Spatial Analysis of Health System Factors in Infectious Disease Management: Lessons Learned from the COVID-19 Pandemic in Korea
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Data Sources
2.2. Variables
2.3. Statistical Analysis
2.4. Ethical Approval
3. Results
3.1. Descriptive Statistics
3.2. Geographical Distribution of Confirmed COVID-19 Cases
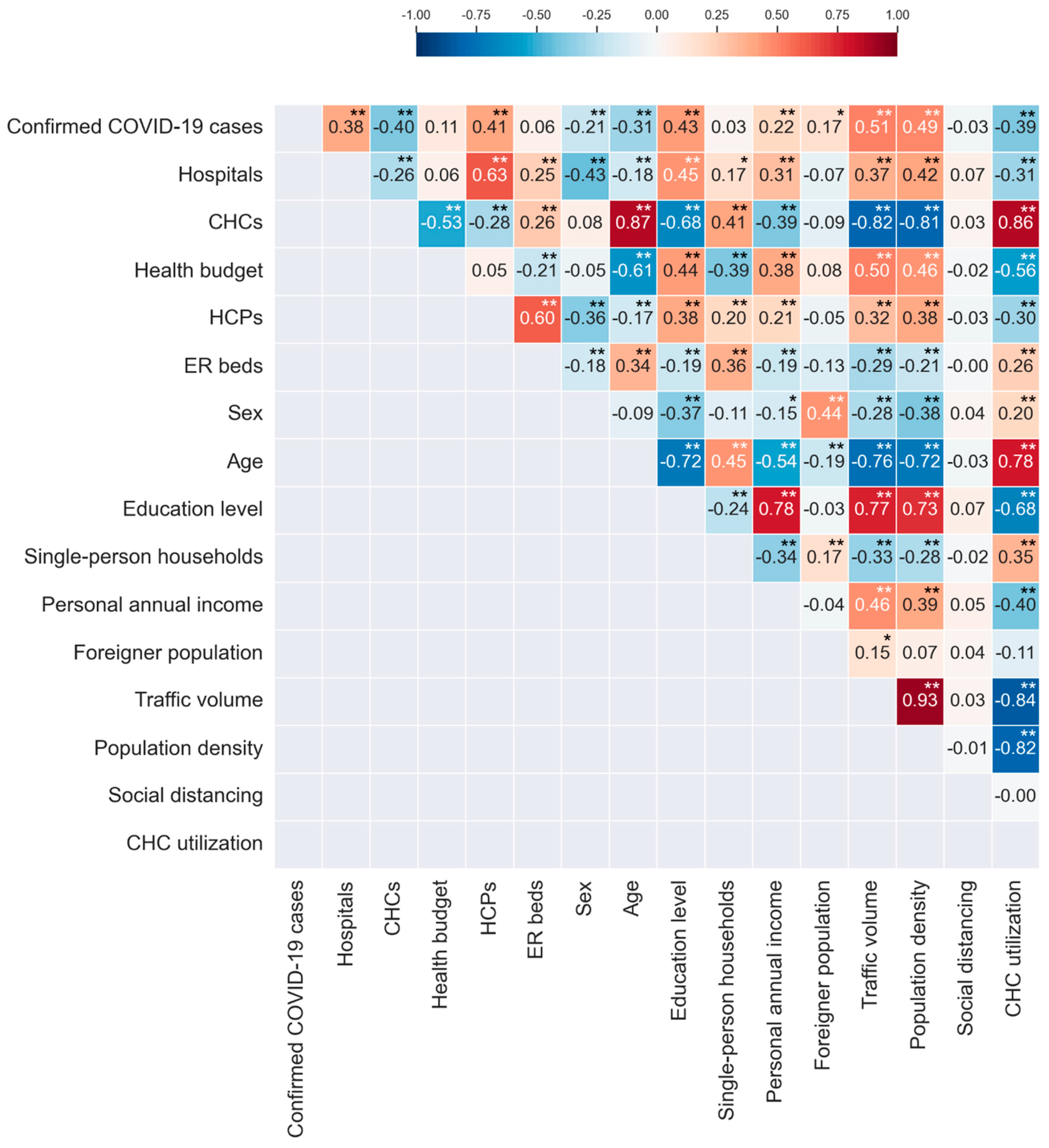
3.3. Correlation Analysis Results
3.4. Regression Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nuzzo, J.B.; Meyer, D.; Snyder, M.; Ravi, S.J.; Lapascu, A.; Souleles, J.; Andrada, C.I.; Bishai, D. What makes health systems resilient against infectious disease outbreaks and natural hazards? Results from a scoping review. BMC Public Health 2019, 19, 1310. [Google Scholar] [CrossRef] [PubMed]
- Burrowes, S.A.; Casey, S.M.; Pierre-Joseph, N.; Talbot, S.G.; Hall, T.; Christian-Brathwaite, N.; Del-Carmen, M.; Garofalo, C.; Lundberg, B.; Mehta, P.K.; et al. COVID-19 pandemic impacts on mental health, burnout, and longevity in the workplace among healthcare workers: A mixed methods study. J. Interprofessional Educ. Pract. 2023, 32, 100661. [Google Scholar] [CrossRef] [PubMed]
- Lazenby, M.; Chambers, S.; Chyun, D.; Davidson, P.; Dithole, K.; Norman, I.; Tlou, S. Clinical nursing and midwifery education in the pandemic age. Int. Nurs. Rev. 2020, 67, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Haldane, V.; De Foo, C.; Abdalla, S.M.; Jung, A.S.; Tan, M.; Wu, S.; Chua, A.; Verma, M.; Shrestha, P.; Singh, S.; et al. Health systems resilience in managing the COVID-19 pandemic: Lessons from 28 countries. Nat. Med. 2021, 27, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Meena, P.; Abdellatif, D.; Tiwari, V.; Chatterjee, S.; Luyckx, V.A. Health Systems Preparedness for Infectious Disease Outbreaks: Relevance for Nephrology. Semin. Nephrol. 2023, 43, 151465. [Google Scholar] [CrossRef] [PubMed]
