditions. BMJ 2012, 344, e4201. [Google Scholar] [CrossRef] [PubMed]
- Steventon, A.; Bardsley, M.; Billings, J.; Dixon, J.; Doll, H.; Hirani, S.; Cartwright, M.; Rixon, L.; Knapp, M.; Henderson, C.; et al. Effect of telehealth on use of secondary care and mortality: Findings from the Whole System Demonstrator cluster randomised trial. BMJ 2012, 344, e3874. [Google Scholar] [CrossRef]
- Shim, J.J.; Kim, G.A.; Oh, C.H.; Kim, J.W.; Myung, J.; Kim, B.H.; Oh, I.H. Reduced liver cancer mortality with regular clinic follow-up among patients with chronic hepatitis B: A nationwide cohort study. Cancer Med. 2020, 9, 7781–7791. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xu, Y.; Xu, T.; Hong, H.; Chen, J.; Qiu, X.; Ding, J.; Huang, C.; Li, L.; Liu, J.; et al. Recommendation for imaging follow-up strategy based on time-specific disease failure for nasopharyngeal carcinoma. Head Neck 2023, 45, 629–637. [Google Scholar] [CrossRef]
- Mai, Z.; Xie, J.; Leng, C.; Xie, X.; Wen, J.; Yang, H.; Liu, Q.; Fu, J. An optimized postsurgery follow-up strategy for patients with esophageal cancer: A cohort study. Int. J. Surg. 2024, 110, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, X.; Yang, Z.; Li, S.; Pan, Y.; Chen, J.; Wang, J.; Hu, D.; Zhou, Z.; Xu, L.; et al. A risk-based postresection follow-up strategy for hepatocellular carcinoma patients. Cancer 2023, 129, 569–579. [Google Scholar] [CrossRef]
- Davidoff, C.; Cheville, A. Telemedicine in Cancer Rehabilitation: Applications and Opportunities across the Cancer Care Continuum. Am. J. Phys. Med. Rehabil. 2024, 103, S52–S57. [Google Scholar] [CrossRef] [PubMed]
- Pennell, N.A.; Dicker, A.P.; Tran, C.; Jim, H.S.L.; Schwartz, D.L.; Stepanski, E.J. mHealth: Mobile Technologies to Virtually Bring the Patient into an Oncology Practice. In American Society of Clinical Oncology Educational Book. American Society of Clinical Oncology. Annual Meeting; ASCO Publications: Alexandria, VA, USA, 2017; Volume 37, pp. 144–154. [Google Scholar] [CrossRef]
- Wosik, J.; Ferranti, J.; Katz, J.N.; Tcheng, J. Telehealth transformation: COVID-19 and the rise of virtual care. J. Am. Med. Inform. Assoc. 2020, 27, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Wang, C.; Wu, S. The internet hospital: An emerging innovation in China. Lancet Glob. Health 2015, 3, e445–e446. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, J.; Wang, Z.; Jia, B.; Zhang, Z.; Guo, J.; Duan, Z.; Lin, X. Postoperative online follow-up improves the quality of life of patients who undergo extraction of impacted madibular third molars: A randomized controlled trial. Clin. Oral Investig. 2021, 25, 993–999. [Google Scholar] [CrossRef]
- Li, L.; Cao, B.; Sun, X.; Yang, C.; Liu, G. Internet follow-up can improve the compliance of sublingual immunotherapy in children with allergic rhinitis: A retrospective cohort study. Transl. Pediatr. 2023, 12, 185–193. [Google Scholar] [CrossRef]