- Craciun, O.M.; Del Rosario Torres, M.; Llanes, A.B.; Romay-Barja, M. Tuberculosis Knowledge, Attitudes, and Practice in Middle- and Low-Income Countries: A Systematic Review. J. Trop. Med. 2023, 2023, 1014666. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Bowers, K.; Zhu, D.; Gao, X.; Cheng, T. Spatio-temporal stratified associations between urban human activities and crime patterns: A case study in San Francisco around the COVID-19 stay-at-home mandate. Comput. Urban Sci. 2022, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, V.; Antony, G.; Wismar, M.; Rechel, B. COVID-19 pandemic: Health impact of staying at home, social distancing and ‘lockdown’ measures—A systematic review of systematic reviews. J. Public Health 2021, 43, e462–e481. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Sarmadi, M.; Marufi, N.; Moghaddam, V.K. Association of COVID-19 global distribution and environmental and demographic factors: An updated three-month study. Environ. Res. 2020, 188, 109748. [Google Scholar] [CrossRef]
- Meyer, D.; Bishai, D.; Ravi, S.J.; Rashid, H.; Mahmood, S.S.; Toner, E.; Nuzzo, J.B. A checklist to improve health system resilience to infectious disease outbreaks and natural hazards. BMJ Glob. Health 2020, 5, e002429. [Google Scholar] [CrossRef]
- KDCA (Korea Disease Control and Prevention Agency). Number of Confirmed COVID-19 Cases by City; KDCA: Cheongju, Republic of Korea, 2024; unpublished raw data.
- KOSIS (Korean Statistical Information Service). 2024. Available online: https://kosis.kr (accessed on 12 June 2024).
- KTDB (Korea Transport Database). Estimated Traffic Volume by Administrative District [Data File]. 2024. Available online: https://www.ktdb.go.kr (accessed on 12 June 2024).
- Cuadros, D.F.; Branscum, A.J.; Mukandavire, Z.; Miller, F.D.; MacKinnon, N. Dynamics of the COVID-19 epidemic in urban and rural areas in the United States. Ann. Epidemiol. 2021, 59, 16–20. [Google Scholar] [CrossRef]
- Yang, S.; Jang, J.; Park, S.; Ahn, S.; Kim, S.; Park, S.; Ryu, B.; Lee, S.; Shin, E.; Kim, N.; et al. Two-year report of COVID-19 outbreak from January 20, 2020 to January 19, 2022 in the Republic of Korea. Public Health Wkly. Rep. 2022, 2022, 414–426. [Google Scholar]
- WHO (World Health Organization). Health Systems Strengthening Glossary. Available online: https://cdn.who.int/media/docs/default-source/documents/health-systems-strengthening-glossary.pdf (accessed on 20 June 2024).