- Viitala, A.; Åstedt-Kurki, P.; Lehto, J.T.; Palonen, M. Online follow-up with a mobile device improves incurable cancer patients’ coping—A qualitative study. Eur. J. Oncol. Nurs. Off. J. Eur. Oncol. Nurs. Soc. 2021, 55, 102047. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.; Wan, W.; Li, J.; Zhang, Y.; Wang, C.; Wang, L. Management of Follow-Up with Preterm Infants during the Outbreak in China. Front. Pediatr. 2021, 9, 637275. [Google Scholar] [CrossRef]
- Han, Y.; Lie, R.K.; Guo, R. The Internet Hospital as a Telehealth Model in China: Systematic Search and Content Analysis. J. Med. Internet Res. 2020, 22, e17995. [Google Scholar] [CrossRef]
- Nakagawa, J.; Fujima, N.; Hirata, K.; Harada, T.; Wakabayashi, N.; Takano, Y.; Homma, A.; Kano, S.; Minowa, K.; Kudo, K. Diagnosis of skull-base invasion by nasopharyngeal tumors on CT with a deep-learning approach. Jpn. J. Radiol. 2024, 42, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Stabile, A.; Giganti, F.; Kasivisvanathan, V.; Giannarini, G.; Moore, C.M.; Padhani, A.R.; Panebianco, V.; Rosenkrantz, A.B.; Salomon, G.; Turkbey, B.; et al. Factors Influencing Variability in the Performance of Multiparametric Magnetic Resonance Imaging in Detecting Clinically Significant Prostate Cancer: A Systematic Literature Review. Eur. Urol. Oncol. 2020, 3, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Deng, Y.; Xia, W.; Qiang, M.; Chen, X.; Liu, K.; Jing, B.; He, C.; Xie, C.; Guo, X.; et al. Development of a self-constrained 3D DenseNet model in automatic detection and segmentation of nasopharyngeal carcinoma using magnetic resonance images. Oral Oncol. 2020, 110, 104862. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, M.; Gallo, C.; Leighl, N.B.; Piccirillo, M.C.; Daniele, G.; Nuzzo, F.; Gridelli, C.; Gebbia, V.; Ciardiello, F.; De Placido, S.; et al. Symptomatic Toxicities Experienced During Anticancer Treatment: Agreement between Patient and Physician Reporting in Three Randomized Trials. J. Clin. Oncol. 2015, 33, 910–915. [Google Scholar] [CrossRef]
- Laugsand, E.A.; Sprangers, M.A.G.; Bjordal, K.; Skorpen, F.; Kaasa, S.; Klepstad, P. Health care providers underestimate symptom intensities of cancer patients: A multicenter European study. Health Qual. Life Outcomes 2010, 8, 104. [Google Scholar] [CrossRef]
- Basch, E.; Iasonos, A.; McDonough, T.; Barz, A.; Culkin, A.; Kris, M.G.; Scher, H.I.; Schrag, D. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: Results of a questionnaire-based study. Lancet Oncol. 2006, 7, 903–909. [Google Scholar] [CrossRef]
- Warrington, L.; Absolom, K.; Conner, M.; Kellar, I.; Clayton, B.; Ayres, M.; Velikova, G. Electronic Systems for Patients to Report and Manage Side Effects of Cancer Treatment: Systematic Review. J. Med. Internet Res. 2019, 21, e10875. [Google Scholar] [CrossRef] [PubMed]