- WHO (World Health Organization). Monitoring the Building Blocks of Health Systems: A Handbook of Indicators and Their Measurement Strategies; World Health Organization: Geneva, Switzerland, 2010.
- Liu, L. Study on the spatial decomposition of the infection probability of COVID-19. Sci. Rep. 2023, 13, 13258. [Google Scholar] [CrossRef] [PubMed]
- Franzese, R.J.; Hays, J.C. Spatial Econometric Models of Cross-Sectional Interdependence in Political Science Panel and Time-Series-Cross-Section Data. Political Anal. 2007, 15, 140–164. [Google Scholar] [CrossRef]
- Anselin, L. Spatial Econometrics. In A Companion to Theoretical Econometrics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 310–330. [Google Scholar] [CrossRef]
- Pace, R.K.; LeSage, J.P. Omitted Variable Biases of OLS and Spatial Lag Models. In Advances in Spatial Science; Springer: Berlin/Heidelberg, Germany, 2009; pp. 17–28. [Google Scholar] [CrossRef]
- Cook, S.J.; An, S.H.; Favero, N. Beyond Policy Diffusion: Spatial Econometric Models of Public Administration. J. Public Adm. Res. Theory 2018, 29, 591–608. [Google Scholar] [CrossRef]
- Anselin, L.; Bera, A.K.; Florax, R.; Yoon, M.J. Simple diagnostic tests for spatial dependence. Reg. Sci. Urban Econ. 1996, 26, 77–104. [Google Scholar] [CrossRef]
- Bera, A.K.; Yoon, M.J. Specification Testing with Locally Misspecified Alternatives. Econom. Theory 1993, 9, 649–658. [Google Scholar] [CrossRef]
- Department of Health and Human Services. Federal Policy for the Protection of Human Subjects. 2017. Available online: https://www.federalregister.gov/d/2017-01058/p-332 (accessed on 28 May 2024).
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Proceedings of the 2nd International Symposium on Information Theory; Petrov, B.N., Csaki, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- LeSage, J.; Pace, R.K. Introduction to Spatial Econometrics; Chapman and Hall: New York, NY, USA; CRC: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Anselin, L. Under the hood: Issues in the specification and interpretation of spatial regression models. Agric. Econ. 2002, 27, 247–267. [Google Scholar] [CrossRef]
- Adetokunboh, O.O.; Are, E.B. Spatial distribution and determinants of HIV high burden in the Southern African sub-region. PLoS ONE 2024, 19, e0301850. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.C.C.; Ferreira, A.F.; Da Silva Filho, J.D.; Da Silveira Lima, M.; Martins-Melo, F.R.; Bezerra, F.S.M.; Sousa, M.S.; Ramos, A.N. Burden of schistosomiasis-related mortality in Brazil: Epidemiological patterns and spatial–temporal distribution, 2003–2018. Trop. Med. Int. Health 2020, 25, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xie, X.; Rao, Z.; Zheng, Z.; Hu, C.; Li, S.; Hu, Z. Spatial Analysis and Comparison of the Economic Burden of Common Diseases: An Investigation of 5.7 Million Rural Elderly Inpatients in Southeast China, 2010–2016. Front. Public Health 2021, 9, 774342. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Rosales, R.; Quintana-Romero, L.; De Jesús-Almonte, L.; De La Cruz Del Río-Rama, M. Spatial spillovers of economic growth and public spending in Mexico: Evidence from a SpVAR model, 1999–2019. Econ. Anal. Policy 2021, 71, 660–673. [Google Scholar] [CrossRef]
- Chih, Y.; Kishan, R.P.; Ojede, A. Be good to thy neighbours: A spatial analysis of foreign direct investment and economic growth in sub-Saharan Africa. World Econ. 2021, 45, 657–701. [Google Scholar] [CrossRef]
- Ren, Y.; Ren, X.; Hu, J. Driving factors of China’s city-level carbon emissions from the perspective of spatial spillover effect. Carbon Manag. 2019, 10, 551–566. [Google Scholar] [CrossRef]
- Wells, K.; O’Hara, R.B.; Morand, S.; Lessard, J.P.; Ribas, A. The importance of parasite geography and spillover effects for global patterns of host–parasite associations in two invasive species. Divers. Distrib. 2014, 21, 477–486. [Google Scholar] [CrossRef]
- Mitchell, J.L. Does Policy Diffusion Need Space? Spatializing the Dynamics of Policy Diffusion. Policy Stud. J. 2017, 46, 424–451. [Google Scholar] [CrossRef]
- Song, Z.; Wang, C.; Bergmann, L. China’s prefectural digital divide: Spatial analysis and multivariate determinants of ICT diffusion. Int. J. Inf. Manag. 2020, 52, 102072. [Google Scholar] [CrossRef]
- Campedelli, G.M.; Favarin, S.; Aziani, A.; Piquero, A.R. Disentangling community-level changes in crime trends during the COVID-19 pandemic in Chicago. Crime Sci. 2020, 9, 21. [Google Scholar] [CrossRef]
- Yang, M.; Chen, Z.; Zhou, M.; Liang, X.; Bai, Z. The Impact of COVID-19 on Crime: A Spatial Temporal Analysis in Chicago. ISPRS Int. J. Geo-Inf. 2021, 10, 152. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Kumari, S. Investigating the Spatial Spillover Effect of Transportation Infrastructure on Green Total Factor Productivity. Energies 2023, 16, 2733. [Google Scholar] [CrossRef]
- Yu, N.; De Jong, M.; Storm, S.; Mi, J. Spatial spillover effects of transport infrastructure: Evidence from Chinese regions. J. Transp. Geogr. 2013, 28, 56–66. [Google Scholar] [CrossRef]
- Kim, D. Exploratory study on the spatial relationship between emerging infectious diseases and urban characteristics: Cases from Korea. Sustain. Cities Soc. 2021, 66, 102672. [Google Scholar] [CrossRef]
- Hamidi, S.; Ewing, R.; Sabouri, S. Longitudinal analyses of the relationship between development density and the COVID-19 morbidity and mortality rates: Early evidence from 1,165 metropolitan counties in the United States. Health Place 2020, 64, 102378. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, A. The socio-economic determinants of COVID-19: A spatial analysis of German county level data. Socio-Econ. Plan. Sci. 2021, 78, 101083. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Villamizar, L.A.; Belalcázar-Ceron, L.C.; Fernández-Niño, J.A.; Marín-Pineda, D.M.; Rojas-Sánchez, O.A.; Acuña-Merchán, L.A.; Ramírez-García, N.; Mangones-Matos, S.C.; Vargas-González, J.M.; Herrera-Torres, J.; et al. Air pollution, sociodemographic and health conditions effects on COVID-19 mortality in Colombia: An ecological study. Sci. Total Environ. 2021, 756, 144020. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Hong, A.; Sung, H. Density or Connectivity: What Are the Main Causes of the Spatial Proliferation of COVID-19 in Korea? Int. J. Environ. Res. Public Health 2021, 18, 5084. [Google Scholar] [CrossRef] [PubMed]
- Van Iseghem, T.; Jacobs, I.; Vanden Bossche, D.; Delobelle, P.; Willems, S.; Masquillier, C.; Decat, P. The role of community health workers in primary healthcare in the WHO-EU region: A scoping review. Int. J. Equity Health 2023, 22, 134. [Google Scholar] [CrossRef]
- Hartzler, A.L.; Tuzzio, L.; Hsu, C.; Wagner, E.H. Roles and Functions of Community Health Workers in Primary Care. Ann. Fam. Med. 2018, 16, 240–245. [Google Scholar] [CrossRef]