- Yunis, R.; Fonda, S.J.; Aghaee, S.; Kubo, A.; Davis, S.W.; Liu, R.; Neeman, E.; Oakley-Girvan, I. Mobile app activity engagement by cancer patients and their caregivers informs remote monitoring. Sci. Rep. 2024, 14, 3375. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring during Routine Cancer Treatment. JAMA 2017, 318, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Ferrua, M.; Fourcade, A.; Mathivon, D.; Duflot-Boukobza, A.; Dumont, S.; Baudin, E.; Delaloge, S.; Malka, D.; Albiges, L.; et al. Digital remote monitoring plus usual care versus usual care in patients treated with oral anticancer agents: The randomized phase 3 CAPRI trial. Nat. Med. 2022, 28, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Izmailova, E.S.; Wagner, J.A.; Bakker, J.P.; Kilian, R.; Ellis, R.; Ohri, N. A proposed multi-domain, digital model for capturing functional status and health-related quality of life in oncology. Clin. Transl. Sci. 2024, 17, e13712. [Google Scholar] [CrossRef]
- Subbiah, V. The next generation of evidence-based medicine. Nat. Med. 2023, 29, 49–58. [Google Scholar] [CrossRef]
- Shih, C.-H.; Chou, P.-C.; Chou, T.-L.; Huang, T.-W. Measurement of Cancer-Related Fatigue Based on Heart Rate Variability: Observational Study. J. Med. Internet Res. 2021, 23, e25791. [Google Scholar] [CrossRef]
- Stam, J.A.V.; Mestrom, E.H.J.; Nienhuijs, S.W.; Hingh, I.; Boer, A.K.; Riel, N.; Groot, K.; Verhaegh, W.; Scharnhorst, V.; Bouwman, R.A. A wearable patch based remote early warning score (REWS) in major abdominal cancer surgery patients. EJSO 2023, 49, 278–284. [Google Scholar] [CrossRef]
- Pfob, A.; Mehrara, B.J.; Nelson, J.A.; Wilkins, E.G.; Pusic, A.L.; Sidey-Gibbons, C. Towards Patient-Centered Decision-Making in Breast Cancer Surgery: Machine Learning to Predict Individual Patient-Reported Outcomes at 1-Year Follow-Up. Ann. Surg. 2023, 277, e144–e152. [Google Scholar] [CrossRef]
- Xu, C.; Subbiah, I.M.; Lu, S.-C.; Pfob, A.; Sidey-Gibbons, C. Machine learning models for 180-day mortality prediction of patients with advanced cancer using patient-reported symptom data. Qual. Life Res. 2023, 32, 713–727. [Google Scholar] [CrossRef]
- Baumgartner, R. Precision medicine and digital phenotyping: Digital medicine’s way from more data to better health. Big Data Soc. 2021, 8, 20539517211066452. [Google Scholar] [CrossRef]
- Vaz-Luis, I.; Masiero, M.; Cavaletti, G.; Cervantes, A.; Chlebowski, R.T.; Curigliano, G.; Felip, E.; Ferreira, A.R.; Ganz, P.A.; Hegarty, J.; et al. ESMO Expert Consensus Statements on Cancer Survivorship: Promoting high-quality survivorship care and research in Europe. Ann. Oncol. 2022, 33, 1119–1133. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Total (N = 1270) | Online Cohort (N = 539) | Traditional Cohort (N = 731) | p Value |
|---|---|---|---|---|
| Age | ||||