- Cook, N.; McGrath, B.M.; Navale, S.M.; Koroukian, S.M.; Templeton, A.R.; Crocker, L.C.; Zyzanski, S.J.; Bensken, W.P.; Stange, K.C. Care Delivery in Community Health Centers Before, During, and After the COVID-19 Pandemic (2019–2022). J. Am. Board Fam. Med. 2023, 36, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Meador, M.; Coronado, F.; Roy, D.; Bay, R.C.; Lewis, J.H.; Chen, J.; Cheung, R.; Utman, C.; Hannan, J.A. Impact of COVID-19-related care disruptions on blood pressure management and control in community health centers. BMC Public Health 2022, 22, 2295. [Google Scholar] [CrossRef]
- De Foo, C.; Verma, M.; Tan, S.Y.; Hamer, J.; Van Der Mark, N.; Pholpark, A.; Hanvoravongchai, P.; Cheh, P.L.J.; Marthias, T.; Mahendradhata, Y.; et al. Health financing policies during the COVID-19 pandemic and implications for universal health care: A case study of 15 countries. Lancet Glob. Health 2023, 11, e1964–e1977. [Google Scholar] [CrossRef] [PubMed]
- Swann, W.L.; DiNardi, M.; Schreiber, T.L. Association Between Interorganizational Collaboration in Opioid Response and Treatment Capacity for Opioid Use Disorder in Counties of Five States: A Cross-Sectional Study. Subst. Abus. 2022, 16, 117822182211119. [Google Scholar] [CrossRef] [PubMed]
- Langabeer, J.R.; Champagne-Langabeer, T.; Helton, J.R.; Segrest, W.; Kash, B.; DelliFraine, J.; Fowler, R. Interorganizational Collaboration in Emergency Cardiovascular Care. Qual. Manag. Health Care 2017, 26, 1–6. [Google Scholar] [CrossRef]
- Boterman, W.R. Urban-Rural Polarisation in Times of the Corona Outbreak? The Early Demographic and Geographic Patterns of the SARS-CoV-2 Epidemic in the Netherlands. Tijdschr. Econ. Soc. Geogr. 2020, 111, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Kane, A.D.; Cook, T.M. Outcomes from intensive care in patients with COVID-19: A systematic review and meta-analysis of observational studies. Anaesthesia 2020, 75, 1340–1349. [Google Scholar] [CrossRef]
- Castillo, R.C.; Staguhn, E.D.; Weston-Farber, E. The effect of state-level stay-at-home orders on COVID-19 infection rates. Am. J. Infect. Control 2020, 48, 958–960. [Google Scholar] [CrossRef]

| Variables | Measurements | Sources |
|---|---|---|
| Confirmed COVID-19 cases | Cumulative number of confirmed COVID-19 cases per 100,000 people in a city from January 2020 to April 2021 | KDCA [12] |
| Hospitals | Number of hospitals in a city per 1000 people | KOSIS [13] |
| CHCs | Number of community health centers in a city per 1000 people | KOSIS [13] |
| Health budget | Budget amount (converted to millions of USD) allocated to the health sector by a city government † | KOSIS [13] |
| HCPs | Number of medical doctors and nurses in a city per 1000 people | KOSIS [13] |
| ER beds | Number of emergency room beds in a city per 1000 people | KOSIS [13] |
| Sex | Male population divided by the female population, multiplied by 100, in a city | KOSIS [13] |
| Age | Median age of city residents | KOSIS [13] |
| Educational level | Percentage of city residents with a bachelor’s degree or higher | KOSIS [13] |
| Single-person households | Percentage of single-person households in a city | KOSIS [13] |
| Personal annual income | Income per worker reported (converted to thousands of USD) during the year-end tax filing for earned income in a city † | KOSIS [13] |
| Foreigner population | Percentage of foreigners living in a city at the time of survey who have lived in Korea for more than 3 months, relative to the total city population | KOSIS [13] |
| Traffic volume | Estimated number of vehicles (cars, buses and trucks) travelling along roads within a city per day | KTDB [14] |
| Population density | Persons living in a city per km2 | KOSIS [13] |
| Social distancing | Percentage of city residents who practiced social distancing in the past week at the time of survey | KOSIS [13] |
| CHC utilization | Percentage of city residents who used community health centers in the past year at the time of survey | KOSIS [13] |
| Variable | Count | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|---|
| Confirmed COVID-19 cases (per 100,000 people) | 225 | 199.66 | 159.19 | 9.58 | 1243.23 |
| Hospitals (per 1000 people) | 225 | 0.66 | 0.36 | 0.13 | 3.70 |
| CHCs (per 1000 people) | 225 | 0.23 | 0.29 | 0.00 | 1.23 |
| Health budget (Million USD) † | 225 | 14.97 | 10.38 | 3.89 | 105.12 |
| HCPs (per 1000 people) | 225 | 5.89 | 6.04 | 0.94 | 50.36 |
| ER beds (per 1000 people) | 225 | 0.21 | 0.16 | 0.00 | 1.11 |
| Sex | 225 | 100.92 | 6.28 | 88.00 | 124.80 |
| Age (years) | 225 | 46.09 | 4.83 | 36.90 | 57.40 |
| Educational level (%) | 225 | 26.24 | 10.09 | 11.50 | 62.75 |
| Single-person households (%) | 225 | 33.48 | 5.06 | 18.26 | 51.91 |
| Personal annual income (Thousand USD) † | 225 | 29.70 | 5.50 | 21.15 | 62.09 |
| Foreigner population (%) | 225 | 3.09 | 2.29 | 0.51 | 12.94 |
| Traffic volume (vehicles/day) | 225 | 6272.99 | 4980.03 | 788.00 | 22,335.00 |
| Population density (persons/km2) | 225 | 3856.49 | 6006.79 | 18.31 | 25,225.51 |
| Social distancing (%) | 225 | 95.35 | 4.61 | 67.20 | 99.90 |
| CHC utilization (%) | 225 | 31.10 | 12.46 | 13.10 | 67.80 |
| Model 1 (OLS) | Model 2 (SLM) | |||||
|---|---|---|---|---|---|---|
| Variable | Coefficient | SE | p | Coefficient | SE | p |
| Spatial Autocorrelation Coefficient for COVID-19 Cases (ρ) | 0.293 | 0.079 | 0.000 ** | |||
| Hospitals | 6.521 | 30.319 | 0.830 | 10.024 | 28.159 | 0.722 |
| CHCs | −177.399 | 73.340 | 0.016 * | −140.806 | 68.334 | 0.039 * |
| Health budget | −44.517 | 18.568 | 0.017 * | −36.967 | 17.333 | 0.033 * |
| HCPs | 4.324 | 2.356 | 0.068 | 3.765 | 2.190 | 0.086 |
| ER beds | 54.143 | 79.222 | 0.495 | 73.261 | 73.603 | 0.320 |
| Sex | 1.653 | 2.127 | 0.438 | 1.191 | 1.978 | 0.547 |
| Age | 4.453 | 3.468 | 0.201 | 2.853 | 3.246 | 0.379 |
| Educational level | 0.788 | 2.209 | 0.722 | 0.188 | 2.058 | 0.927 |
| Single-person households | 4.177 | 2.094 | 0.047 * | 3.106 | 1.952 | 0.112 |
| Personal annual income | 46.619 | 95.008 | 0.624 | 35.393 | 88.239 | 0.688 |
| Foreigner population | 3.361 | 4.564 | 0.462 | 3.051 | 4.243 | 0.472 |
| Traffic volume | 25.873 | 30.270 | 0.394 | 19.207 | 28.119 | 0.495 |
| Population density | 5.327 | 10.713 | 0.620 | 4.311 | 10.089 | 0.669 |
| Social distancing | −1.677 | 1.651 | 0.311 | −2.302 | 1.534 | 0.133 |
| CHC utilization | 2.179 | 1.361 | 0.111 | 1.965 | 1.266 | 0.121 |
| Seoul Capital Area | 180.980 | 25.005 | 0.000 ** | 137.451 | 25.604 | 0.000 ** |
| Daegu Metropolitan City | 300.299 | 43.431 | 0.000 ** | 226.423 | 44.492 | 0.000 ** |
| Constant | −612.624 | 544.290 | 0.262 | −345.673 | 506.731 | 0.495 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lee, S. Spatial Analysis of Health System Factors in Infectious Disease Management: Lessons Learned from the COVID-19 Pandemic in Korea. Healthcare 2024, 12, 1484. https://doi.org/10.3390/healthcare12151484
Lee J, Lee S. Spatial Analysis of Health System Factors in Infectious Disease Management: Lessons Learned from the COVID-19 Pandemic in Korea. Healthcare. 2024; 12(15):1484. https://doi.org/10.3390/healthcare12151484
Chicago/Turabian StyleLee, Jeongwook, and SangA Lee. 2024. "Spatial Analysis of Health System Factors in Infectious Disease Management: Lessons Learned from the COVID-19 Pandemic in Korea" Healthcare 12, no. 15: 1484. https://doi.org/10.3390/healthcare12151484