| Median age (years) | 46 (37 to 54) | 44 (36 to 52) | 47 (39 to 56) | <0.001 |
| Sex | 0.638 | |||
| Male | 922 (73%) | 395 (73%) | 527 (72%) | |
| Female | 348 (27%) | 144 (27%) | 204 (28%) | |
| Pre-existing conditions | ||||
| Hypertension | 112 (9%) | 34 (6%) | 78 (11%) | 0.007 |
| Coronary heart disease | 8 (<1%) | 4 (<1%) | 4 (<1%) | 0.664 |
| Diabetes | 47 (4%) | 11 (2%) | 36 (5%) | 0.007 |
| Malignant tumors | 7 (<1%) | 2 (<1%) | 5 (<1%) | 0.457 |
| Infectious diseases | ||||
| Hepatitis | 107 (8%) | 36 (7%) | 71 (10%) | 0.054 |
| Tuberculosis | 28 (2%) | 13 (2%) | 15 (2%) | 0.666 |
| T category | 0.859 | |||
| T1 | 59 (5%) | 23 (4%) | 36 (5%) | |
| T2 | 136 (11%) | 54 (10%) | 82 (11%) | |
| T3 | 752 (59%) | 324 (60%) | 428 (59%) | |
| T4 | 323 (25%) | 138 (26%) | 185 (25%) | |
| N category | 0.217 | |||
| N0 | 114 (9%) | 53 (10%) | 61 (8%) | |
| N1 | 419 (33%) | 166 (31%) | 253 (35%) | |
| N2 | 494 (39%) | 206 (38%) | 288 (39%) | |
| N3 | 243 (19%) | 114 (21%) | 129 (18%) | |
| Clinical stage | 0.694 | |||
| I | 25 (2%) | 9 (2%) | 16 (2%) | |
| II | 76 (6%) | 29 (5%) | 47 (7%) | |
| III | 635 (50%) | 267 (50%) | 368 (50%) | |
| IV | 534 (42%) | 234 (43%) | 300 (41%) | |
| EBV DNA | 0.121 | |||
| ≤2000 copies/mL | 940 (74%) | 411 (76%) | 529 (72%) | |
| >2000 copies/mL | 330 (26%) | 128 (24%) | 202 (28%) | |
| Distance from home to the center (km) | 350 (100 to 680) | 390 (140 to 680) | 350 (100 to 580) | 0.040 |
| Characteristics | Total (N = 666) | Online Cohort (N = 333) | Traditional Cohort (N = 333) | p Value |
|---|---|---|---|---|
| Age | ||||
| Median age (years) | 48 (39 to 55) | 46 (39 to 55) | 46 (37 to 55) | 0.541 |
| Sex | 0.678 | |||
| Male | 453 (68%) | 229 (69%) | 224 (67%) | |
| Female | 213 (32%) | 104 (31%) | 109 (33%) | |
| Pre-existing conditions | ||||
| Hypertension | 74 (11%) | 32 (10%) | 42 (13%) | 0.218 |
| Coronary heart disease | 6 (1%) | 3 (1%) | 3 (1%) | >0.999 |
| Diabetes | 23 (3%) | 11 (3%) | 12 (4%) | 0.832 |
| Malignant tumors | 6 (1%) | 2 (1%) | 4 (1%) | 0.412 |
| Infectious diseases | ||||
| Hepatitis | 70 (11%) | 35 (11%) | 35 (11%) | >0.999 |
| Tuberculosis | 18 (3%) | 10 (3%) | 8 (2%) | 0.633 |
| T category | 0.460 | |||
| T1 | 40 (6%) | 18 (5%) | 22 (7%) | |
| T2 | 78 (12%) | 34 (10%) | 44 (13%) | |
| T3 | 383 (57%) | 200 (60%) | 183 (55%) | |
| T4 | 165 (25%) | 81 (25%) | 84 (25%) | |
| N category | 0.217 | |||
| N0 | 57 (9%) | 27 (8%) | 30 (9%) | |
| N1 | 242 (36%) | 115 (35%) | 127 (38%) | |
| N2 | 239 (36%) | 118 (35%) | 121 (36%) | |
| N3 | 128 (19%) | 73 (22%) | 55 (17%) | |
| Clinical stage | 0.336 | |||
| I | 17 (2%) | 8 (2%) | 9 (3%) | |
| II | 59 (9%) | 23 (7%) | 36 (11%) | |
| III | 311 (47%) | 157 (47%) | 154 (46%) | |
| IV | 279 (42%) | 145 (44%) | 134 (40%) | |
| EBV DNA | 0.561 | |||
| ≤2000 copies/mL | 499 (75%) | 246 (74%) | 253 (76%) | |
| >2000 copies/mL | 167 (25%) | 87 (26%) | 80 (24%) | |
| Distance from home to center (km) | 350 (100 to 680) | 380 (100 to 680) | 350 (100 to 680) | 0.602 |
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95%CI) | p Value | OR (95%CI) | p Value | |
| Form of follow-up (Online, Traditional) | 6.45 (4.99–8.34) | <0.001 | 6.32 (4.88–8.18) | <0.001 |
| Age (≥45, <45) | 0.67 (0.53–0.85) | 0.001 | 0.78 (0.60–1.00) | 0.052 |
| Sex (Male, Female) | 1.04 (0.80–1.35) | 0.771 | ||
| Clinical stage | 1.17 (0.98–1.40) | 0.077 | ||
| Pre-existing condition (Yes, No) | 0.93 (0.73–1.19) | 0.586 | ||
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95%CI) | p Value | OR (95%CI) | p Value | |
| Form of follow-up (Online, Traditional) | 6.24 (4.35–8.96) | <0.001 | 6.81 (4.69–9.90) | <0.001 |
| Age (≥45, <45) | 0.94 (0.68–1.30) | 0.703 | 0.66 (0.45–0.96) | 0.029 |
| Sex (Male, Female) | 0.87 (0.62–1.22) | 0.429 | ||
| Clinical stage | 1.07 (0.86–1.33) | 0.561 | ||
| Pre-existing condition (Yes, No) | 0.98 (0.71–1.35) | 0.903 | ||
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Compliance (Good, Poor) | 1.63 (0.95–2.80) | 0.077 | ||
| Form of follow-up (Online, Traditional) | 0.39 (0.20–0.74) | 0.004 | 0.40 (0.21–0.76) | 0.005 |
| Age (≥45, <45) | 1.78 (1.00–3.16) | 0.051 | 1.67 (0.93–2.97) | 0.084 |
| Sex (Male, Female) | 1.88 (0.92–3.84) | 0.086 | ||
| Clinical stage | 2.19 (1.33–3.60) | 0.002 | 2.25 (1.37–3.70) | 0.001 |
| Pre-existing condition (Yes, No) | 1.34 (0.78–2.32) | 0.289 | ||
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Compliance (Good, Poor) | 1.25 (0.58–2.69) | 0.571 | ||
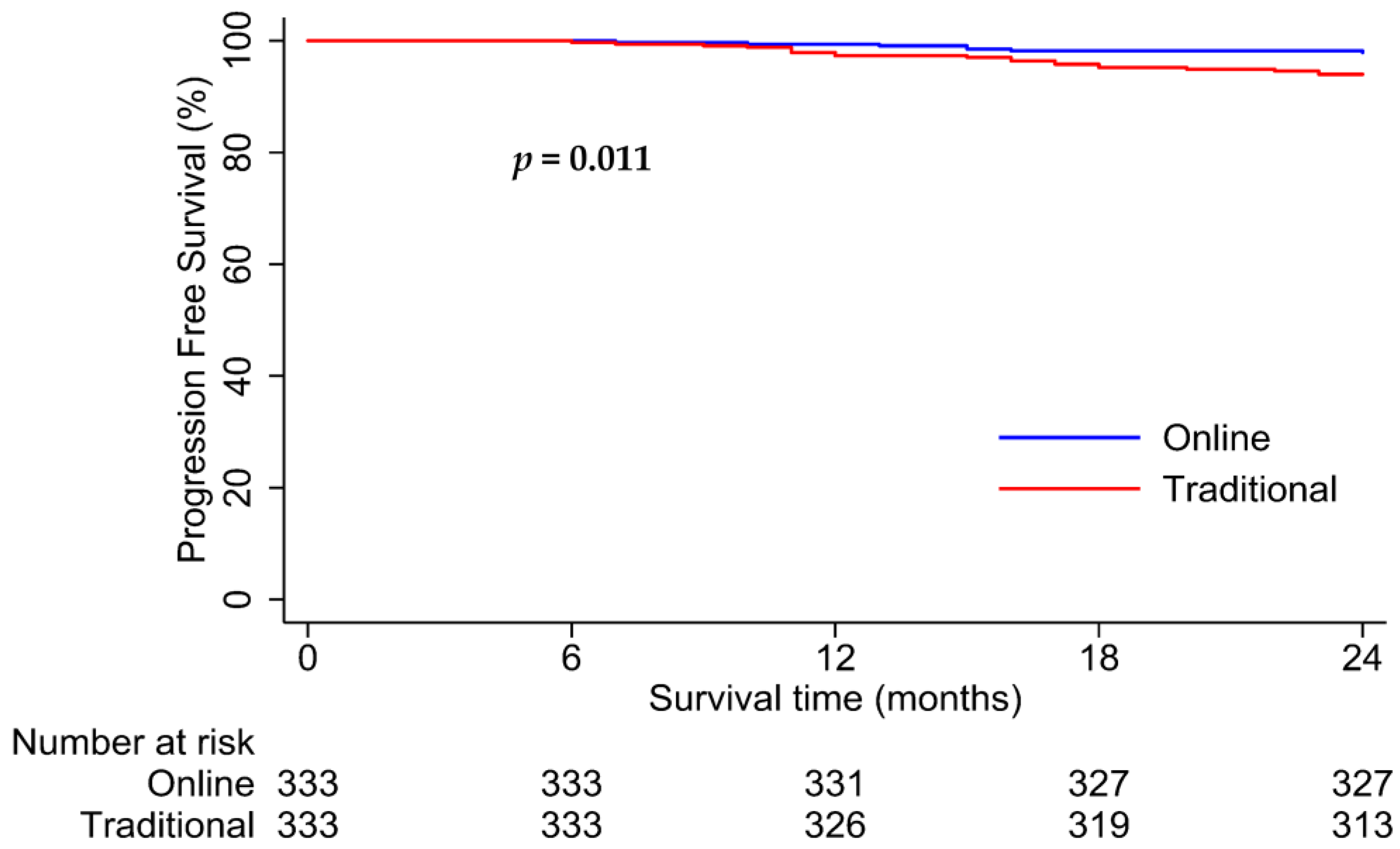
| Form of follow-up (Online, Traditional) | 0.34 (0.15–0.81) | 0.015 | 0.33 (0.14–0.78) | 0.011 |
| Age (≥45, <45) | 1.21 (0.54–2.68) | 0.647 | ||
| Sex (Male, Female) | 1.67 (0.67–4.13) | 0.270 | ||
| Clinical stage | 1.92 (1.01–3.63) | 0.045 | 1.99 (1.05–3.75) | 0.034 |
| Pre-existing condition (Yes, No) | 1.05 (0.49–2.27) | 0.896 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Feng, C.; Sun, P.; Zhang, J.; Liang, H. Effect of Online Clinic on Follow-Up Compliance and Survival Outcomes in Nasopharyngeal Carcinoma: Real-World Cohort Study from Endemic Area. Healthcare 2024, 12, 1452. https://doi.org/10.3390/healthcare12141452
Chen S, Feng C, Sun P, Zhang J, Liang H. Effect of Online Clinic on Follow-Up Compliance and Survival Outcomes in Nasopharyngeal Carcinoma: Real-World Cohort Study from Endemic Area. Healthcare. 2024; 12(14):1452. https://doi.org/10.3390/healthcare12141452
Chicago/Turabian StyleChen, Siqi, Chenyang Feng, Peng Sun, Jingrong Zhang, and Hu Liang. 2024. "Effect of Online Clinic on Follow-Up Compliance and Survival Outcomes in Nasopharyngeal Carcinoma: Real-World Cohort Study from Endemic Area" Healthcare 12, no. 14: 1452. https://doi.org/10.3390/healthcare12141452





